Effects of Host Plants on Bacterial Community Structure in Larvae Midgut of Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing Conditions
2.2. Gut Dissection and 16S rRNA Gene Sequencing
2.3. Operational Taxonomic Unit (OTU) Clustering and Annotation
2.4. Sequencing Data Analyses and Function Prediction
2.5. Statistical Analyses
3. Results
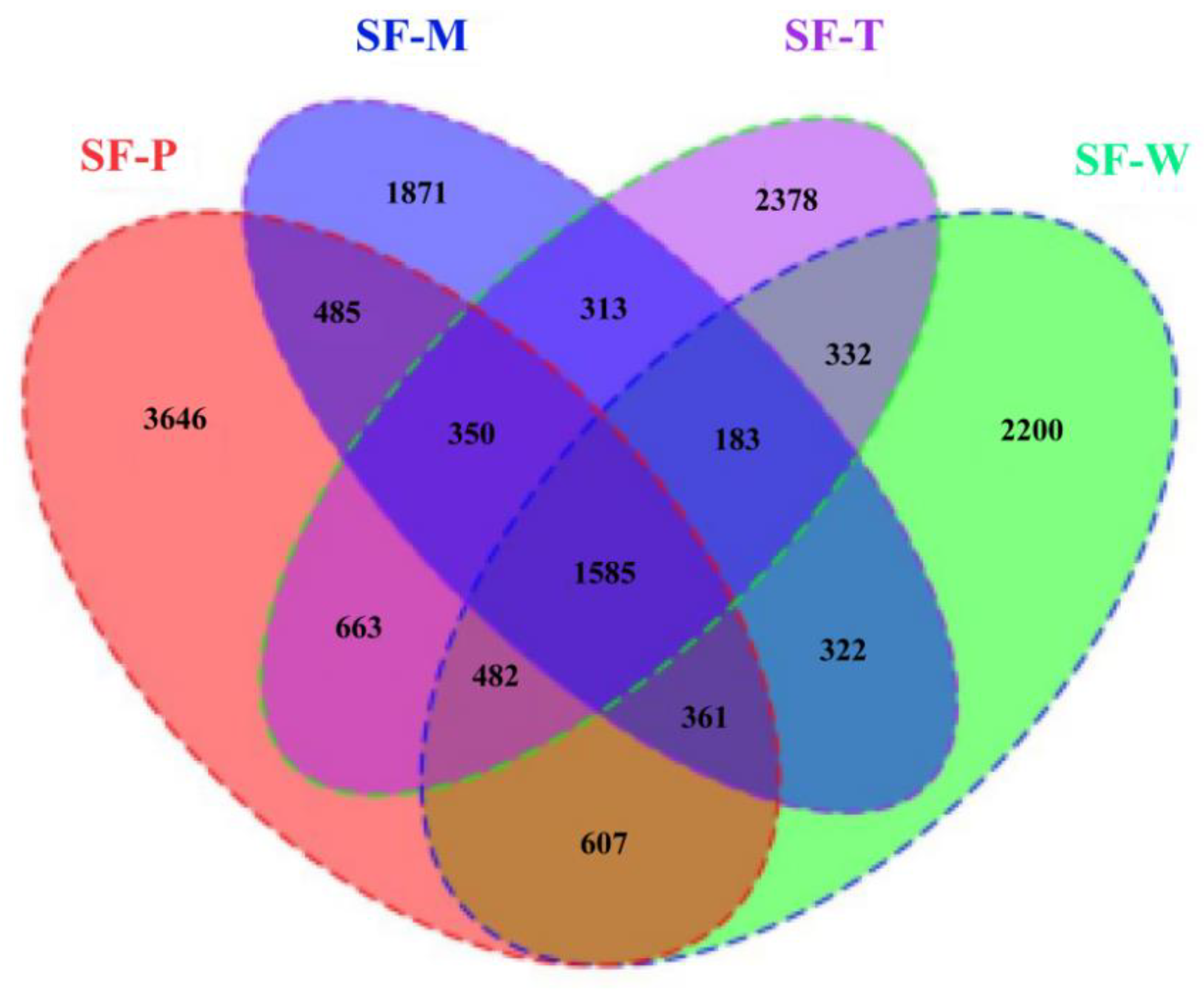
3.1. Sequencing Data Statistics and Clustering
3.2. Alpha Diversity of Gut Flora
3.3. Beta Diversity of Gut Flora
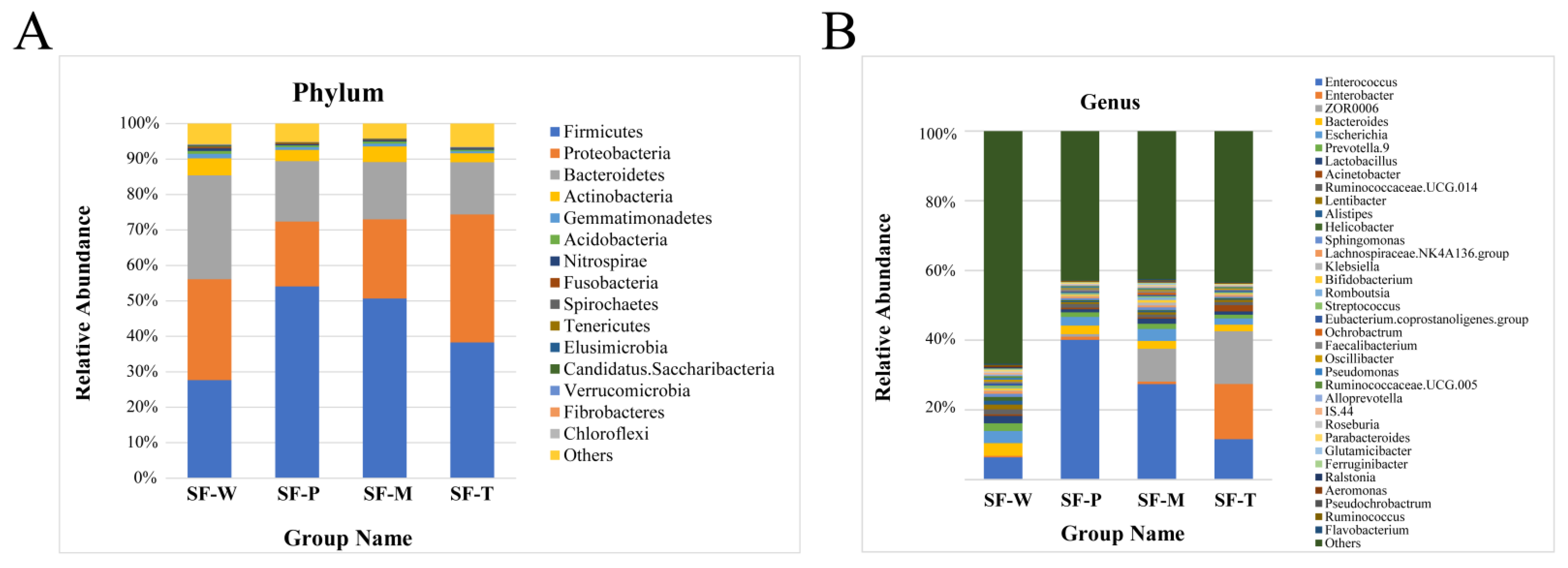
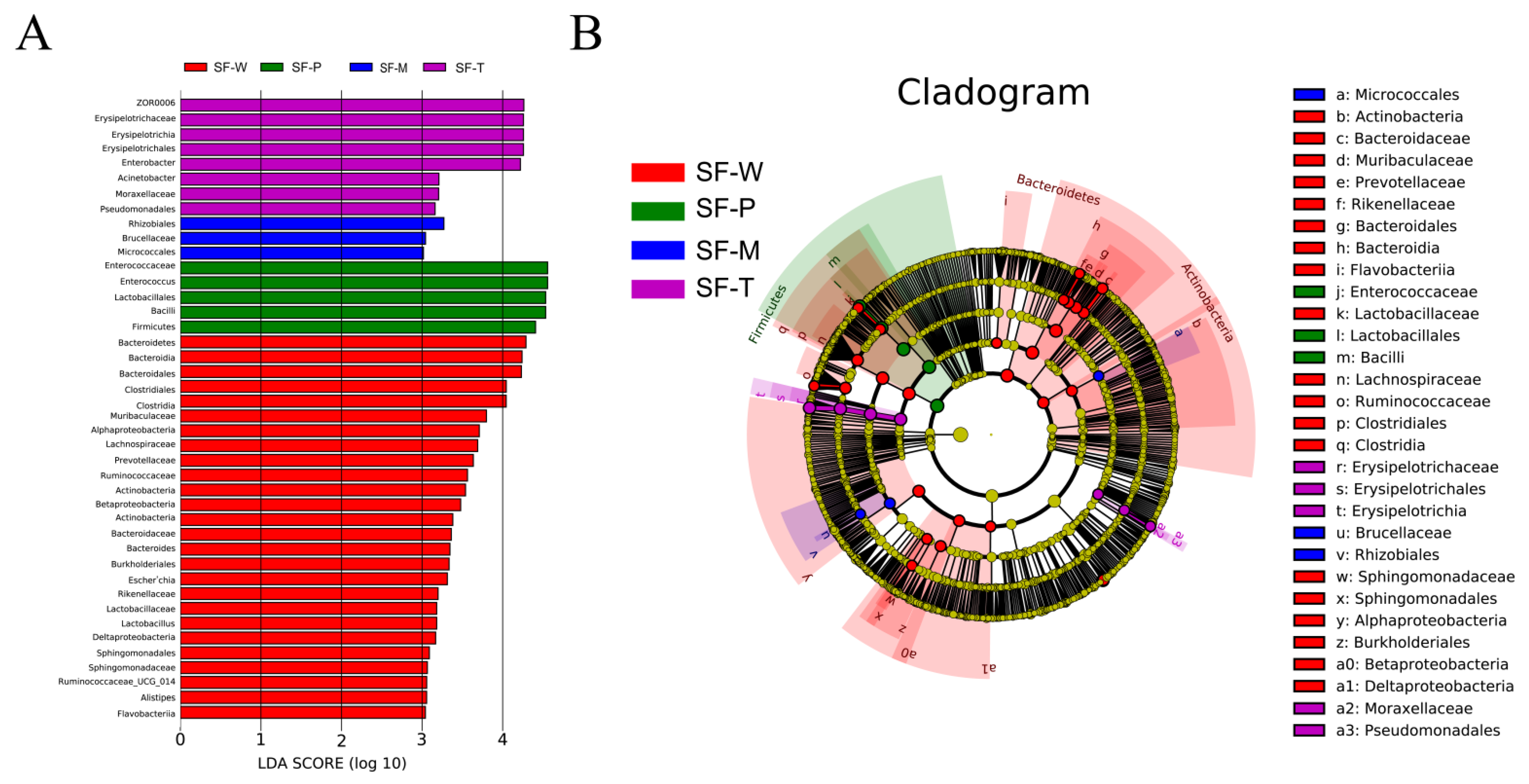
3.4. Differences in Community Structure of Gut Flora
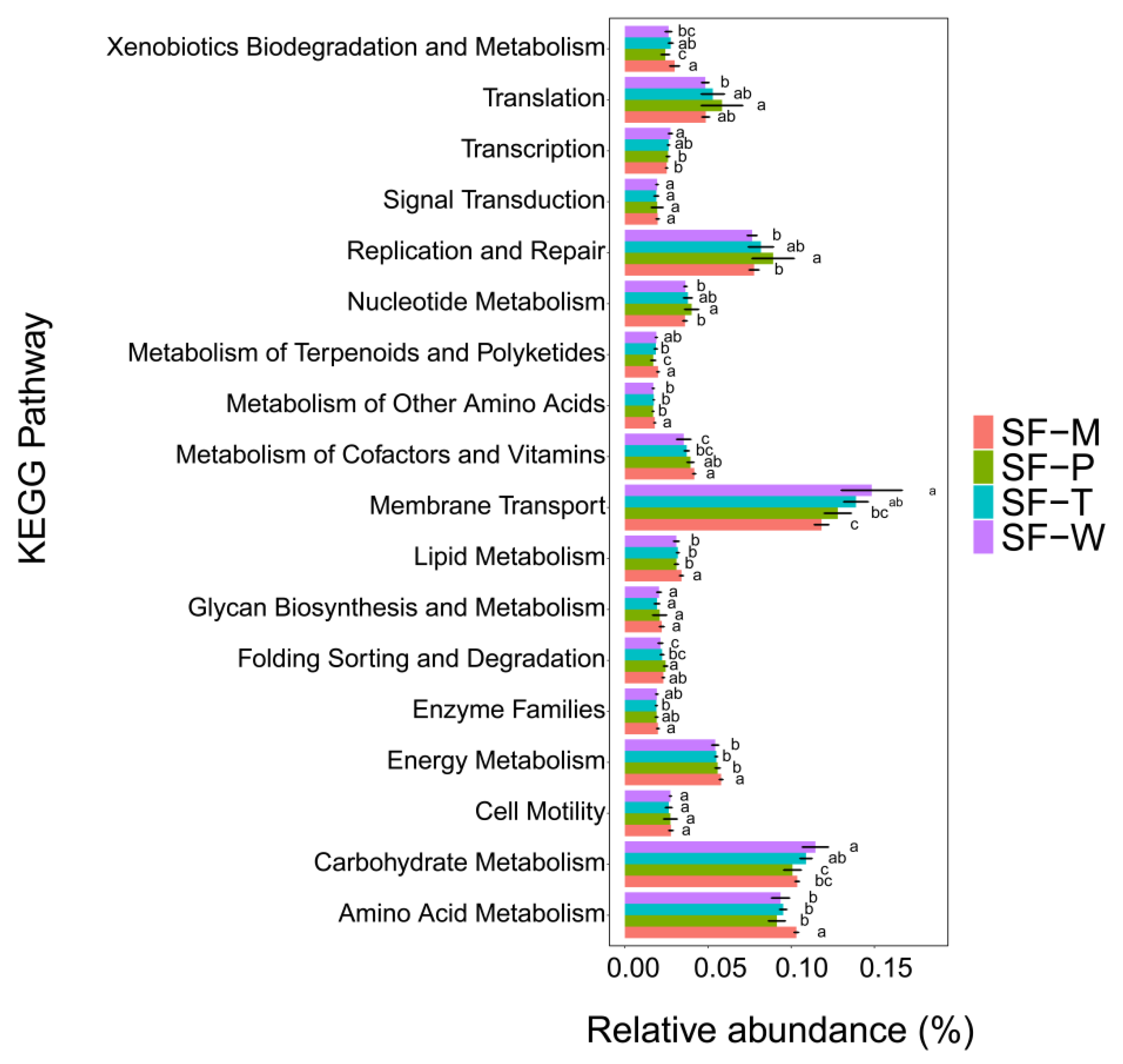
3.5. Functional Prediction of Gut Flora and Their Difference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinds, W.E.; Dew, J.A. The grass worm or fall army worm. Ala. Agric. Exp. Sta. Bull. 1915, 186, 61–92. [Google Scholar]
- Montezano, D.G.; Spechr, A.; Sosa-Gonez, D.R.; Roque-Spechi, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–86. [Google Scholar] [CrossRef]
- Casmuz, A.; Juάrez, M.L.; Socías, M.G.; Murua, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminza, G. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 2010, 69, 209–231. [Google Scholar]
- Hardke, J.T.; Lorenz, G.M.; Leonard, B.R. Fall armyworm (Lepidoptera: Noctuidae) ecology in south-eastern cotton. J. Integr. Pest Manag. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Gui, F.R.; Lan, T.M.; Zhao, Y.; Guo, W.; Dong, Y.; Fang, D.M.; Liu, H.; Li, H.; Wang, H.L.; Hao, R.S.; et al. Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Pashley, D.P. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): A sibling species complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Pashley, D.P. Quantitative genetics, development, and physiological adaptation in host strains of fall armyworm. Evolution 1988, 42, 93–102. [Google Scholar]
- Silva-Brandão, K.L.; Horikoshi, R.J.; Bernardi, D.; Omoto, C.; Figueira, A.; Brandão, M.M. Transcript expression plasticity as a response to alternative larval host plants in the speciation process of corn and rice strains of Spodoptera frugiperda. BMC Genom. 2017, 18, 792. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Ray, S.; Meagher, R.; Luthe, D.S.; Felton, G.W. Intraspecific differences in plant defense induction by fall armyworm strains. New Phytol. 2018, 218, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Moné, Y.; Nhim, S.; Gimenez, S.; Legeai, F.; Seninet, I.; Parrinello, H.; Nègre, N.; d’Alençon, E. Characterization and expression profiling of microRNAs in response to plant feeding in two host-plant strains of the lepidopteran pest Spodoptera frugiperda. BMC Genom. 2018, 19, 804. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Carrière, Y.; Crickmore, N.; Tabashnik, B.E. Insect resistance to Bt crops: Lessons from the first billion acres. Nat. Biotechnol. 2013, 31, 510–521. [Google Scholar]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Phys. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Magoč, C.T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Warnecke, F.; Luginbühl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; McHardy, A.C.; Djordjevic, G.; Aboushadi, N.; et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 2007, 450, 560–565. [Google Scholar] [CrossRef]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Ryu, J.H.; Ha, E.M.; Lee, W.J. Innate immunity and gut-microbe mutualism in Drosophila. Dev. Comp. Immunol. 2010, 34, 369–376. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiontmediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella L. Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Segal, D.; Ringo, J.M.; Hefetz, A.; Zilber-Rosenberg, I.; Rosenberg, E. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 107, 20051–20056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, G.; Segal, D.; Zilber-Rosenberg, I.; Rosenberg, E. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes 2011, 2, 190–192. [Google Scholar] [CrossRef] [Green Version]
- Montagna, M.; Chouaia, B.; Mazza, G.; Prosdocimi, E.M.; Crotti, E.; Mereghetti, V.; Vacchini, V.; Giorgi, A.; De Biase, A.; Longo, S.; et al. Effects of the diet on the microbiota of the red palm weevil (Coleoptera: Dryophthoridae). PLoS ONE 2015, 10, e0117439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudakaran, S.; Retz, F.; Kikuchi, Y.; Kost, C.; Kaltenpoth, M. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 2015, 9, 2587–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikaelyan, A.; Dietrich, C.; Köhler, T.; Poulsen, M.; Sillam-Dussès, D.; Brune, A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Jo, A.; Lee, K.A.; Han, H.J.; Kim, Y.J.; Kim, H.Y.; Lee, G.R.; Kim, M.; Park, Y.; Kang, Y.S.; et al. Effects of diet type, developmental stage, and gut compartment in the gut bacterial communities of two Cerambycidae species (Coleoptera). Microbiology 2017, 55, 21–30. [Google Scholar] [CrossRef]
- Cristina, V.; Joaquín, B.; Amparo, L.; Manuel, P. The generalist inside the specialist: Gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front. Microbiol. 2016, 7, 1005. [Google Scholar]
- Nuringtyas, T.R.; Verpoorte, R.; Klinkhamer, P.G.L.; van Oers, M.M.; Leiss, K.A. Toxicity of pyrrolizidine alkaloids to spodoptera exigua using insect cell lines and injection bioassays. J. Chem. Ecol. 2014, 40, 609–616. [Google Scholar] [CrossRef]
- Lu, Z.H.; Chen, Y.P.; Zhou, A.C.; He, S.Q.; Li, H.; Bao, Y.Y.; Gui, F.R. Effects of multi-generation feeding with different host plants on activities of enzyme in Spodoptera frugiperda larvae. J. Environ. Entomol. 2020, 42, 1361–1368. (In Chinese) [Google Scholar]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin, and starch and their impact on digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, K.; Watanabe, H.; Tokuda, G.; Kitamoto, K.; Arioka, M. Purification and characterization of termite endogenous beta-1,4-endoglucanases produced in As pergillus oryzae. Biosci. Biotech. Bioch. 2010, 74, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.C.; Tang, Y.L.; Wu, Y.Y.; Niu, X.H.; Li, Q.Y.; Guo, Z.B.; Xiang, L.; Jiang, R.X.; Lei, Y.F.; Liu, X. Comparison of gut bacteria between Spodoptera frugiperda and Mythimna separate feeding on sorghum in Chongqing area. J. Southwest Univ. 2019, 41, 6–13. (In Chinese) [Google Scholar]
- Xu, T.M.; Fu, C.Y.; Su, Z.T.; Xiao, G.L.; Li, W.W.; Chen, B. Comparison and diversity of gut bacteria community of Spodoptera frugiperda form the first invasion site and the dispersal area in Yunnan province. Plant Prot. 2020, 46, 116–125. (In Chinese) [Google Scholar]
- Acevedo, F.E.; Peiffer, M.; Tan, C.W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D.S.; et al. Fall armyworm-associated gut bacteria modulate plant defense responses. Mol. Plant Microb. Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.F.F.; Omoto, C.; Cônsoli, F.L. Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J. Pest Sci. 2020, 93, 833–851. [Google Scholar] [CrossRef]
- Lv, D.; Liu, X.; Dong, Y.; Yan, Z.; Zhang, X.; Wang, P.; Yuan, X.; Li, Y. Comparison of Gut Bacterial Communities of Fall Armyworm (Spodoptera frugiperda) Reared on Different Host Plants. Int. J. Mol. Sci. 2021, 22, 1266. [Google Scholar] [CrossRef]
- Su, X.N.; Li, C.Y.; Huang, S.H.; Liu, W.L.; Zhang, Y.P.; Pan, Z.P. Optimization of artificial diet and rearing condition of fall armyworm, Spodoptera frugiperda (J. E. Smith). J. Environ. Entomol. 2019, 41, 992–998. (In Chinese) [Google Scholar]
- Yang, L.; Bian, G.; Su, Y.; Zhu, W. Comparison of faecal microbial community of lantang, bama, erhualian, meishan, xiaomeishan, duroc, landrace, and yorkshire sows. Asian-Australas. J. Anim. Sci. 2014, 27, 898–906. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database, and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarridge III, J.E. Impact of 16S rRNA gene sequence analysis for identifification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004, 10, 840–862. [Google Scholar] [CrossRef] [Green Version]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities. Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Dantur, K.I.; Enrique, R.; Welin, B.; Castagnaro, A.P. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 2015, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, N.; Martens, R.; Tebbe, C.C. Origin and diversity of metabolically active gut bacteria from laboratory-bred larvae of Manduca sexta (Sphingidae, Lepidoptera, Insecta). Appl. Environ. Microbiol. 2008, 74, 7189–7196. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.; Couture, J.; Raffa, K.F. Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia 2014, 175, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Du, K.; Sun, C.; Vimalanathan, A.; Liang, X.; Li, Y.; Wang, B.; Lu, X.; Li, L.; Shao, Y. Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME. J. 2018, 12, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef]
- Rezaei, F.; Xing, D.F.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microb. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.F.; Gurr, G.M.; Vasseur, L.; Zhang, D.D.; Zhong, H.Z.; Qin, B.C.; Lin, J.H.; Wang, Y.; Song, F.Q.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Wang, J.H.; He, H.Z.; Wang, M.Z.; Wang, S.; Zhang, J.; Wei, W.; Xu, H.X.; Lv, Z.M.; Shen, D.S. Bioaugmentation of activated sludge with Acinetobacter sp. TW enhances nicotine degradation in a synthetic tobacco wastewater treatment system. Bioresource Technol. 2013, 142, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Raff, K.F.; Goodman, R.M.; Handelsman, J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, P.R.; Crickmore, N. Gut bacteria are not required for the insecticidal activity of bacillus thuringiensis toward the tobacco Hornworm, Manduca sexta. Appl. Environ. Microb. 2009, 75, 5094–5099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tago, K.; Kikuchi, Y.; Nakaoka, S.; Katsuyama, C.; Hayatsu, M. Insecticide applications to soil contribute to the development of Burkholderia mediating insecticide resistance in stinkbugs. Mol. Ecol. 2015, 24, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.E.A.; Pétriacq, P.; Cameron, D.D.; Meselmani, M.A.; Schwarzenbacher, R.; Rolfe, S.A.; Ton, J. Metabolic regulation of the maize rhizobiome by benzoxazinoid. ISME J. 2019, 13, 1647–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sample | Raw_Reads_R | Clean_Reads | Total_Tag | Taxon_Tag | Unique_Tag | OTU_Num. |
|---|---|---|---|---|---|---|
| SF-W | 79,873.25 ± 546.44 | 70,602.38 ± 3294.11 | 63,671.63 ± 3377.64 | 62,599.25 ± 3342.82 | 971.13 ± 84.94 | 1324.25 ± 199.73 |
| SF-M | 80,458.00 ± 398.37 | 73,055.25 ± 515.94 | 65,966.75 ± 988.87 | 65,031.88 ± 1056.30 | 830.38 ± 71.51 | 1313.50 ± 74.87 |
| SF-P | 80,129.88 ± 521.60 | 73,838.75 ± 581.67 | 63,773.63 ± 876.75 | 62,810.50 ± 936.74 | 906.50 ± 82.30 | 1873.00 ± 190.66 |
| SF-T | 79,677.75 ± 476.71 | 73,560.88 ± 571.33 | 66,188.75 ± 1040.84 | 65,275.75 ± 1108.93 | 873.63 ± 106.75 | 1435.25 ± 139.87 |
| Shannon Index | Simpson Index | Chao1 Index | Ace Index | |
|---|---|---|---|---|
| SF-W | 8.90 ± 0.14 a | 0.99 ± 0.00 a | 1348.62 ± 184.46 a | 1371.17 ± 177.76 a |
| SF-P | 6.54 ± 0.59 b | 0.81 ± 0.06 b | 1906.92 ± 184.31 a | 1862.35 ± 181.10 a |
| SF-M | 6.87 ± 0.24 b | 0.90 ± 0.02 ab | 1383.32 ± 71.24 a | 1344.99 ± 62.78 a |
| SF-T | 6.31 ± 0.65 b | 0.87 ± 0.04 ab | 1471.53 ± 147.96 a | 1390.33 ± 136.05 a |
| F | 6.61 | 4.38 | 2.81 | 2.82 |
| df | 3 | 3 | 3 | 3 |
| p | 0.002 | 0.012 | 0.058 | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-P.; Li, Y.-H.; Sun, Z.-X.; Du, E.-W.; Lu, Z.-H.; Li, H.; Gui, F.-R. Effects of Host Plants on Bacterial Community Structure in Larvae Midgut of Spodoptera frugiperda. Insects 2022, 13, 373. https://doi.org/10.3390/insects13040373
Chen Y-P, Li Y-H, Sun Z-X, Du E-W, Lu Z-H, Li H, Gui F-R. Effects of Host Plants on Bacterial Community Structure in Larvae Midgut of Spodoptera frugiperda. Insects. 2022; 13(4):373. https://doi.org/10.3390/insects13040373
Chicago/Turabian StyleChen, Ya-Ping, Ya-Hong Li, Zhong-Xiang Sun, E-Wei Du, Zhi-Hui Lu, Hao Li, and Fu-Rong Gui. 2022. "Effects of Host Plants on Bacterial Community Structure in Larvae Midgut of Spodoptera frugiperda" Insects 13, no. 4: 373. https://doi.org/10.3390/insects13040373
APA StyleChen, Y.-P., Li, Y.-H., Sun, Z.-X., Du, E.-W., Lu, Z.-H., Li, H., & Gui, F.-R. (2022). Effects of Host Plants on Bacterial Community Structure in Larvae Midgut of Spodoptera frugiperda. Insects, 13(4), 373. https://doi.org/10.3390/insects13040373






