Acclimation Effects of Natural Daily Temperature Variation on Longevity, Fecundity, and Thermal Tolerance of the Diamondback Moth (Plutella xylostella)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Protocol
2.3. Statistical Analysis
3. Results
3.1. Correlation Analysis among P. xylostella Traits and Temperature Factors
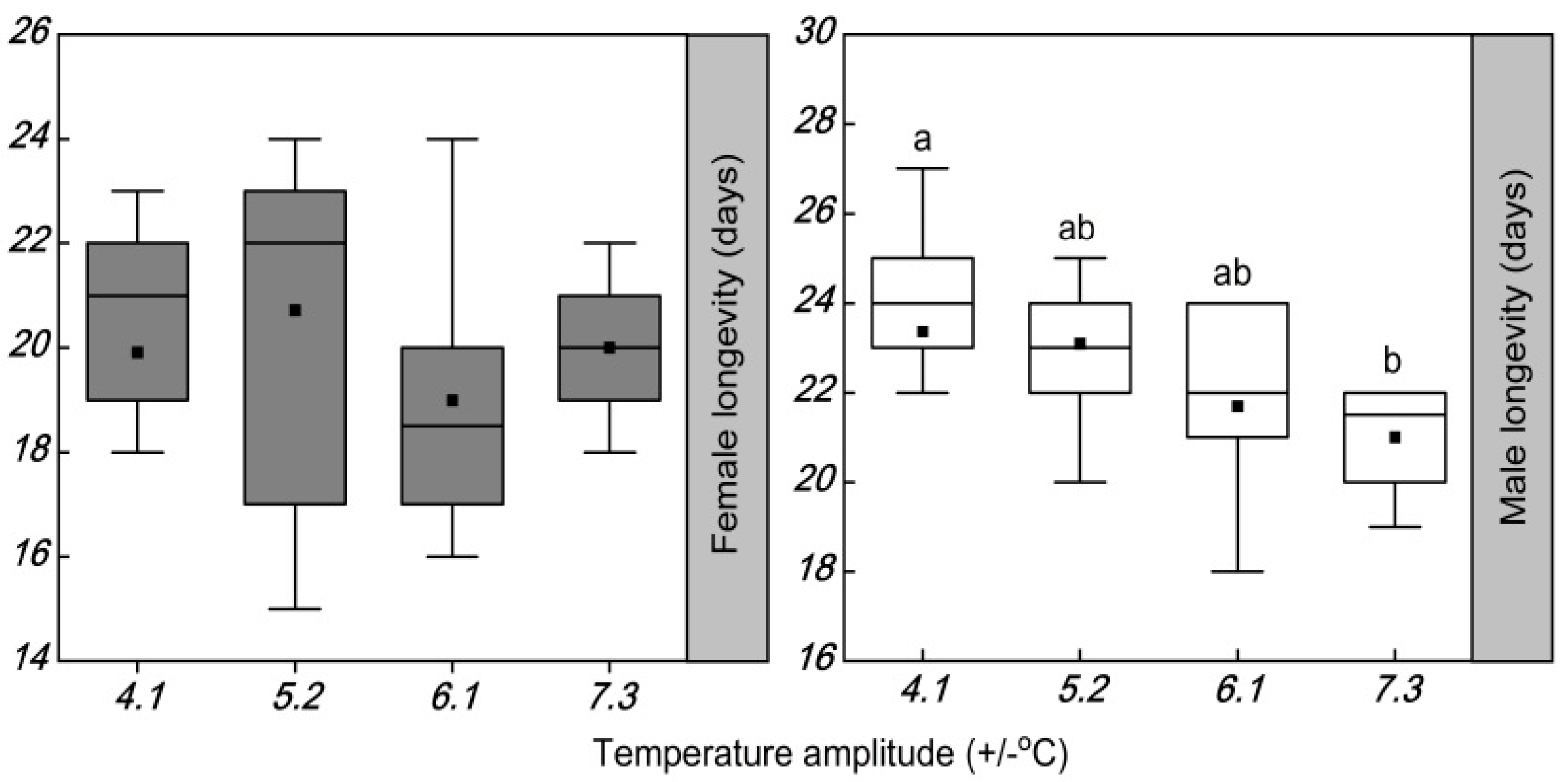
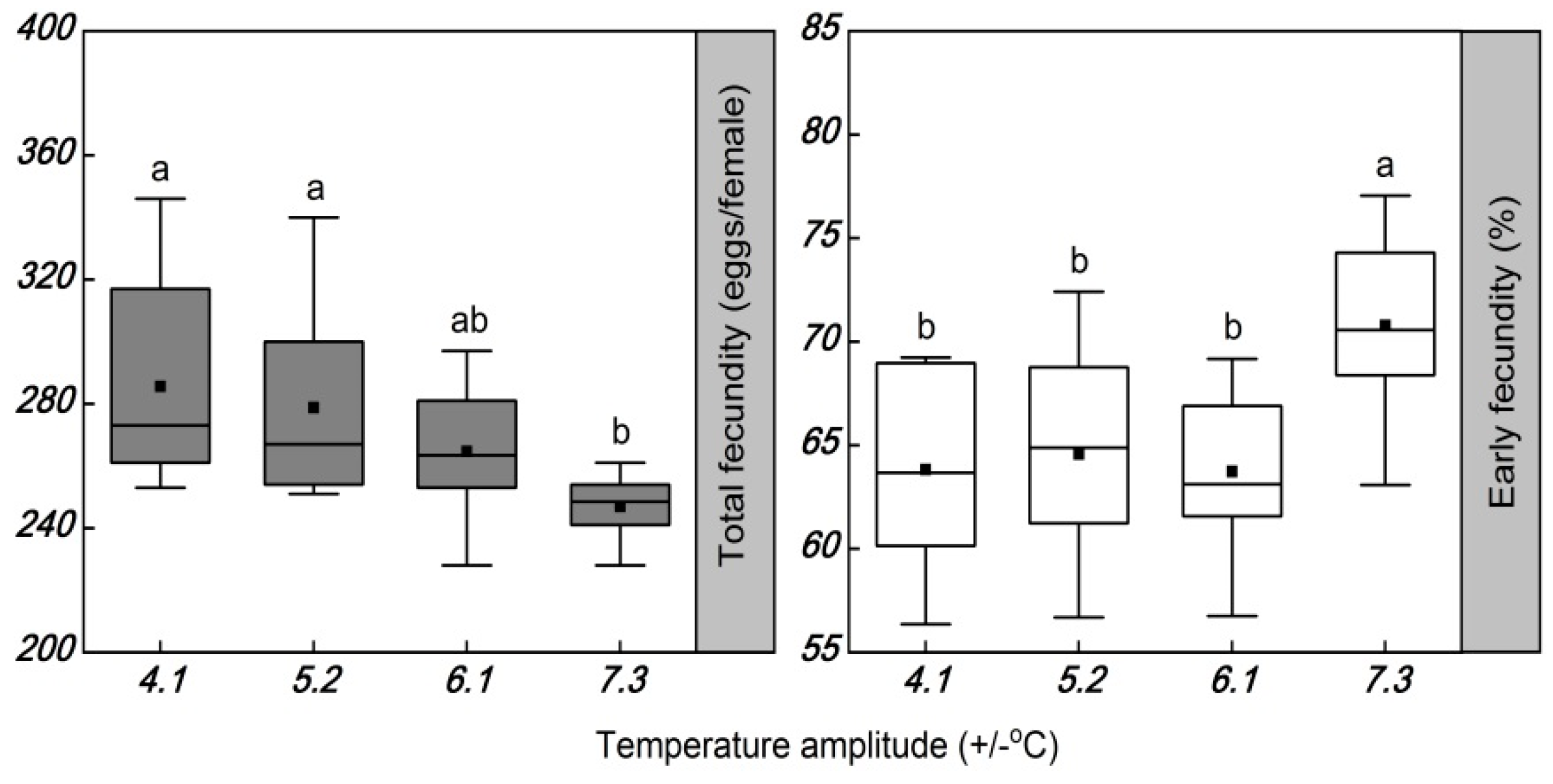
3.2. Influence of Temperature Amplitude on the Longevity and Fecundity
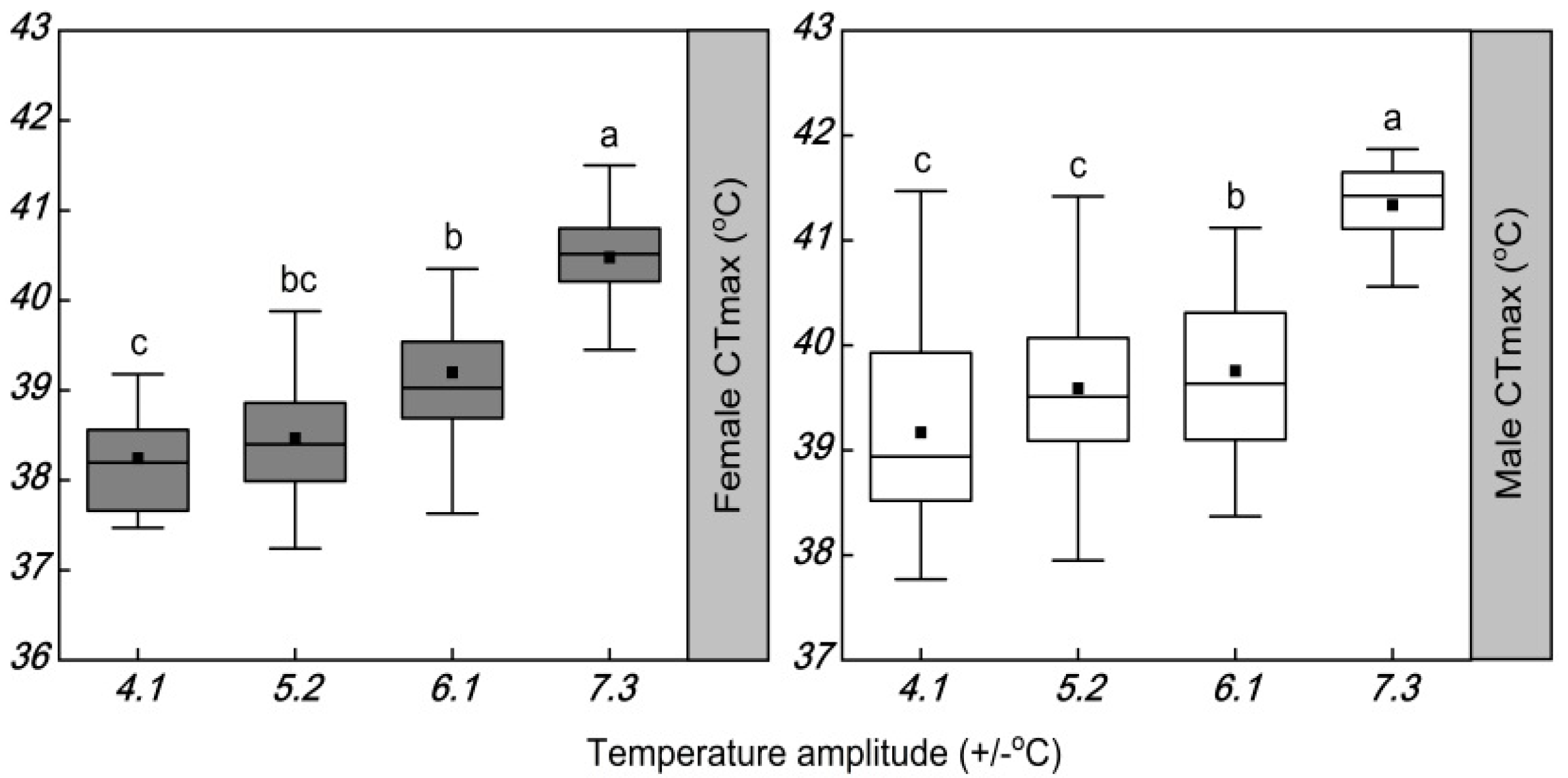
3.3. Influence of Temperature Amplitude on the Thermal Tolerance
4. Discussion
4.1. Influence of Temperature Amplitudes on Longevity and Fecundity
4.2. Influence of Temperature Amplitude on Thermal Tolerance
4.3. Implications for Pest Population Modeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.M.; Gillooly, J.F.; Brown, J.H.; West, G.B.; Charnov, E.L. Effects of body size and temperature on population growth. Am. Nat. 2004, 163, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Schmid, W.D. Survival of frogs in low temperature. Science 1982, 215, 697–698. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, J.Y.; Chen, H.S.; Wan, F.H. Effects of temperature on survival, development, longevity, and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a potential biological control agent against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 2010, 39, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Schoeller, E.N.; Redak, R.A. Temperature-dependent development and survival of giant whitefly Aleurodicus dugesii (Hemiptera: Aleyrodidae) under constant temperatures. Environ. Entomol. 2018, 47, 1586–1595. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef]
- Simmonds, E.G.; Sheldon, B.C.; Coulson, T.; Cole, E. Incubation behavior adjustments, driven by ambient temperature variation, improve synchrony between hatch dates and caterpillar peak in a wild bird population. Ecol. Evol. 2017, 7, 9415–9425. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, D.A.; DeLong, J.P.; Gilbert, B.; Greig, H.S.; Harley, C.D.G.; McCann, K.S.; Savage, V.; Tunney, T.D.; O’Connor, M.I. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. 2014, 281, 20132612. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; SøRensen, J.G.; Loeschcke, V. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 2003, 28, 175–216. [Google Scholar] [CrossRef]
- Schou, M.F.; Mouridsen, M.B.; SøRensen, J.G.; Loeschcke, V. Linear reaction norms of thermal limits in Drosophila: Predictable plasticity in cold but not in heat tolerance. Funct. Ecol. 2017, 31, 934–945. [Google Scholar] [CrossRef]
- Niehaus, A.C.; Angilletta, M.J.; Sears, M.W.; Franklin, C.E.; Wilson, R.S. Predicting the physiological performance of ectotherms in fluctuating thermal environments. J. Exp. Biol. 2012, 215, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2014, 60, 123–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, K.S.; Padash, M.; Carter, A.W.; Marshall, K.E. Different amplitudes of temperature fluctuation induce distinct transcriptomic and metabolomic responses in the dung beetle Phanaeus vindex. J. Exp. Biol. 2020, 223, jeb.233239. [Google Scholar] [CrossRef]
- Hoffmann, A.A. Acclimation: Increasing survival at a cost. Trends Ecol. Evol. 1995, 10, 1–2. [Google Scholar]
- Falfushynska, H.I.; Phan, T.; Sokolova, I.M. Long-term acclimation to different thermal regimes affects molecular responses to heat stress in a freshwater clam Corbicula Fluminea. Sci. Rep. 2016, 6, 39476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zari, T.A. Seasonal metabolic acclimatization in the herbivorous desert lizard Uromastyx philbyi (Reptilia: Agamidea) from western Saudi Arabia. J. Therm. Biol. 2016, 60, 180–185. [Google Scholar] [CrossRef]
- Thompson, L.J.; Brown, M.; Downs, C.T. Thermal acclimation in a small afrotropical bird. Behav. Processes 2016, 128, 113–118. [Google Scholar] [CrossRef]
- Moyano, M.; Candebat, C.; Ruhbaum, Y.; Álvarez-Fernández, S.; Claireaux, G.; Zambonino-Infante, J.-L.; Peck, M.A. Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS ONE 2017, 12, e0179928. [Google Scholar] [CrossRef]
- Shah, A.A.; Funk, W.C.; Ghalambor, C.K. Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integr. Comp. Biol. 2017, 57, 977–987. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Kavallieratos, N.G.; Hartzer, K.L. To acclimate or not to acclimate? Simultaneous positive and negative effects of acclimation on susceptibility of Tribolium confusum (Coleoptera: Tenebrionidae) and Oryzaephilus surinamsis (Coleoptera: Silvanidae) to low temperatures. J. Econ. Entomol. 2019, 112, 2441–2449. [Google Scholar] [CrossRef]
- Jensen, K.; Kristensen, T.N.; Overgaard, J.; Toft, S.; Sørensen, J.G.; Holmstrup, M. Cold acclimation reduces predation rate and reproduction but increases cold- and starvation tolerance in the predatory mite Gaeolaelaps aculeifer Canestrini. Biol. Control 2017, 114, 150–157. [Google Scholar] [CrossRef]
- Chown, S.L.; Haupt, T.M.; Sinclair, B.J. Similar metabolic rate-temperature relationships after acclimation at constant and fluctuating temperatures in caterpillars of a sub-Antarctic moth. J. Insect Physiol. 2016, 85, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, P.P.; Burkert, L.H.; Abou, B.; Federle, W.; Menzel, F. Coping with the climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 2018, 221, jeb171488. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.A.; Hoffmann, A.A. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 2010, 24, 694–700. [Google Scholar] [CrossRef]
- Ma, G.; Hoffmann, A.A.; Ma, C.S. Daily temperature extremes play an important role in predicting thermal effects. J. Exp. Biol. 2015, 218, 2289–2296. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.Y.; Xing, K.; Liu, H.P.; Zhao, F. Effects of developmental acclimation on fitness costs differ between two aphid species. J. Therm. Biol. 2018, 78, 58–64. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C.S. Effect of acclimation on heat-escape temperatures of two aphid species: Implications for estimating behavioral response of insects to climate warming. J. Insect Physiol. 2012, 58, 303–309. [Google Scholar] [CrossRef]
- Ma, G.; Rudolf, V.H.W.; Ma, C.S. Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob. Chang. Biol. 2015, 21, 1794–1808. [Google Scholar] [CrossRef]
- Chidawanyika, F.; Terblanche, J.S. Costs and benefits of thermal acclimation for codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Implications for pest control and the sterile insect release programme. Ecol. Appl. 2011, 4, 534–544. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Pijpe, J.; Zwaan, B.J. Developmental plasticity and acclimation both contribute to adaptive responses to alternating seasons of plenty and of stress in Bicyclus butterflies. J. Biosci. 2007, 32, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Li, Y.; Goto, M. Physiological and biochemical changes in summer and winter diapause and non-diapause pupae of the cabbage armyworm, Mamestra brassicae L. during long-term cold acclimation. J. Insect Physiol. 2003, 49, 1153–1159. [Google Scholar] [CrossRef]
- Slabber, S.; Chown, S.L. Differential responses of thermal tolerance to acclimation in the sub-Antarctic rove beetle Halmaeusa atriceps. Physiol. Entomol. 2010, 30, 195–204. [Google Scholar] [CrossRef]
- Parker, D.J.; Vesala, L.; Ritchie, M.G.; Laiho, A.; Hoikkala, A.; Kankare, M. How consistent are the transcriptome changes associated with cold acclimation in two species of the Drosophila virilis group? Heredity 2015, 115, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Manenti, T.; Loeschcke, V.; Sørensen, J.G. Constitutive up-regulation of Turandot genes rather than changes in acclimation ability is associated with the evolutionary adaptation to temperature fluctuations in Drosophila simulans. J. Insect Physiol. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Chapman, J.W.; Nesbit, R.L.; Burgin, L.E.; Reynolds, D.R.; Smith, A.D.; Middleton, D.R.; Hill, J.K. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 2010, 327, 682–685. [Google Scholar] [CrossRef]
- Golizadeh, A.; Kamali, K.; Fathipour, Y.; Abbasipour, H. Temperature-dependent development of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) on two brassicaceous host plants. Insect Sci. 2007, 14, 309–316. [Google Scholar] [CrossRef]
- Guo, S.; Qin, Y. Effects of temperature and humidity on emergence dynamics of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2010, 103, 2028–2033. [Google Scholar] [CrossRef] [Green Version]
- Golizadeh, A.; Kamali, K.; Fathipour, Y.; Abbasipour, H. Effect of temperature on life table parameters of Plutella xylostella (Lepidoptera: Plutellidae) on two brassicaceous host plants. J. Asia Pac. Entomol. 2009, 12, 207–212. [Google Scholar] [CrossRef]
- Coulson, S.J.; Hodkinson, I.D.; Webb, N.R.; Mikkola, K.; Harrison, A.; Pedgley, D.E. Aerial colonization of high Arctic islands by invertebrates: The diamondback moth Plutella xylostella (Lepidoptera: Yponomeutidae) as a potential indicator species. Divers. Distrib. 2010, 8, 327–334. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Foerster, L.A. Development and survival of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae) as a function of temperature: Effect on the number of generations in tropical and subtropical regions. Neotrop. Entomol. 2011, 40, 533–541. [Google Scholar]
- Xing, K.; Ma, C.S.; Zhao, F.; Han, J.C. Effects of large temperature fluctuations on hatching and subsequent development of the diamondback moth (Lepidoptera: Plutellidae). Fla. Entomol. 2015, 98, 651–659. [Google Scholar] [CrossRef]
- Xing, K.; Hoffmann, A.A.; Ma, C.S. Does thermal variability experienced at the egg stage influence life history traits across life cycle stages in a small invertebrate. PLoS ONE 2014, 9, e99500. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Zhao, F.; Ma, C.S. Effects of temperature fluctuation on life history traits of different developmental stages of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2015, 58, 160–168. [Google Scholar]
- Xing, K.; Hoffmann, A.A.; Zhao, F.; Ma, C.S. Wide diurnal temperature variation inhibits larval development and adult reproduction in the diamondback moth. J. Therm. Biol. 2019, 84, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, Y.; Zhao, X.J.; Hoffmann, A.A.; Li, R.; Ma, C.S. Emergence of the overwintering generation of peach fruit moth (Carposina sasakii) depends on diapause and spring soil temperatures. J. Insect Physiol. 2016, 86, 32–39. [Google Scholar] [CrossRef]
- 4Krechemer, F.S.; Foerster, L.A. Influence of biotic and abiotic factors on the population fluctuation of Tuta absoluta (Lepidoptera: Gelechiidae) in an organic tomato farming. Int. J. Trop. Insect Sci. 2020, 40, 199–208. [Google Scholar] [CrossRef]
- Li, H.B.; Shi, L.; Lu, M.X.; Wang, J.J.; Du, Y.Z. Thermal tolerance of Frankliniella occidentalis: Effects of temperature, exposure time, and gender. J. Therm. Biol. 2011, 36, 437–442. [Google Scholar] [CrossRef]
- Yamada, H.; Kawasaki, K. The effect of temperature and humidity on the development, fecundity and multiplication of the diamondback moth, Plutella xylostella (L.). Jpn. J. Appl. Entomol. Zool. 1983, 27, 17–21. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- David, J.R.; Araripe, L.O.; Chakir, M.; Legout, H.; Lemos, B.; Pétavy, G.; Rohmer, C.; Joly, D.; Moreteau, B. Male sterility at extreme temperatures: A significant but neglected phenomenon for understanding Drosophila climatic adaptations. J. Evol. Biol. 2005, 18, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Dmitriew, C.; Rowe, L. Effects of early resource limitation and compensatory growth on lifetime fitness in the ladybird beetle (Harmonia axyridis). J. Evol. Biol. 2007, 20, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.A.; Davidowitz, G.; Woods, H.A. Cross-stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 2011, 25, 548–556. [Google Scholar] [CrossRef]
- Block, M.D.; Stoks, R. Compensatory growth and oxidative stress in a damselfly. Proc. R. Soc. B Biol. Sci. 2008, 275, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Rudolf, V.H.W.; Ma, C.S. Stage-specific heat effects: Timing and duration of heat waves alter demographic rates of a global insect pest. Oecologia 2015, 179, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Monaghan, P. Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 2001, 16, 254–260. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.X.; Shen, Y.; Huang, L.H.; Feng, Q.L. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol. Biol. 2012, 21, 535–543. [Google Scholar] [CrossRef]
- Tomanek, L.; Zuzow, M.J. The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: Implications for thermal tolerance limits and metabolic costs of thermal stress. J. Exp. Biol. 2010, 213, 3559–3574. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.H.; Bing, C.; Kang, L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 2007, 53, 1199–1205. [Google Scholar] [CrossRef]
- Javois, J.; Tammaru, T. Reproductive decisions are sensitive to cues of life expectancy: The case of a moth. Anim. Behav. 2004, 68, 249–255. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V.; Schou, M.F. No trade-off between high and low temperature tolerance in a winter acclimatized danish Drosophila subobscura population. J. Insect Physiol. 2015, 77, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Clusella-Trullas, S.; Chown, S.L. The effects of acclimation and rates of temperature change on critical thermal limits in Tenebrio molitor (Tenebrionidae) and Cyrtobagous salviniae (Curculionidae). J. Insect Physiol. 2012, 58, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.Q.; Ma, C.S.; Zhang, S.; Liang, L. Thermal tolerance of diamondback moth Plutella xylostella. J. Appl. Ecol. 2012, 23, 772–778. [Google Scholar]
- Terblanche, J.S.; Nyamukondiwa, C.; Kleynhans, E. Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomol. Exp. Appl. 2010, 137, 304–315. [Google Scholar] [CrossRef]
- Garcia, C.M.; Morrell, J.J. Development of the powderpost beetle (coleoptera: Bostrichidae) at constant temperatures. Environ. Entomol. 2009, 38, 478–483. [Google Scholar] [CrossRef]
- Cocuzza, G.E.; Clercq, P.D.; Lizzio, S.; Veire, M.; Tirry, L.; Degheele, D.; Vacante, V. Life tables and predation activity of Orius laevigatus and O. albidipennis at three constant temperatures. Entomol. Exp. Appl. 1997, 85, 189–198. [Google Scholar] [CrossRef]
- O’Neal, M.J.; Headrick, D.H.; Montez, G.H.; Grafton-Cardwell, E.E. Temperature thresholds and degree-day model for Marmara gulosa (Lepidoptera: Gracillariidae). J. Econ. Entomol. 2011, 104, 1286–1293. [Google Scholar] [CrossRef]
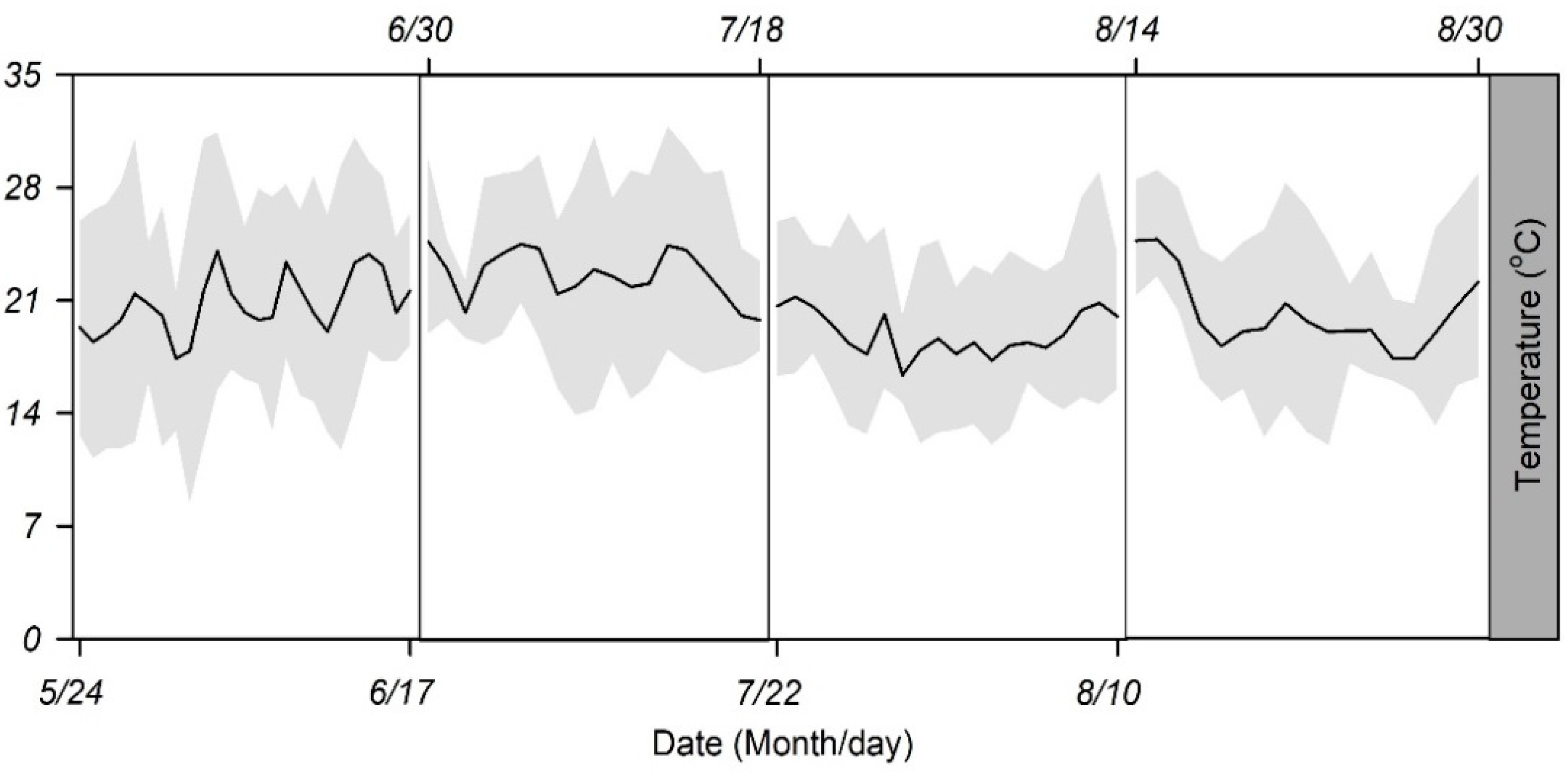
| Group | Duration | Actual Recorded Temperature | |||
|---|---|---|---|---|---|
| DMT 1 (°C) | DTA (±°C) | DHT (°C) | DLT (°C) | ||
| 1 | 5/24–6/17 | 20.8 ± 1.8 b | 7.3 ± 1.7 a | 27.6 ± 2.3 a | 14.2 ± 2.6 b |
| 2 | 6/30–7/18 | 22.6 ± 1.5 a | 5.2 ± 1.6 ab | 28.0 ± 2.6 a | 17.3 ± 1.9 a |
| 3 | 7/22–8/10 | 22.8 ± 1.6 a | 6.1 ± 1.2 ab | 29.3 ± 2.4 a | 17.3 ± 1.9 a |
| 4 | 8/14–8/30 | 20.2 ± 2.3 b | 4.1 ± 1.4 b | 25.4 ± 2.7 b | 16.0 ± 3.0 ab |
| df | 3.25 | 3.19 | 3.20 | 3.17 | |
| MS | 33.012 | 38.271 | 47.493 | 51.252 | |
| F | 9.809 | 16.893 | 7.658 | 9.120 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | |
| Trait | DMT (°C) | DTA (±°C) | DHT (°C) | DLT (°C) |
|---|---|---|---|---|
| Female longevity | −0.12 | −0.22 | −0.35 | −0.09 |
| Male longevity | −0.07 | −0.96 *1 | −0.56 | +0.52 |
| Total fecundity | +0.07 | −0.97 * | −0.43 | +0.66 |
| Early fecundity | −0.39 | +0.79 | +0.02 | −0.88 |
| Female CTmax | −0.20 | +0.92 | +0.30 | −0.71 |
| Male CTmax | −0.13 | +0.96 * | +0.51 | −0.46 |
| Female CTmin | −0.32 | +0.70 | +0.01 | −0.80 |
| Male CTmin | +0.24 | +0.90 | +0.54 | −0.42 |
| Mode | df | MS | F | p |
|---|---|---|---|---|
| y1 1 = −0.441 x1 2 | 1.42 | 36.823 | 9.658 | 0.0033 |
| y2 = −0.479 x1 | 1.42 | 7751.408 | 11.902 | 0.001 |
| y3 = −0.497 x1 | 1.42 | 0.027 | 12.140 | 0.001 |
| y4 = 5.962 x1 − 5.102 x2 + 4.433 x3 | 3.120 | 24.755 | 41.917 | <0.001 |
| y5 = 0.658 x1 | 1.120 | 62.018 | 89.926 | <0.001 |
| Trait | df | MS | F | p |
|---|---|---|---|---|
| Female longevity | 3.42 | 0.071 | 1.081 | 0.369 |
| Male longevity | 3.42 | 0.118 | 4.309 | 0.0101 |
| Total fecundity | 3.42 | 2752.413 | 4.094 | 0.013 |
| Early fecundity | 3.42 | 116.564 | 6.033 | 0.002 |
| Female CTmax | 3.120 | 0.165 | 44.374 | <0.001 |
| Male CTmax | 3.120 | 30.194 | 53.481 | <0.001 |
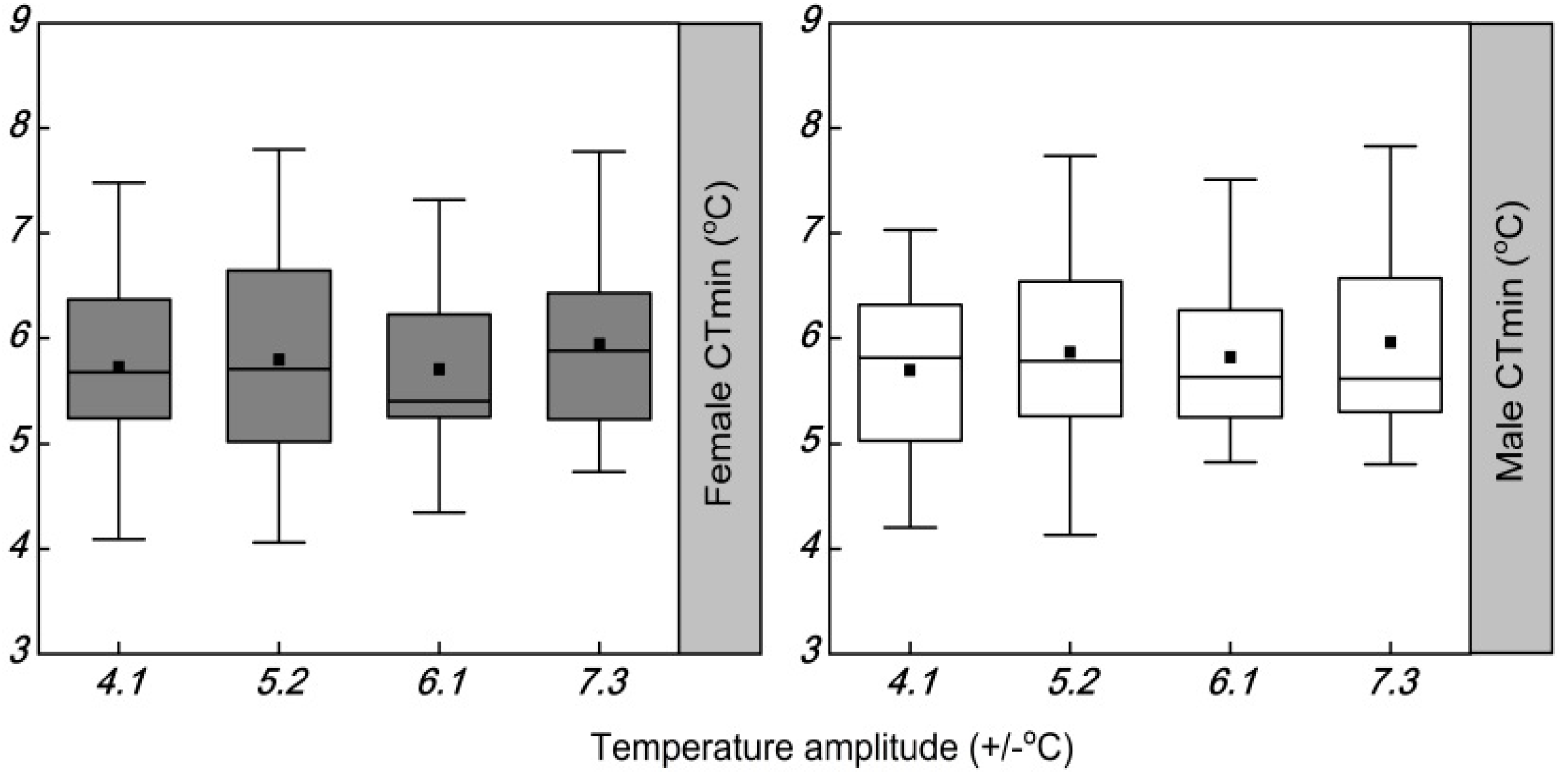
| Female CTmin | 3.120 | 0.015 | 0.502 | 0.682 |
| Male CTmin | 3.120 | 0.341 | 0.374 | 0.772 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, K.; Zhao, F. Acclimation Effects of Natural Daily Temperature Variation on Longevity, Fecundity, and Thermal Tolerance of the Diamondback Moth (Plutella xylostella). Insects 2022, 13, 309. https://doi.org/10.3390/insects13040309
Xing K, Zhao F. Acclimation Effects of Natural Daily Temperature Variation on Longevity, Fecundity, and Thermal Tolerance of the Diamondback Moth (Plutella xylostella). Insects. 2022; 13(4):309. https://doi.org/10.3390/insects13040309
Chicago/Turabian StyleXing, Kun, and Fei Zhao. 2022. "Acclimation Effects of Natural Daily Temperature Variation on Longevity, Fecundity, and Thermal Tolerance of the Diamondback Moth (Plutella xylostella)" Insects 13, no. 4: 309. https://doi.org/10.3390/insects13040309
APA StyleXing, K., & Zhao, F. (2022). Acclimation Effects of Natural Daily Temperature Variation on Longevity, Fecundity, and Thermal Tolerance of the Diamondback Moth (Plutella xylostella). Insects, 13(4), 309. https://doi.org/10.3390/insects13040309





