Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing Conditions
2.2. Preparation of E1800
2.3. Acute Toxicity Assay: 1st Instar Larvae
2.4. Acute Toxicity Assay: 3rd Instar Larvae and Pupae
2.5. Stability of Acute Toxicity of E1800 against 1st Instar Larvae
2.6. Chronic Toxicity and Development Assay: 1st Instar Larvae
2.7. Physical Characterization of E1800 Suspensions
2.8. Statistical Analysis
3. Results
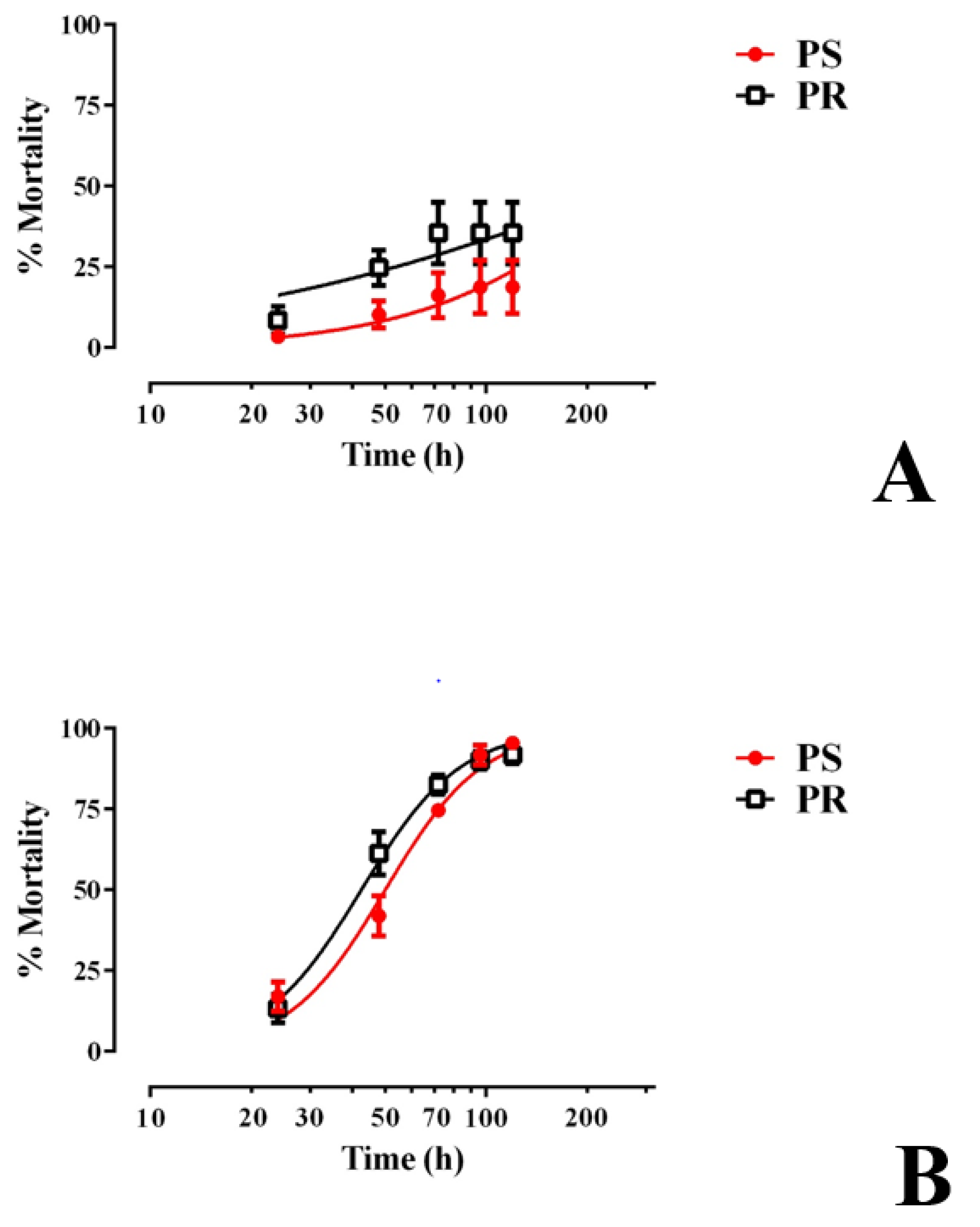
3.1. E1800 Kills 1st Instar Larvae of PS and PR Strains within 48 h
3.2. Stability of the Larvicidal Activity of E1800 Suspensions
3.3. E1800 Is Toxic to Larvae within 120 h of Exposure and Does Not Alter Development Thereafter
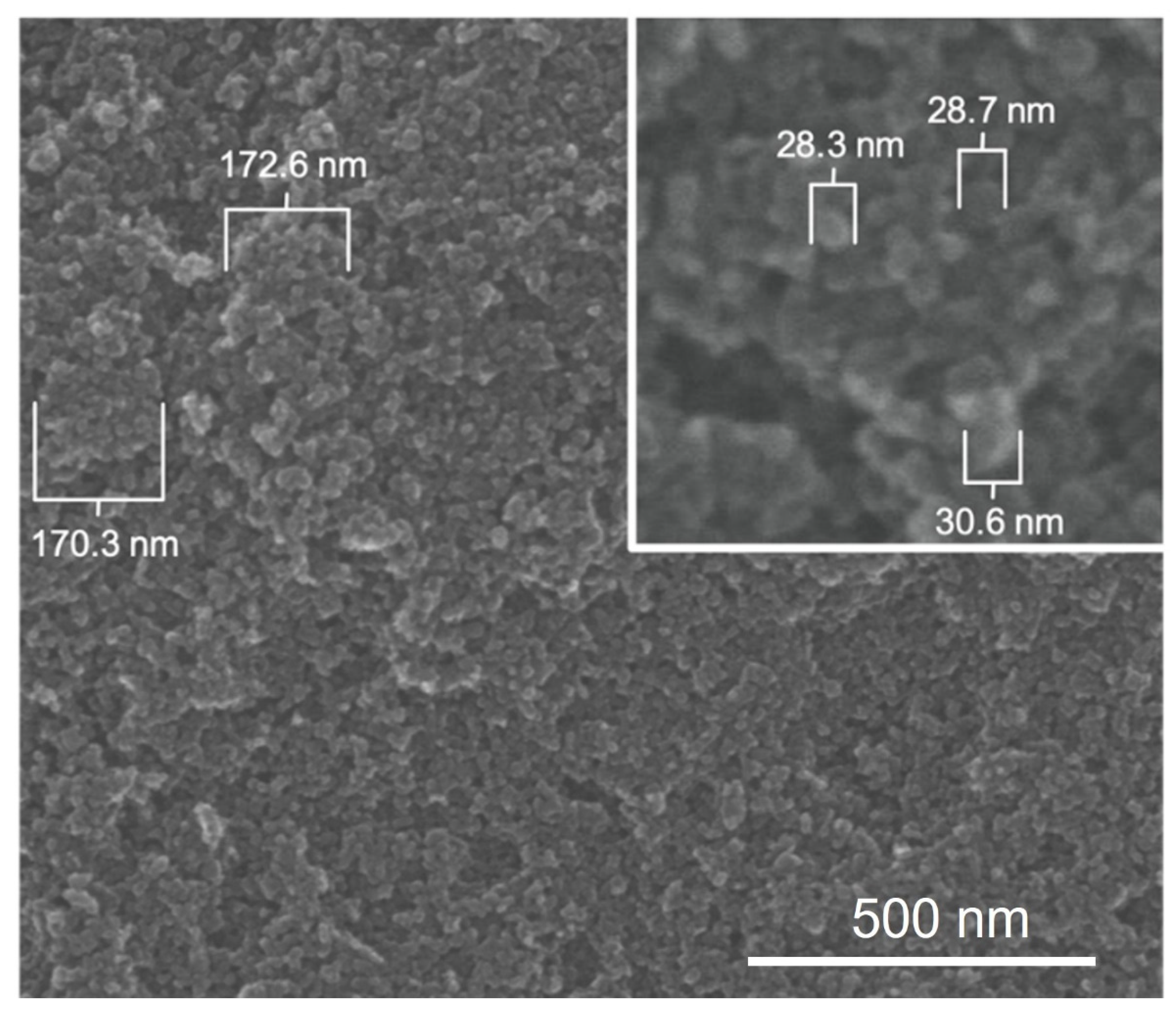
3.4. E1800 Suspensions Contain Nanoparticles That Form Fundamental Aggregates
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnett, E.D. Yellow Fever: Epidemiology and Prevention. Clin. Infect. Dis. 2007, 44, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E. Reemergence of Chikungunya Virus. J. Virol. 2014, 88, 11644–11647. [Google Scholar] [CrossRef] [Green Version]
- Weaver, S.C. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLoS Negl. Trop. Dis. 2014, 8, e2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Prim. 2016, 2, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Dermody, T.S. The Journal of Clinical Investigation Ecology and Epidemiology. J. Clin. Invest. 2017, 127, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.R.; Catteruccia, F. Vector Biology Meets Disease Control: Using Basic Research to Fight Vector-Borne Diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide Resistance in the Major Dengue Vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Murugan, K.; Aruna, P.; Panneerselvam, C.; Madhiyazhagan, P.; Paulpandi, M.; Subramaniam, J.; Rajaganesh, R.; Wei, H.; Alsalhi, M.S.; Devanesan, S.; et al. Fighting Arboviral Diseases: Low Toxicity on Mammalian Cells, Dengue Growth Inhibition (in Vitro), and Mosquitocidal Activity of Centroceras clavulatum-Synthesized Silver Nanoparticles. Parasitol. Res. 2016, 115, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Paquette, C.C.H.; Phanse, Y.; Perry, J.L.; Sanchez-Vargas, I.; Airs, P.M.; Dunphy, B.M.; Xu, J.; Carlson, J.O.; Luft, J.C.; DeSimone, J.M.; et al. Biodistribution and Trafficking of Hydrogel Nanoparticles in Adult Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phanse, Y.; Dunphy, B.M.; Perry, J.L.; Airs, P.M.; Paquette, C.C.H.; Carlson, J.O.; Xu, J.; Luft, J.C.; DeSimone, J.M.; Beaty, B.J.; et al. Biodistribution and Toxicity Studies of PRINT Hydrogel Nanoparticles in Mosquito Larvae and Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003735. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Sowmiya, R.; Balasubramani, G.; Aiswarya, D.; Arul, D.; Josebin, M.P.D.; Perumal, P. Mosquito-Larvicidal Efficacy of Gold Nanoparticles Synthesized from the Seaweed, Turbinaria ornata (Turner) J.Agardh 1848. Part. Sci. Technol. 2018, 36, 974–980. [Google Scholar] [CrossRef]
- Murugan, K.; Dinesh, D.; Kavithaa, K.; Paulpandi, M.; Ponraj, T.; Alsalhi, M.S.; Devanesan, S.; Subramaniam, J.; Rajaganesh, R.; Wei, H.; et al. Hydrothermal Synthesis of Titanium Dioxide Nanoparticles: Mosquitocidal Potential and Anticancer Activity on Human Breast Cancer Cells (MCF-7). Parasitol. Res. 2016, 115, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J.; et al. Green-Synthesized Silver Nanoparticles as a Novel Control Tool against Dengue Virus (DEN-2) and Its Primary Vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharreddy, P.; Rani, P.U. Biofabrication of Ag Nanoparticles Using Sterculia foetida L. Seed Extract and Their Toxic Potential against Mosquito Vectors and HeLa Cancer Cells. Mater. Sci. Eng. C 2014, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Nataraj, D.; Madhiyazhagan, P.; Sujitha, V.; Chandramohan, B.; Panneerselvam, C.; Dinesh, D.; Chandirasekar, R.; Kovendan, K.; Suresh, U.; et al. Carbon and Silver Nanoparticles in the Fight against the Filariasis Vector Culex quinquefasciatus: Genotoxicity and Impact on Behavioral Traits of Non-Target Aquatic Organisms. Parasitol. Res. 2016, 115, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Flannery, E.M.; Tomchaney, M.; Severson, D.W.; Duman-Scheel, M. Disruption of Aedes aegypti Olfactory System Development through Chitosan/SiRNA Nanoparticle Targeting of Semaphorin-1a. PLoS Negl. Trop. Dis. 2013, 7, e2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/Double-Stranded RNA Nanoparticle-Mediated RNA Interference to Silence Chitin Synthase Genes through Larval Feeding in the African Malaria Mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Airs, P.M.; Bartholomay, L.C. Molecular and Nano-Scale Alternatives to Traditional Insecticides for in Situ Control of Mosquito Vectors. ACS Symp. Ser. 2018, 1289, 75–99. [Google Scholar] [CrossRef]
- Ramanibai, R.; Velayutham, K. Bioactive Compound Synthesis of Ag Nanoparticles from Leaves of Melia azedarach and its Control for Mosquito Larvae. Res. Vet. Sci. 2014, 98, 82–88. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Facile Biosynthesis of Silver Nanoparticles Using Barleria cristata: Mosquitocidal Potential and Biotoxicity on Three Non-Target Aquatic Organisms. Parasitol. Res. 2015, 115, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K.M.; Haldar, B.; Chandra, G. Fabrication, Characterization and Mosquito Larvicidal Bioassay of Silver Nanoparticles Synthesized from Aqueous Fruit Extract of Putranjiva, Drypetes roxburghii (Wall). Parasitol. Res. 2013, 112, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Rawani, A.; Ghosh, A.; Chandra, G. Mosquito Larvicidal and Antimicrobial Activity of Synthesized Nano-Crystalline Silver Particles Using Leaves and Green Berry Extract of Solanum nigrum L. (Solanaceae: Solanales). Acta Trop. 2013, 128, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.D.; Borase, H.P. Larvicidal Activity of Silver Nanoparticles Synthesized Using Pergularia daemia Plant Latex against Aedes aegypti and Anopheles stephensi and Nontarget Fish Poecillia reticulata. Parasitol. Res. 2012, 111, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Subarani, S.; Sabhanayakam, S. Studies on the Impact of Biosynthesized Silver Nanoparticles (AgNPs) in Relation to Malaria and Filariasis Vector Control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2013, 112, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Mode of Action of Nanoparticles against Insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.C.; Jana, N.R. Different Synthesis Process of Carbon Nanomaterials for Biological Applications. In Carbon Nanomaterials for Biological and Medical Applications; Ray, S.C., Jana, N.R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–41. [Google Scholar]
- Adohi, J.-P.; Mdarhri, A.; Prunier, C.; Haidar, B.; Brosseau, C. A Comparison between Physical Properties of Carbon Black-Polymer and Carbon Nanotubes-Polymer Composites. J. Appl. Phys. 2010, 108, 074108. [Google Scholar] [CrossRef]
- Benelli, G.; Caselli, A.; Canale, A. Nanoparticles for Mosquito Control: Challenges and Constraints Nanoparticles for Mosquito Control. J. King Saud Univ. - Sci. 2017, 29, 424–435. [Google Scholar] [CrossRef]
- Saxena, M.; Sonkar, S.K.; Sarkar, S. Water Soluble Nanocarbons Arrest the Growth of Mosquitoes. RSC Adv. 2013, 3, 22504–22508. [Google Scholar] [CrossRef]
- ORION Engineered Carbons LLC. What Is Carbon Black? 2014. Available online: https://www.thecarycompany.com/media/pdf/specs/orion-what-is-carbon-black.pdf (accessed on 16 March 2022).
- CABOT Corporation. EMPEROR® 1800 Specialty Carbon Black 2018. Available online: https://www.cabotcorp.com.ar/-/media/files/guides/specialty-carbon-blacks/processing-and-formulation-guide-emperor-1800-specialty-carbon-blacks-water-based-coatings.pdf (accessed on 17 March 2022).
- Sharma, M. Understanding the Mechanism of Toxicity of Carbon Nanoparticles in Humans in the New Millennium: A Systemic Review. Indian J. Occup. Environ. Med. 2010, 14, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-Based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Saha, D.; Heldt, C.L.; Gencoglu, M.F.; Vijayaragavan, K.S.; Chen, J.; Saksule, A. A Study on the Cytotoxicity of Carbon-Based Materials. Mater. Sci. Eng. C 2016, 68, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piermarini, P.M.; Hine, R.M.; Schepel, M.; Miyauchi, J.; Beyenbach, K.W. Role of an Apical K, Cl Cotransporter in Urine Formation by Renal Tubules of the Yellow Fever Mosquito (Aedes aegypti). Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2011, 301, 1318–1337. [Google Scholar] [CrossRef] [Green Version]
- Inocente, E.A.; Shaya, M.; Acosta, N.; Rakotondraibe, L.H.; Piermarini, P.M. A Natural Agonist of Mosquito TRPA1 from the Medicinal Plant Cinnamosma fragrans that Is Toxic, Antifeedant, and Repellent to the Yellow Fever Mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Ramos, A.P. Dynamic Light Scattering Applied to Nanoparticle Characterization. In Nanocharacterization, Techniques; Da Róz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; Elsevier/William Andrew Publishing: Oxford, UK, 2017; pp. 99–110. [Google Scholar]
- de Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of Nanotechnology for the Encapsulation of Botanical Insecticides for Sustainable Agriculture: Prospects and Promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Retamal Marín, R.R.; Babick, F.; Hillemann, L. Zeta Potential Measurements for Non-Spherical Colloidal Particles – Practical Issues of Characterisation of Interfacial Properties of Nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 516–521. [Google Scholar] [CrossRef]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The Effects of Carboxylic Acids on the Aqueous Dispersion and Electrophoretic Deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Maggi, F.; Pavela, R.; Murugan, K.; Govindarajan, M.; Vaseeharan, B.; Petrelli, R.; Cappellacci, L.; Kumar, S.; Hofer, A.; et al. Mosquito Control with Green Nanopesticides: Towards the One Health Approach? A Review of Non-Target Effects. Environ. Sci. Pollut. Res. 2018, 25, 10184–10206. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Raul, P.K.; Goswami, D.; Das, B.; Gogoi, H.K.; Raju, P.S. Nanoweapon: Control of Mosquito Breeding Using Carbon-Dot-Silver Nanohybrid as a Biolarvicide. Environ. Chem. Lett. 2018, 16, 1017–1023. [Google Scholar] [CrossRef]
- Benelli, G. Plant-Mediated Biosynthesis of Nanoparticles as an Emerging Tool against Mosquitoes of Medical and Veterinary Importance: A Review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; de Oliveira, J.L.; Abrantes, D.C.; Rogério, C.B.; Bueno, C.; Miranda, V.R.; Monteiro, R.A.; Fraceto, L.F. Recent Developments in Nanotechnology for Detection and Control of Aedes aegypti-Borne Diseases. Front. Bioeng. Biotechnol. 2020, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Barik, T.K.; Kamaraju, R.; Gowswami, A. Silica Nanoparticle: A Potential New Insecticide for Mosquito Vector Control. Parasitol. Res. 2012, 111, 1075–1083. [Google Scholar] [CrossRef]
- Sundararajan, B.; Ranjitha Kumari, B.D. Novel Synthesis of Gold Nanoparticles Using Artemisia vulgaris L. Leaf Extract and Their Efficacy of Larvicidal Activity against Dengue Fever Vector Aedes aegypti L. J. Trace Elem. Med. Biol. 2017, 43, 187–196. [Google Scholar] [CrossRef]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Sharmili, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Bacterial Exopolysaccharide (EPS)-Coated ZnO Nanoparticles Showed High Antibiofilm Activity and Larvicidal Toxicity against Malaria and Zika Virus Vectors. J. Trace Elem. Med. Biol. 2018, 45, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile Green Synthesis of Zinc Oxide Nanoparticles Using Ulva lactuca Seaweed Extract and Evaluation of Their Photocatalytic, Antibiofilm and Insecticidal Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wei, S.; Wei, S.; Qi, Y.; Qi, Y.; Ma, L.; Liu, Y.; Li, G.; Sang, N.; Liu, S.; et al. Ageing Remarkably Alters the Toxicity of Carbon Black Particles towards Susceptible Cells: Determined by Differential Changes of Surface Oxygen Groups. Environ. Sci. Nano 2020, 7, 1633–1641. [Google Scholar] [CrossRef]
- Freeman, J.C.; Smith, L.B.; Silva, J.J.; Fan, Y.; Sun, H.; Scott, J.G. Fitness Studies of Insecticide Resistant Strains: Lessons Learned and Future Directions. Pest Manag. Sci. 2021, 77, 3847–3856. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Rodríguez, E.J.; Evans, P.; Kalsi, M.; Rosenblatt, N.; Stanley, M.; Piermarini, P.M. Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti. Insects 2022, 13, 307. https://doi.org/10.3390/insects13030307
Martínez Rodríguez EJ, Evans P, Kalsi M, Rosenblatt N, Stanley M, Piermarini PM. Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti. Insects. 2022; 13(3):307. https://doi.org/10.3390/insects13030307
Chicago/Turabian StyleMartínez Rodríguez, Erick J., Parker Evans, Megha Kalsi, Noah Rosenblatt, Morgan Stanley, and Peter M. Piermarini. 2022. "Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti" Insects 13, no. 3: 307. https://doi.org/10.3390/insects13030307
APA StyleMartínez Rodríguez, E. J., Evans, P., Kalsi, M., Rosenblatt, N., Stanley, M., & Piermarini, P. M. (2022). Larvicidal Activity of Carbon Black against the Yellow Fever Mosquito Aedes aegypti. Insects, 13(3), 307. https://doi.org/10.3390/insects13030307






