Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Beet Armyworm Colony
2.2. Resistance Stability
2.3. Fitness of Beet Armyworm
2.4. Statistical Analyses
3. Results
3.1. Resistance Stability
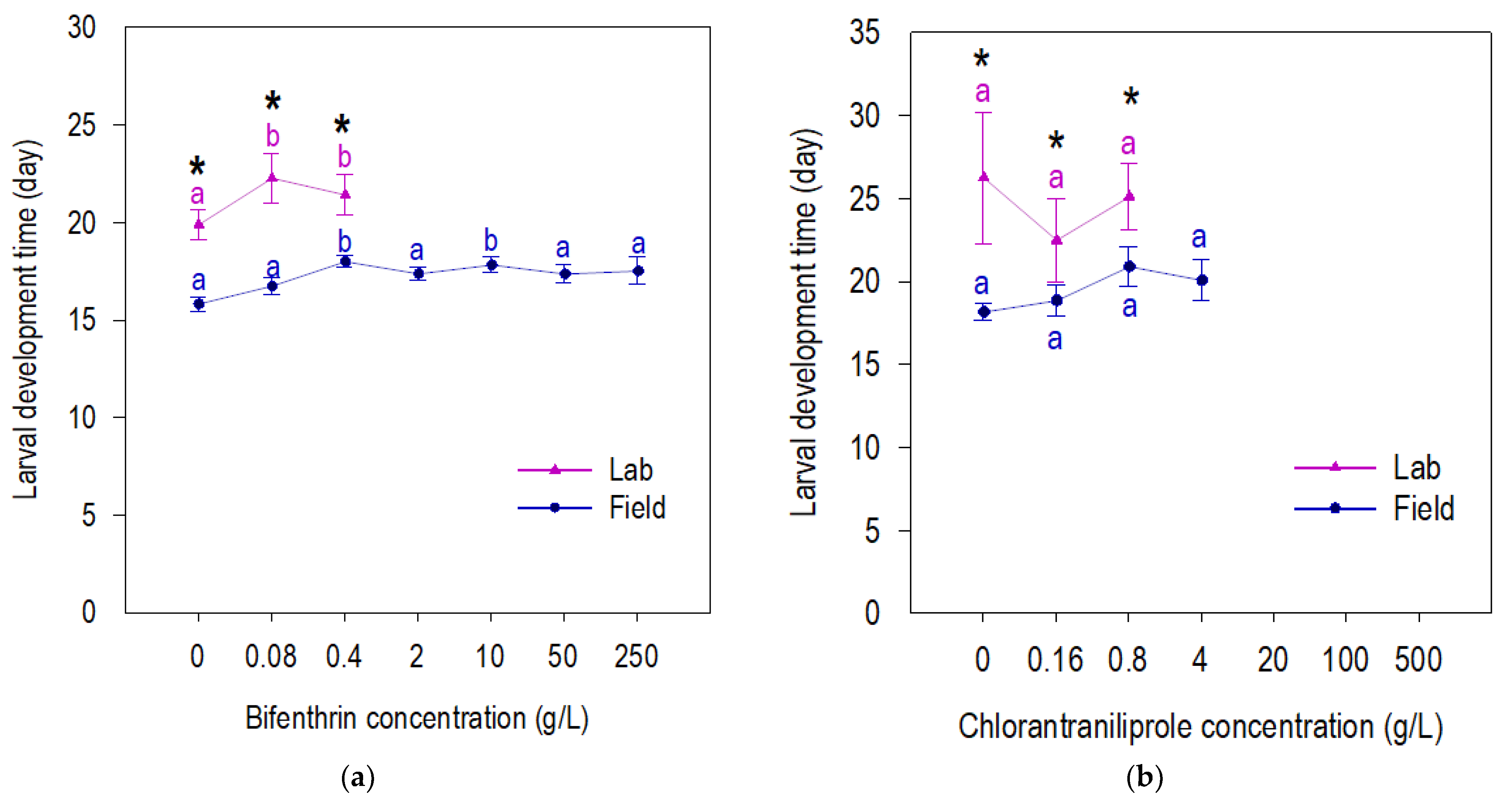
3.2. Fitness of Beet Armyworm
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pogue, M. A World Revision of the Genus Spodoptera Guenée (Lepidoptera: Noctuidae); American Entomological Society: Philadelphia, PA, USA, 2002. [Google Scholar]
- Zheng, X.L.; Cong, X.P.; Wang, X.P.; Lei, C.L. A Review of Geographic Distribution, Overwintering and Migration in Spodoptera exigua Hübner (Lepidoptera: Noctuidae). J. Entomol. Res. Soc. 2011, 13, 39–48. [Google Scholar]
- Mascarenhas, V.J.; Leonard, B.R.; Burris, E.; Graves, J.B. Beet Armyworm (Lepidoptera: Noctuidae) Control on Cotton in Louisiana. Fla. Entomol. 1996, 79, 336. [Google Scholar] [CrossRef]
- Stewart, S.D.; Adamczyk, J.J.; Knighten, K.S.; Davis, F.M. Impact of Bt Cottons Expressing One or Two Insecticidal Proteins of Bacillus thuringiensis Berliner on Growth and Survival of Noctuid (Lepidoptera) Larvae. J. Econ. Entomol. 2001, 94, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Li, J.; Su, J. Monitoring of Beet Armyworm Spodoptera exigua (Lepidoptera: Noctuidae) Resistance to Chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Paula-Moraes, S.V.; Pereira, E.J.G.; Siegfried, B.D. Contrasting Susceptibility of Lepidopteran Pests to Diamide and Pyrethroid Insecticides in a Region of Overwintering and Migratory Intersection. Pest Manag. Sci. 2020, 76, 4240–4247. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Ullah, F.; Khan, M.M.; Li, X.; Zhang, Z.; Shah, S.; Imran, M.; Assiri, M.A.; Fernández-Grandon, G.M.; Desneux, N. Metabolic-Based Insecticide Resistance Mechanism and Ecofriendly Approaches for Controlling of Beet Armyworm Spodoptera exigua: A Review. Environ. Sci. Pollut. Res. 2021, 29, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Huang, F. Detection and Monitoring of Insect Resistance to Transgenic Bt Crops. Insect Sci. 2006, 13, 73–84. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.L.; Luo, H.B.; Zhang, S.; Si, S.Y. Monitoring the Resistance of the Beet Armyworm (Lepidoptera: Noctuidae) to Four Insecticides in Southern China from 2014 to 2018. J. Econ. Entomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide Resistance Status of Field Populations of Spodoptera exigua (Lepidoptera: Noctuidae) From China. J. Econ. Entomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the Ryanodine Receptor Mutation I4743M and Its Contribution to Diamide Insecticide Resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic Actions of Pyrethroid Insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Ottea, J.A.; Holloway, J.W. Target-Site Resistance to Pyrethroids in Heliothis virescens (F.) and Helicoverpa zea (Boddie). Pestic. Biochem. Physiol. 1998, 61, 155–167. [Google Scholar] [CrossRef]
- Montezano, D.G.; Hunt, T.E.; Souza, D.; Vieira, B.C.; Vélez, A.M.; Kruger, G.R.; Zukoff, S.N.; Bradshaw, J.D.; Peterson, J.A. Bifenthrin Baseline Susceptibility and Evaluation of Simulated Aerial Applications in Striacosta albicosta (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Foster, R.; Krupke, C.; Hutchison, W.; Pittendrigh, B.; Weinzierl, R. Resistance to Pyrethroid Insecticides in Helicoverpa zea (Lepidoptera: Noctuidae) in Indiana and Illinois. J. Econ. Entomol. 2009, 102, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.A.; Andaloro, J.T. Diamide Insecticides: Global Efforts to Address Insect Resistance Stewardship Challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. RynaxypyrTM: A New Insecticidal Anthranilic Diamide That Acts as a Potent and Selective Ryanodine Receptor Activator. Bioorganic Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef]
- Reisig, D.; Kerns, D.; Gore, J.; Musser, F. Managing Pyrethroid- and Bt-Resistant Bollworm in Southern U.S. Cotton. Crops Soils 2019, 52, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, M.M.; Paula-Moraes, S.V.; Pereira, E.J.G.; Siegfried, B.D. Demographic Performance of Helicoverpa zea Populations on Dual and Triple-Gene Bt Cotton. Toxins 2020, 12, 551. [Google Scholar] [CrossRef]
- Baldwin, J.M.; Paula-Moraes, S.V.; Mulvaney, M.J.; Meagher, R.L. Occurrence of Arthropod Pests Associated with Brassica Carinata and Impact of Defoliation on Yield. GCB Bioenergy 2021, 13, 570–581. [Google Scholar] [CrossRef]
- Freeman, J.C.; Smith, L.B.; Silva, J.J.; Fan, Y.; Sun, H.; Scott, J.G. Fitness Studies of Insecticide Resistant Strains: Lessons Learned and Future Directions. Pest Manag. Sci. 2021, 77, 3847–3856. [Google Scholar] [CrossRef]
- Belinato, T.A.; Martins, A.J. Insecticide Resistance and Fitness Cost. In Insecticides Resistance; IntechOpen: London, UK, 2016; pp. 243–261. [Google Scholar]
- Santos-Amaya, O.F.; Tavares, C.S.; Rodrigues, J.V.C.; Campos, S.O.; Guedes, R.N.C.; Alves, A.P.; Pereira, E.J.G. Fitness Costs and Stability of Cry1Fa Resistance in Brazilian Populations of Spodoptera frugiperda. Pest Manag. Sci. 2017, 73, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliot, A.; Ghanim, M. Fitness Costs Associated with Insecticide Resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness Costs of Insect Resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Jakka, S.R.K.; Knight, V.R.; Jurat-Fuentes, J.L. Fitness Costs Associated With Field-Evolved Resistance to Bt Maize in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2014, 107, 342–351. [Google Scholar] [CrossRef]
- Von Kanel, M.B.; Gore, J.; Catchot, A.; Cook, D.; Musser, F.; Caprio, M. Influence of Dual-Bt Protein Corn on Bollworm, Helicoverpa zea (Boddie), Survivorship on Bollgard II Cotton. J. Econ. Entomol. 2016, 109, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yao, X.; Xiang, X.; Yang, Q.; Wang, X.; Xin, T.; Yu, S. Fitness Costs Associated with Chlorantraniliprole Resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manag. Sci. 2021, 77, 1739–1747. [Google Scholar] [CrossRef]
- Bourguet, D.; Genissel, A.; Raymond, M. Insecticide Resistance and Dominance Levels. J. Econ. Entomol. 2000, 93, 1588–1595. [Google Scholar] [CrossRef]
- Raymond, B.E.N.; Sayyed, A.H.; Hails, R.S.; Wright, D.J. Exploiting Pathogens and Their Impact on Fitness Costs to Manage the Evolution of Resistance to Bacillus thuringiensis. J. Appl. Ecol. 2007, 44, 768–780. [Google Scholar] [CrossRef]
- Santos-Amaya, O.F.; Rodrigues, J.V.C.; Souza, T.C.; Tavares, C.S.; Campos, S.O.; Guedes, R.N.C.; Pereira, E.J.G. Resistance to Dual-Gene Bt Maize in Spodoptera frugiperda: Selection, Inheritance, and Cross-Resistance to Other Transgenic Events. Sci. Rep. 2015, 5, 18243. [Google Scholar] [CrossRef]
- Carrière, Y.; Deland, J.-P.; Roff, D.A.; Vincent, C. Life-History Costs Associated with the Evolution of Insecticide Resistance. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 258, 35–40. [Google Scholar]
- Tabashnik, B.E.; Van Rensburg, J.B.J.; Carrière, Y. Field-Evolved Insect Resistance to Bt Crops: Definition, Theory, and Data. J. Econ. Entomol. 2009, 102, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Endersby, N.M.; Ridland, P.M.; Hoffmann, A.A. The Effects of Local Selection versus Dispersal on Insecticide Resistance Patterns: Longitudinal Evidence from Diamondback Moth (Plutella xylostella (Lepidoptera: Plutellidae)) in Australia Evolving Resistance to Pyrethroids. Bull. Entomol. Res. 2008, 98, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Shad, S.A. Stability and Fitness Cost Associated with Spirotetramat Resistance in Oxycarenus Hyalinipennis Costa (Hemiptera: Lygaeidae). Pest Manag. Sci. 2021, 78, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Pampel, F. Probit Analysis. In Logistic Regression; SAGE Publications, Inc.: Newbury Park, CA, USA, 2011. [Google Scholar]
- Software, L. Polo Plus: Probit and Logit Analysis; Version 1.0; Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 2002. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1315356279. [Google Scholar]
- Basit, M.; Saeed, S.; Saleem, M.A.; Denholm, I.; Shah, M. Detection of Resistance, Cross-Resistance, and Stability of Resistance to New Chemistry Insecticides in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2013, 106, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.S. Development and Stability of Insecticide Resistance in the Leafminer Liriomyza trifolii (Diptera: Agromyzidae) to Cyromazine, Abamectin, and Spinosad. J. Econ. Entomol. 2004, 97, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groeters, F.R.; Tabashnik, B.E.; Finson, N.; Johnson, M.W. Fitness Costs of Resistance to Bacillus thuringiensis in the Diamondback Moth (Plutella xylostella). Evolution 1994, 48, 197–201. [Google Scholar] [PubMed]
- Ahmad, M.; Sayyed, A.H.; Crickmore, N.; Saleem, M.A. Genetics and Mechanism of Resistance to Deltamethrin in a Field Population of Spodoptera Litura (Lepidoptera: Noctuidae). Pest Manag. Sci. 2007, 63, 1002–1010. [Google Scholar] [CrossRef]
- Abbas, I.; Liu, J.; Faheem, M.; Noor, R.S.; Shaikh, S.A.; Solangi, K.A.; Raza, S.M. Different Real-Time Sensor Technologies for the Application of Variable-Rate Spraying in Agriculture. Sens. Actuators A Phys. 2020, 316, 112265. [Google Scholar] [CrossRef]
- Groeters, F.R.; Tabashnik, B.E. Roles of Selection Intensity, Major Genes, and Minor Genes in Evolution of Insecticide Resistance. J. Econ. Entomol. 2000, 93, 1580–1587. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide Resistance: 10 Years of Lessons from Lepidopteran Pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef] [Green Version]
- Rivero, A.; Magaud, A.; Nicot, A.; Vézilier, J. Energetic Cost of Insecticide Resistance in Culex pipiens Mosquitoes. J. Med. Entomol. 2011, 48, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Steinbach, D. Resistance to Diamide Insecticides in Lepidopteran Pests. In Advances in Insect Control and Resistance Management; Springer: Cham, Switzerland, 2016; pp. 219–240. [Google Scholar]
- Zuo, Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 Mediated G4946E Substitution in the Ryanodine Receptor of Spodoptera exigua Confers High Levels of Resistance to Diamide Insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.H. Novel Diamide Resistance-Linked Mutation in Korean Spodoptera exigua and a LAMP Assay Based on a Mutation-Associated Intronic InDel. J. Pest Sci. 2021, 94, 1017–1029. [Google Scholar] [CrossRef]
- Huang, J.-M.; Zhao, Y.-X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.-F.; Wu, S.-F. Monitoring and Mechanisms of Insecticide Resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with Special Reference to Diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef]
- Shimada, K.; Natsuhara, K.; Oomori, Y.; Miyata, T. Permethrin Resistance Mechanisms in the Beet Armyworm (Spodoptera exigua (Hübner)). J. Pestic. Sci. 2005, 30, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.L.; Liu, S.W.; Baerson, S.R.; Qin, Z.; Ma, Z.H.; Su, Y.J.; Zhang, J.E. Identification and Functional Analysis of a Novel Cytochrome P450 Gene CYP9A105 Associated with Pyrethroid Detoxification in Spodoptera exigua Hübner. Int. J. Mol. Sci. 2018, 19, 737. [Google Scholar] [CrossRef] [Green Version]
- Carrière, Y.; Tabashnik, B.E. Reversing Insect Adaptation to Transgenic Insecticidal Plants. Proc. R. Soc. B Biol. Sci. 2001, 268, 1475–1480. [Google Scholar] [CrossRef] [Green Version]



| Population | Generation (Year) | N a | Equation | χ2 | pc | LC50 (95% CL) b | RR (95% CL) d |
|---|---|---|---|---|---|---|---|
| Field | F2 (2018) | 300 | y = −7.28 + 1.76x | 2.04 | 0.56 | 3310.00 (2085–15,520) | 10,071 (4426–22,916) |
| F13(2019) | 200 | y = −0.70 + −0.08x | nc e | nc | >250.00 | >2083 | |
| F27(2020) | 148 | y = −2.07 + 0.16x | nc | nc | >250.00 | >1666 | |
| Laboratory | F2 (2018) | 300 | y = −0.26 + 1.78x | 0.02 | 0.99 | 0.32 (0.16–0.53) | 1 |
| F13(2019) | 250 | y = −0.98 + 1.10x | 1.79 | 0.77 | 0.12 (0.03–0.27) | 1 | |
| F27(2020) | 150 | y = 1.00 + 1.24x | 0.84 | 0.42 | 0.15 (0.06–0.27) | 1 |
| Population | Generation (Year) | N a | Equation | χ2 | pc | LC50 (95% CL) b | RR (95% CL) d |
|---|---|---|---|---|---|---|---|
| Field | F2 (2018) | 144 | y = −3.06 + 0.07x | nc e | nc | >139.92 | 629 (13–22,215) |
| F13(2019) | 200 | y = −1.80 + 0.86x | 2.81 | 0.42 | 121.93 (32.00–273.00) | 80 (25–252) | |
| F27(2020) | 127 | y = −0.64 + 0.53x | 0.87 | 0.97 | 15.92 (1.79–81.81) | 15 (1–123) | |
| Laboratory | F2 (2018) | 300 | y = −0.39 + 1.19x | 2.29 | 0.51 | 2.12 (1.31–3.46) | 1 |
| F13(2019) | 250 | y = −0.16 + 0.92x | 3.05 | 0.21 | 1.51 (0.11–8.18) | 1 | |
| F27(2020) | 122 | y = −0.09 + 1.07x | 1.69 | 0.42 | 1.02 (0.19–2.79) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, M.M.; Santos, I.B.; Paula-Moraes, S.V. Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States. Insects 2022, 13, 365. https://doi.org/10.3390/insects13040365
Rabelo MM, Santos IB, Paula-Moraes SV. Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States. Insects. 2022; 13(4):365. https://doi.org/10.3390/insects13040365
Chicago/Turabian StyleRabelo, Marcelo M., Izailda B. Santos, and Silvana V. Paula-Moraes. 2022. "Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States" Insects 13, no. 4: 365. https://doi.org/10.3390/insects13040365
APA StyleRabelo, M. M., Santos, I. B., & Paula-Moraes, S. V. (2022). Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States. Insects, 13(4), 365. https://doi.org/10.3390/insects13040365







