Impact of the Timing and Use of an Insecticide on Arthropods in Cover-Crop-Corn Systems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Field Characteristics
2.2. Arthropod Sampling
2.3. Corn Injury Assessment
2.4. Agronomic Parameters
2.5. Statistical Analysis
2.5.1. Arthropod Activity
2.5.2. Agronomic Parameters
3. Results
3.1. Arthropod Activity
3.1.1. 2019 Growing Season
3.1.2. 2020 Growing Season
3.1.3. 2021 Growing Season
3.2. Agronomic Parameters
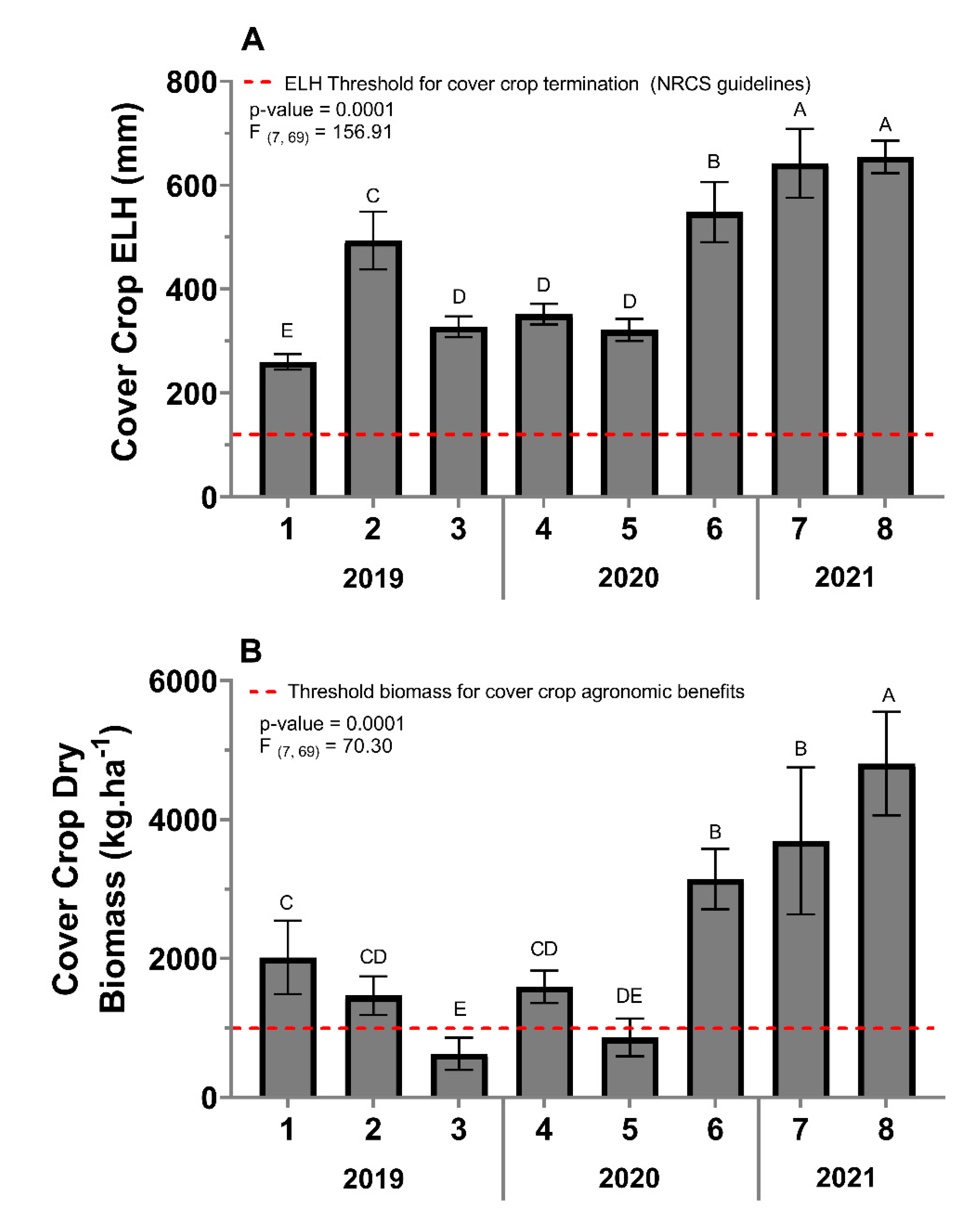
3.2.1. Cover-Crop Extended Leaf Height
3.2.2. Cover-Crop Biomass
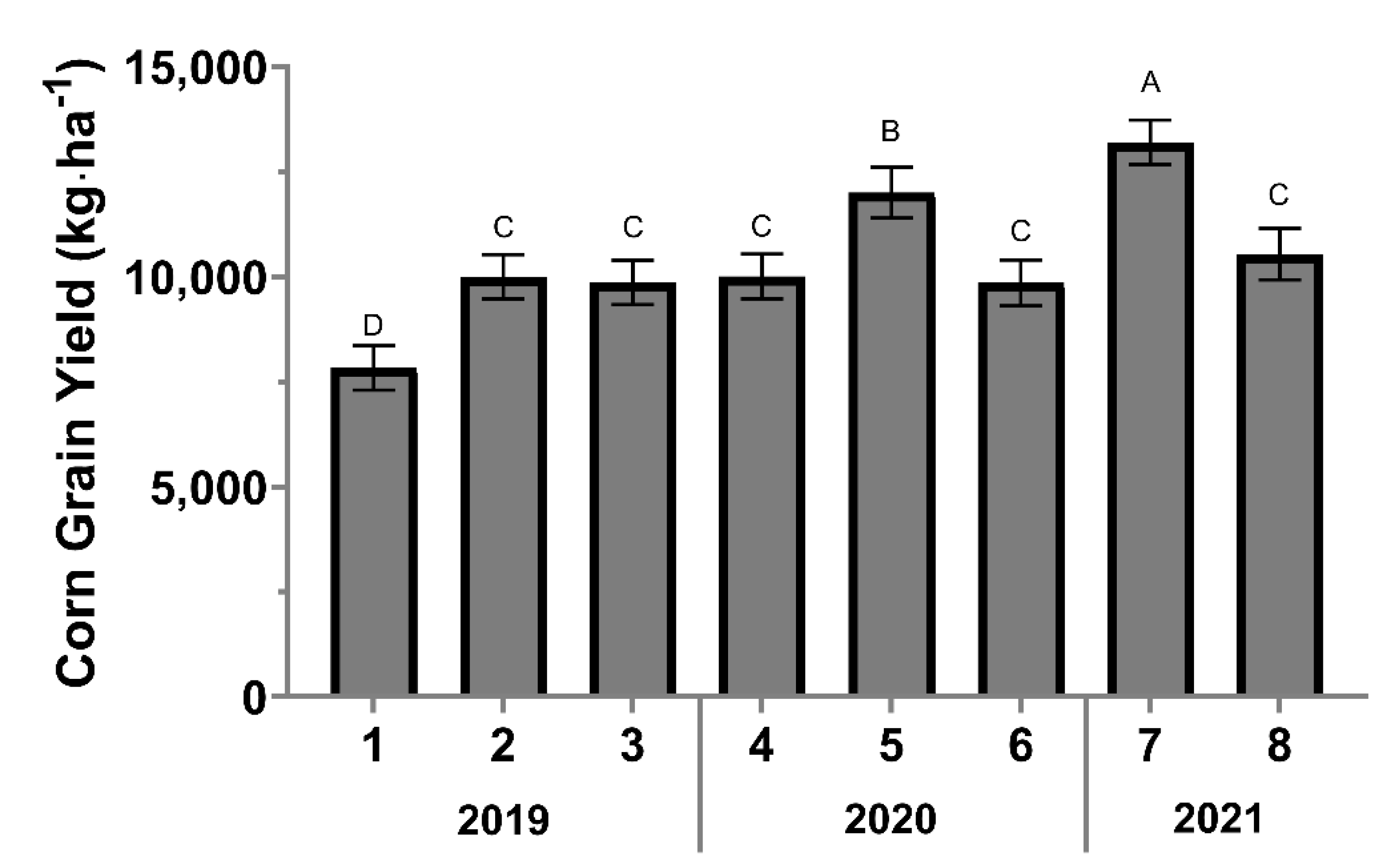
3.2.3. Corn Grain Yield
3.3. Environmental Conditions during the Pitfall Trap Sample Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Year | Site | Sampling Period | Cumulative Avg Temperature (°C) | Cumulative Precipitation (mm) |
|---|---|---|---|---|
| 2019 | 1 | 1 | 127.5 | 19.30 |
| 2 | 210.6 | 43.94 | ||
| 3 | 198.3 | 18.96 | ||
| 2 | 1 | 140.3 | 1.27 | |
| 2 | 130.3 | 1.27 | ||
| 3 | 131.7 | 2.54 | ||
| 3 | 1 | 166.1 | 29.46 | |
| 2 | 166.3 | 43.43 | ||
| 3 | 192.2 | 34.80 | ||
| 2020 | 4 | 1 | 196.7 | 3.56 |
| 2 | 196.1 | 0.00 | ||
| 3 | 200.0 | 8.64 | ||
| 5 | 1 | 125.3 | 0.00 | |
| 2 | 181.7 | 5.59 | ||
| 3 | 183.9 | 7.87 | ||
| 6 | 1 | 125.3 | 0.00 | |
| 2 | 188.9 | 6.60 | ||
| 3 | 188.3 | 10.92 | ||
| 2021 | 7 | 1 | 79.4 | 0.00 |
| 2 | 181.7 | 5.59 | ||
| 3 | 183.9 | 7.87 | ||
| 8 | 1 | 79.4 | 0.00 | |
| 2 | 184.7 | 7.62 | ||
| 3 | 194.4 | 0.00 |
References
- (USDA, NRCS) United States Department of Agriculture, Natural Resource Conservation Service. Acreage; Agricultural Statistics Board: Washington, BD, USA, 2017; ISSN 1949-1522.
- Hamilton, A.V.; Mortensen, D.A.; Allen, M.K. The state of the cover crop nation and how to set realistic future goals for the popular conservation practice. J. Soil Conserv. 2017, 75, 111A–115A. [Google Scholar] [CrossRef]
- (CTIC) Conservation Technology Information Center. Report of the 2016–2017 National Cover Crop Survey; Joint publication of the Conservation Technology Information Center, The North Central Region Sustainable Agriculture Research and Education Program, and the American Seed Trade Association: West Lafayette, IN, USA, 2017. [Google Scholar]
- Bottenberg, H.; Masiunas, J.; Eastman, C.; Eastburn, D.M. The Impact of Rye Cover Crops on Weeds, Insects, and Diseases in Snap Bean Cropping Systems. J. Sustain. Agric. 1997, 9, 131–155. [Google Scholar] [CrossRef]
- Shearin, A.F.; Reberg-Horton, S.C.; Gallandt, E.R. Cover Crop Effects on the Activity-Density of the Weed Seed Predator Harpalus rufipes(Coleoptera: Carabidae). Weed Sci. 2008, 56, 442–450. [Google Scholar] [CrossRef]
- Ward, M.; Ryan, M.; Curran, W.; Barbercheck, M.; Mortensen, D. Cover crops and disturbance influence activity-density of weed seed predators Amara aenea and Harpalus pensylvanicus (Coleoptera: Carabidae). Weed Sci. 2011, 59, 76–81. [Google Scholar] [CrossRef]
- Dunbar, M.W.; Gassmann, A.J.; O’Neal, M.E. Limited impact of fall-Seeded, spring-terminated rye cover crop on beneficial arthropods. J. Environ. Entomol. 2017, 46, 284–290. [Google Scholar] [CrossRef]
- Nichols, V.; Martinez-Feria, R.; Weisberger, D.; Carlson, S.; Basso, B.; Basche, A. Cover crops and weed suppression in the U.S. Midwest: A meta-analysis and modeling study. Agric. Environ. Lett. 2020, 5, e20022. [Google Scholar] [CrossRef]
- (USDA), U.S. Department of Agriculture. Natural Resource Conservation Service: Cover Crops. 2014. Available online: https://plants.usda.gov/about_cover_crops.html (accessed on 15 September 2020).
- Casey, P.A. Plant Guide for Cereal Rye (Secale Cereale); United States Department of Agriculture, Natural Resource Conservation Service, Plant Materials Center: Elsberry, MO, USA, 2002.
- Carmona, G.I.; Delserone, L.M.; Duarte, J.N.C.; de Almeida, T.F.; Ozório, R.D.V.B.; Wright, R.; McMechan, A.J. Does cover crop management affect arthropods in the subsequent corn and soybean crops in the United States? A systematic review. Ann. Entomol. Soc. Am. 2021, 2, 151–162. [Google Scholar] [CrossRef]
- Dunbar, M.W.; O’Neal, M.E.; Gassmann, A.J. Increased risk of insect injury to corn following rye cover crop. J. Econ. Entomol. 2016, 109, 1691–1697. [Google Scholar] [CrossRef]
- Carmona, G.I.; Rees, J.; Seymour, R.; Wright, R.; McMechan, A.J. Wheat Stem Maggot (Diptera: Chloropidae): An Emerging Pest of Cover Crop to Corn Transition Systems. Plant Health Prog. 2019, 20, 147–154. [Google Scholar] [CrossRef]
- Song, F.; Swinton, S.M. Returns to integrated pest management research and outreach for soybean aphid. J. Econ. Entomol. 2009, 102, 2116–2125. [Google Scholar] [CrossRef] [Green Version]
- Shelton, A.M.; Naranjo, S.E.; Romeis, J.; Hellmich, R.L.; Wolt, J.D.; Federici, B.A.; Albajes, R.; Bigler, F.; Burgess, E.P.J.; Dively, G.P.; et al. Setting the record straight: A rebuttal to an erroneous analysis on transgenic insecticidal crops and natural enemies. Transgenic Resear. 2009, 18, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Castle, S.; Naranjo, S.E. E. Sampling plans, selective insecticides and sustainability: The case for IPM as ‘informed pest management’. Pest Manag. Sci. 2009, 65, 1321–1328. [Google Scholar] [CrossRef]
- SAS Institute. PROC User’s Manual; Version 9.4; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- Coaker, T.H. The effect of soil insecticides on the predators and parasites of the cabbage root fly (Erioischia brassicae (Bouché)) and on the subsequent damage caused by the pest. Ann. Appl. Biol. 1966, 57, 397–407. [Google Scholar] [CrossRef]
- Chiverton, P.A. Pitfall-trap catches of the carabid beetle Pterostichus melanarius, in relation to gut contents and prey densities, in insecticide treated and untreated spring barley. Entomol. Exp. Appl. 1984, 36, 23–30. [Google Scholar] [CrossRef]
- Heneghan, P.A. Assessing the effects of an insecticide on the activity of predatory ground beetles, in Interpretation of Pesticide Effects on Beneficial Arthropods. Associ. Appl. Biologists. 1992, 31, 113–119. [Google Scholar]
- Bel’Skaya, E.A.; Zinov’Ev, E.V.; Kozyrev, M.A. Carabids in a Spring Wheat Agrocenosis to the South of Sverdlovsk Oblast and the Effect of Insecticide Treatment on Their Populations. Russ. J. Ecol. 2002, 33, 38–44. [Google Scholar] [CrossRef]
- Calabrese, E.J. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ. Pollut. 2005, 138, 379–411. [Google Scholar] [CrossRef]
- Cohen, E. Pesticide-mediated hemeostatic modulation in arthropods. Pestic. Biochem. Physiol. 2006, 85, 21–27. [Google Scholar] [CrossRef]
- Cutler, G.C. Insects, Insecticides and Hormesis: Evidence and Considerations for Study. Dose-Response 2013, 11, 154–177. [Google Scholar] [CrossRef]
- Lopez, M.D.; Prasifka, J.R.; Bruck, D.J.; Lewis, L.C. Utility of ground beetle species as indicators of potential non-target effects of Bt crops. Environ. Entomol. 2005, 34, 1317–1324. [Google Scholar] [CrossRef]
- Campos, J.N.D. The Impacts of Cover Crops Species and Termination Dates on Arthropod Activity in a Corn Production System. Master’s Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, August 2021. [Google Scholar]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-Induced Stress in Arthropod Pests for Optimized Integrated Pest Management Programs. Ann. Entomol. Soc. Am. 2016, 61, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Readshaw, J.L. Biological control of orchard mites in Australia with insecticide-resistant predator. J. Aust. Inst. Agricul. Sci. 1975, 41, 213–214. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I. Biodiversity and Pest Management in Agrosystems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Dutcher, J.D. A Review of Resurgence and Replacement Causing Pest Outbreaks in IPM. In General Concepts in Integrated Pest and Disease Management; Springer: Dordrecht, The Netherlands, 2007; pp. 27–43. [Google Scholar]
- Gross, K.; Rosenheim, J.A. Quantifying secondary pest outbreaks in cotton and their monetary cost with causal-inference statistics. Ecol. Appl. 2011, 21, 2770–2780. [Google Scholar] [CrossRef]
- Hill, M.P.; MacFadyen, S.; Nash, M.A. Broad spectrum pesticide application alters natural enemy communities and may facilitate secondary pest outbreaks. PeerJ 2017, 5, e4179. [Google Scholar] [CrossRef] [Green Version]
- Edwards, O.R.; Franzmann, B.; Thackray, D.; Micic, S. Insecticide resistance and implications for future aphid management in Australian grains and pastures: A review. Aust. J. Exp. Agric. 2008, 48, 1523–1530. [Google Scholar] [CrossRef]

| Year | Site | Nebraska County | Cover Crop Management | Corn Management | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cover Crop Planting Dates | Seed Rate (kg·ha−1) | Row Spacing (cm) | Corn Planting | Seed Rate (seeds·ha−1) | Corn Hybrid | Corn Harvest | |||
| 2019 | 1 | Saunders | 24 October 2018 | 106 | 19 | 28 April 2019 | 79,040 | P1197AM | 24 October 2019 |
| 2 | Saunders | Late November, 2018 | 67 | 19 | 28 April 2019 | 74,131 | DKC60-88 | 20 October 2019 | |
| 3 | Lancaster | Late November, 2018 | 73 | 38 | 15 April 2019 | 86,486 | DKC63-90 RIB | 9 October 2019 | |
| 2020 | 4 | Lancaster | Late October, 2019 | 67 | 19 | 23 April 2020 | 74,131 | DKC60-88 | 15 October 2020 |
| 5 | Saunders | Late October, 2019 | 73 | Fly | 30 April 2020 | 79,040 | P1366AM | 11 October 2020 | |
| 6 | Saunders | Late September, 2019 | 67 | 19 | 28 April 2020 | 86,486 | DKC63-90 RIB | 24 October 2020 | |
| 2021 | 7 | Saunders | Late October, 2020 | 67 | 38 | 26 April 2021 | 79,040 | P1366AM | 4 October 2021 |
| 8 | Lancaster | Late October, 2020 | 78 | Fly | 1 May 2021 | 86,486 | DKC63-90 RIB | 4 October 2021 | |
| Year | Site | Nebraska County | Treatment Applications | Measurements | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cover-Crop termination and Tank Mix Application | Late Insecticide Application | Insecticide Rate (g·ha−1) | Plot Size (m × m) | Sampling Periods (Pitfall Traps) | Corn Injury Assessment | Cover-Crop biomass | |||||
| I | II | III | |||||||||
| 2019 | 1 | Saunders | 6 May 2019 | 22 May 2019 | 142 | 9.14 × 9.14 | 24–28 April 2019 | 6–13 May 2019 | Lost | 30 May 2019 | 6 May 2019 |
| 2 | Saunders | 2 May 2019 | 1 June 2019 | 142 | 12.16 × 12.16 | 20–24 April 2019 | 2–6 May 2019 | 13–17 May 2019 | 30 May 2019 | 2 May 2019 | |
| 3 | Lancaster | 2 April 2019 | 3 June 2019 | 142 | 9.14 × 9.14 | 1–5 May 2019 | 22–17 May 2019 | 3–7 June 2019 | 10 June 2019 | 22 May 2019 | |
| 2020 | 4 | Lancaster | 27 April 2020 | 11 May 2020 | 142 | 27.4 × 30.48 | 30 March–6 April 2020 | 27 April–2 May 2020 | 11–17 May 2020 | 29 May 2020 | 27 April 2020 |
| 5 | Saunders | 1 May 2020 | 18 May 2020 | 142 | 27.4 × 30.48 | 6–10 April 2020 | 1–6 May 2020 | 18–23 May 2020 | 4 June 2020 | 1 May 2020 | |
| 6 | Saunders | 1 May 2020 | 18 May 2020 | 142 | 27.43 × 27.43 | 6–10 April 2020 | 1–6 May 2020 | 18–23 May 2020 | 4 June 2020 | 1 May 2020 | |
| 2021 | 7 | Saunders | 30 April 2021 | 22 May 2021 | 283 | 27.43 × 27.43 | 12–15 April 2021 | 29 April–4 May 2021 | 22–26 May 2021 | 1 June 2021 | 30 April 2021 |
| 8 | Lancaster | 13 May 2021 | 3 June 2021 | 283 | 27.4 x 30.48 | 12–15 April 2021 | 12–17 May 2021 | 3–7 June 2021 | 7 June 2021 | 13 May 2021 | |
| Year | Class | Insecta | Arachnida | Chilopoda | Collembola | Total Arthropods | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Hemiptera | Diptera | Coleoptera | ||||||||||||
| Family | Aphididae | Anthomyiidae | Sciaridae | Zopheridae | Staphylinidae | Nitidulidae | Carabidae | Larvae | Araneae | Acari | |||||
| 2019 | Site 1 | Number | 24 | 43 | 67 | 58 | 86 | 64 | 36 | 50 | 68 | 1629 | 7 | 574 | 2880 |
| % | 0.8 | 1.5 | 2.3 | 2 | 3 | 2.2 | 1.3 | 1.7 | 2.4 | 56.6 | 0.2 | 19.9 | 100 | ||
| Site 2 | Number | 0 | 145 | 35 | 79 | 135 | 27 | 40 | 39 | 43 | 2130 | 13 | 4442 | 7245 | |
| % | 0 | 2 | 0.5 | 1.1 | 1.9 | 0.4 | 0.6 | 0.5 | 0.6 | 29.4 | 0.2 | 61.3 | 100 | ||
| Site 3 | Number | 571 | 50 | 2 | 504 | 30 | 174 | 127 | 131 | 104 | 957 | 0 | 281 | 3137 | |
| % | 18.2 | 1.6 | 0.1 | 16.1 | 1 | 5.5 | 4 | 4.2 | 3.3 | 30.5 | 0 | 9 | 100 | ||
| 2020 | Site 4 | Number | 1139 | 0 | 20 | 1463 | 39 | 451 | 145 | 31 | 211 | 220 | 6 | 1067 | 5029 |
| % | 22.6 | 0 | 0.4 | 29.1 | 0.8 | 9 | 2.9 | 0.6 | 4.2 | 4.4 | 0.1 | 21.2 | 100 | ||
| Site 5 | Number | 843 | 316 | 22 | 1191 | 37 | 941 | 65 | 16 | 133 | 274 | 83 | 1870 | 5875 | |
| % | 14.3 | 5.4 | 0.4 | 20.3 | 0.6 | 16 | 1.1 | 0.3 | 2.3 | 4.7 | 1.4 | 31.8 | 100 | ||
| Site 6 | Number | 154 | 104 | 25 | 345 | 35 | 931 | 26 | 326 | 119 | 48 | 39 | 914 | 3124 | |
| % | 4.9 | 3.3 | 0.8 | 11 | 1.1 | 29.8 | 0.8 | 10.4 | 3.8 | 1.5 | 1.2 | 29.3 | 100 | ||
| 2021 | Site 7 | Number | 3 | 24 | 95 | 255 | 5 | 107 | 69 | 7 | 135 | 154 | 101 | 1869 | 2870 |
| % | 0.1 | 0.8 | 3.3 | 8.9 | 0.2 | 3.7 | 2.4 | 0.2 | 4.7 | 5.4 | 3.5 | 65.1 | 100 | ||
| Site 8 | Number | 41 | 4 | 266 | 108 | 13 | 206 | 21 | 0 | 71 | 874 | 0 | 1405 | 3157 | |
| % | 1.3 | 0.1 | 8.4 | 3.4 | 0.4 | 6.5 | 0.7 | 0 | 2.2 | 27.7 | 0 | 44.5 | 100 | ||
| Year | Class | Insecta | Arachnida | Collembola | Total Arthropods | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Order | Hemiptera | Diptera | Coleoptera | - | - | ||||||||||
| Family | Aphididae | Anthomyiidae | Sciaridae | Zopheridae | Staphalynidae | Nitidulidae | Carabidae | Larvae | Araneae | Acari | - | ||||
| 2019 | Site 1 | SMP | F | n/a | 10.78 | n/a | 12.59 | 16.68 | 3.58 | 8.19 | 4.91 | 1.11 | 85.41 | 20.17 | 66.98 |
| df 2 | 1, 13 | 1, 13 | 1, 13 | 1, 12 | 1, 13 | 1, 13 | 1, 13 | 1, 13 | 1, 13 | 1, 12 | |||||
| P | 0.009 | 0.006 | 0.001 | 0.083 | 0.013 | 0.095 | 0.310 | <0.0001 | 0.001 | <0.0001 | |||||
| Trt | F | 0.54 | 0.91 | 0.88 | 0.64 | 0.34 | 2.58 | 1.88 | 0.21 | 1.45 | 0.58 | ||||
| df | 2, 13 | 2, 13 | 2, 13 | 2, 12 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | |||||
| P | 0.612 | 0.466 | 0.452 | 0.544 | 0.715 | 0.114 | 0.192 | 0.813 | 0.270 | 0.573 | |||||
| SMP*Trt | F | 0.09 | 0.16 | 1.2 | 0.12 | 0.66 | 0.81 | 0.07 | 0.01 | 3.2 | 0.67 | ||||
| df | 2, 13 | 2, 13 | 2, 13 | 2, 12 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | 2, 13 | |||||
| P | 0.979 | 0.856 | 0.333 | 0.885 | 0.533 | 0.464 | 0.928 | 0.991 | 0.074 | 0.527 | |||||
| Site 2 | SMP | F | n/a | 0.87 | n/a | 8.11 | 7.61 | 5.75 | 2.75 | 5.42 | n/a | 0.36 | 4.84 | 5.43 | |
| df | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | ||||||
| P | 0.3763 | 0.019 | 0.022 | 0.040 | 0.132 | 0.0450 | 0.565 | 0.055 | 0.045 | ||||||
| Trt | F | 4.4 | 1.17 | 1.59 | 0.54 | 0.11 | 3.57 | 2.46 | 1.74 | 3.87 | |||||
| df | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | ||||||
| P | 0.097 | 0.354 | 0.256 | 0.601 | 0.894 | 0.072 | 0.142 | 0.230 | 0.081 | ||||||
| SMP*Trt | F | 5.08 | 0.23 | 2.06 | 0.2 | 0.3 | 0.81 | 1.21 | 0.05 | 0.41 | |||||
| df | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | ||||||
| P | 0.083 | 0.796 | 0.183 | 0.825 | 0.746 | 0.475 | 0.343 | 0.947 | 0.674 | ||||||
| Site 3 1 | SMP | F | 2.75 | 15.85 | n/a | 0.83 | 4.69 | 18.65 | 51.02 | 22.95 | 25.14 | 116.07 | 95.92 | 247.11 | |
| df | 1, 9 | 2, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | 1, 9 | ||||
| P | 0.132 | 0.003 | 0.387 | 0.059 | 0.002 | <0.0001 | 0.001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | ||||
| Trt | F | 4.19 | 0.61 | 1.09 | 1.17 | 1.68 | 0.52 | 2.93 | 0.87 | 2.13 | 0.67 | 4.52 | |||
| df | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | ||||
| P | 0.052 | 0.565 | 0.376 | 0.353 | 0.240 | 0.610 | 0.105 | 0.452 | 0.175 | 0.536 | 0.044 | ||||
| SMP*Trt | F | 2.08 | 0.28 | 1.41 | 0.73 | 1.28 | 0.4 | 2.27 | 0.56 | 0.22 | 0.91 | 5.57 | |||
| df | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2, 9 | 2. 9 | 2, 9 | 2, 9 | 2, 9 | ||||
| P | 0.187 | 0.766 | 0.294 | 0.509 | 0.323 | 0.682 | 0.159 | 0.452 | 0.810 | 0.438 | 0.027 | ||||
| 2020 | Site 4 | SMP | F | 34.45 | n/a | n/a | 42.42 | n/a | 61.51 | 0.05 | n/a | 47.29 | n/a | 17.02 | 0.67 |
| df | 1, 15 | 1, 12 | 1, 12 | 1, 12 | 1, 15 | 1, 12 | 1, 15 | ||||||||
| P | <0.0001 | <0.0001 | <0.0001 | 0.0945 | <0.0001 | 0.002 | 0.001 | ||||||||
| Trt | F | 2.3 | 0.63 | 0.94 | 0.64 | 0.96 | 2.31 | 0.24 | |||||||
| df | 2, 15 | 2, 12 | 2, 12 | 2. 12 | 2, 15 | 2, 12 | 2, 15 | ||||||||
| P | 0.119 | 0.612 | 0.468 | 0.601 | 0.437 | 0.157 | 0.843 | ||||||||
| SMP*Trt | F | 1.3 | 0.26 | 1.24 | 2.8 | 0.17 | 0.42 | 0.13 | |||||||
| df | 2, 15 | 2, 12 | 2, 12 | 2, 12 | 2, 15 | 2, 15 | 2, 15 | ||||||||
| P | 0.311 | 0.855 | 0.345 | 0.078 | 0.904 | 0.743 | 0.938 | ||||||||
| Site 5 | SMP | F | 112.17 | 9.77 | n/a | 0.06 | n/a | 31.79 | 0.84 | n/a | 0.42 | 15.78 | 31.52 | 0.14 | |
| df | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | ||||||
| P | <0.0001 | 0.012 | 0.817 | <0.0001 | 0.382 | 0.633 | 0.001 | <0.0001 | 0.712 | ||||||
| Trt | F | 6.8 | 0.5 | 1.86 | 0.15 | 2.16 | 0.76 | 1.57 | 0.54 | 1.69 | |||||
| df | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | ||||||
| P | 0.006 | 0.623 | 0.219 | 0.860 | 0.151 | 0.630 | 0.109 | 0.489 | 0.245 | ||||||
| SMP*Trt | F | 4.42 | 2.56 | 1.41 | 0.34 | 8.66 | 0.31 | 0.16 | 0.12 | 2.62 | |||||
| df | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | ||||||
| P | 0.026 | 0.141 | 0.290 | 0.718 | 0.010 | 0.422 | 0.856 | 0.982 | 0.127 | ||||||
| Site 6 | SMP | F | 18.45 | 3.88 | n/a | 5.71 | n/a | 2.34 | 0.03 | n/a | 11.59 | 1.01 | 10.21 | 0.73 | |
| df | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 10 | 1, 15 | 1, 10 | 1, 10 | 1, 10 | ||||||
| P | 0.001 | 0.068 | 0.042 | 0.161 | 0.897 | 0.004 | 0.246 | 0.012 | 0.005 | ||||||
| Trt | F | 2.26 | 2.18 | 2.04 | 0.03 | 0.16 | 0.2 | 0.98 | 0.54 | 3.38 | |||||
| df | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 15 | 2, 10 | 2, 10 | 2, 10 | ||||||
| P | 0.167 | 0.148 | 0.204 | 0.967 | 0.870 | 0.821 | 0.810 | 0.604 | 0.085 | ||||||
| SMP*Trt | F | 0.86 | 1.01 | 1.68 | 2.01 | 1.58 | 3.51 | 0.66 | 0.95 | 2.14 | |||||
| df | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 10 | 2, 15 | 2, 10 | 2, 10 | 2, 10 | ||||||
| P | 0.444 | 0.389 | 0.242 | 0.191 | 0.350 | 0.096 | 0.583 | 0.425 | 0.172 | ||||||
| 2021 | Site 7 | SMP | F | n/a | 0.22 | 0.6158 | 7.48 | n/a | 0.17 | 1.86 | n/a | 1.01 | 9.6 | 38.56 | 22.3 |
| df | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 15 | 1, 6 | 1, 6 | ||||||
| P | 0.659 | 0.616 | 0.015 | 0.688 | 0.193 | 0.332 | 0.029 | 0.001 | 0.005 | ||||||
| Trt | F | 1.27 | 1.2 | 0.79 | 1.72 | 0.21 | 1.15 | 0.33 | 0.56 | 0.44 | |||||
| df | 2, 15 | 2, 12 | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 6 | 2, 6 | ||||||
| P | 0.313 | 0.341 | 0.471 | 0.244 | 0.813 | 0.344 | 0.737 | 0.600 | 0.667 | ||||||
| SMP*Trt | F | 1.65 | 0.55 | 0.52 | 2.46 | 0.92 | 0.1 | 0.68 | 0.62 | 0.35 | |||||
| df | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 15 | 2, 6 | 2, 6 | ||||||
| P | 0.212 | 0.604 | 0.603 | 0.119 | 0.420 | 0.903 | 0.554 | 0.568 | 0.723 | ||||||
| Site 8 | SMP | F | n/a | 0.13 | 2.17 | 17.68 | n/a | 26.06 | 0.03 | n/a | 3.17 | 57.72 | 58.26 | 3.33 | |
| df | 1, 11 | 1, 11 | 1, 11 | 1, 11 | 1, 11 | 1, 11 | 1, 11 | 1, 11 | 1, 11 | ||||||
| P | 0.815 | 0.099 | 0.002 | 0.001 | 0.882 | 0.326 | <0.0001 | <0.0001 | 0.101 | ||||||
| Trt | F | 0.8 | 0.15 | 0.3 | 1.11 | 0.4 | 1.89 | 1.25 | 0.3 | 0.73 | |||||
| df | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | ||||||
| P | 0.764 | 0.865 | 0.746 | 0.355 | 0.745 | 0.459 | 0.403 | 0.745 | 0.543 | ||||||
| SMP*Trt | F | 0.98 | 1.11 | 0.17 | 1.05 | 1.51 | 1.42 | 1.15 | 8.66 | 12.13 | |||||
| df | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | 2, 11 | ||||||
| P | 0.697 | 0.451 | 0.843 | 0.382 | 0.499 | 0.511 | 0.367 | 0.006 | 0.003 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, G.I.; Robinson, E.; Campos, J.N.D.; McMechan, A.J. Impact of the Timing and Use of an Insecticide on Arthropods in Cover-Crop-Corn Systems. Insects 2022, 13, 348. https://doi.org/10.3390/insects13040348
Carmona GI, Robinson E, Campos JND, McMechan AJ. Impact of the Timing and Use of an Insecticide on Arthropods in Cover-Crop-Corn Systems. Insects. 2022; 13(4):348. https://doi.org/10.3390/insects13040348
Chicago/Turabian StyleCarmona, Gabriela Inveninato, Emily Robinson, Julia Nogueira Duarte Campos, and Anthony Justin McMechan. 2022. "Impact of the Timing and Use of an Insecticide on Arthropods in Cover-Crop-Corn Systems" Insects 13, no. 4: 348. https://doi.org/10.3390/insects13040348
APA StyleCarmona, G. I., Robinson, E., Campos, J. N. D., & McMechan, A. J. (2022). Impact of the Timing and Use of an Insecticide on Arthropods in Cover-Crop-Corn Systems. Insects, 13(4), 348. https://doi.org/10.3390/insects13040348






