Seed Dispersal by Ants in Three Early-Flowering Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Experimental Procedure
2.4. Statistical Analyses
3. Results
3.1. Ants Participating in Seed Dispersal
3.2. Absolute and Relative Differences of Diaspore/Elaiosome Weight among Plants
3.3. Effects of Treatment on Diaspore/Elaiosome Weight
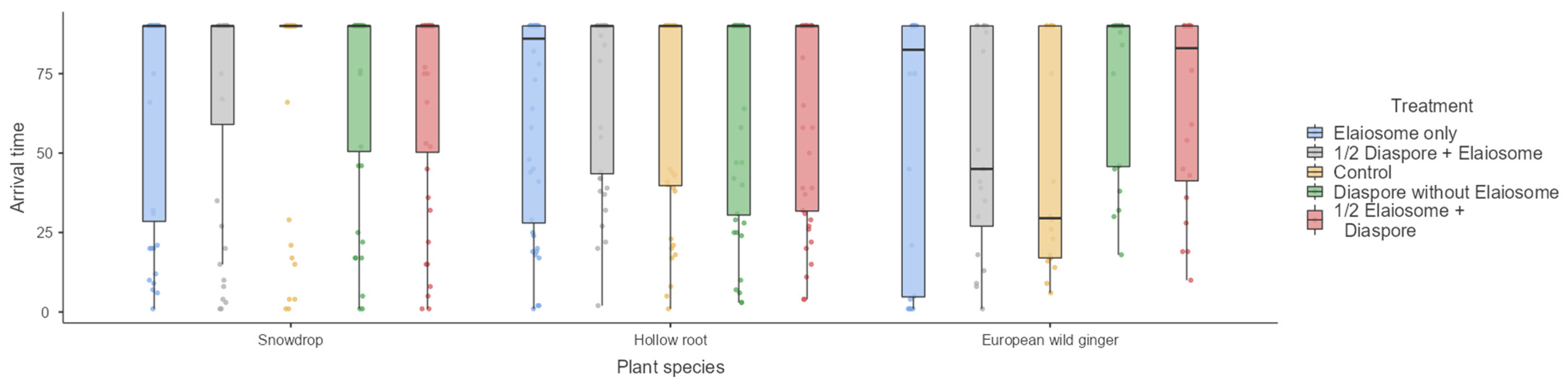
3.4. First Ant Arrival Time
3.5. Maximum Number of Ants
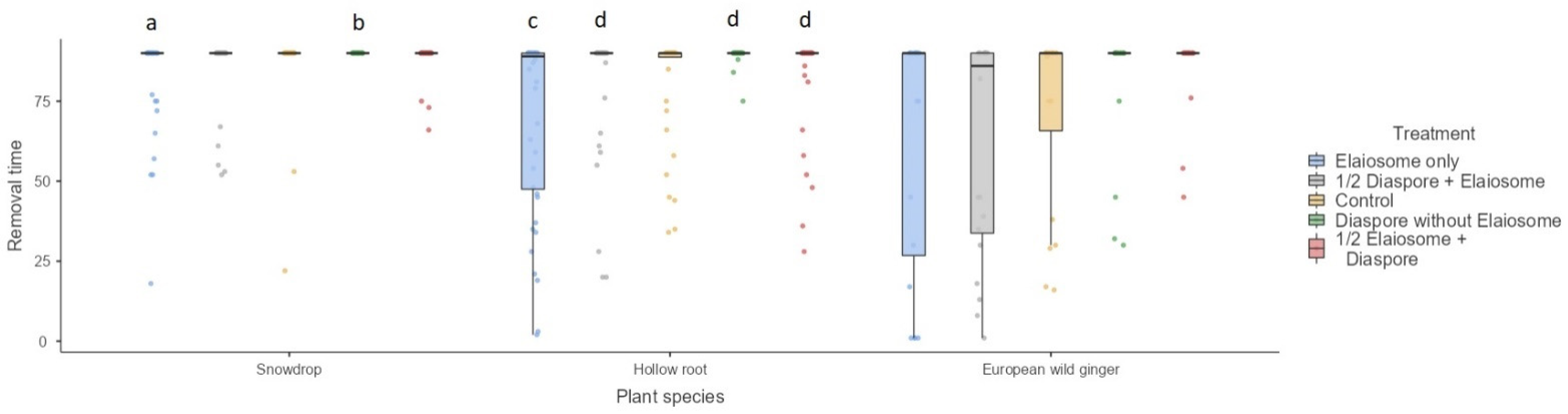
3.6. Diaspore/Elaiosome Removal
3.7. Associations between Ants and Diaspore/Elaiosome Displacement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, C.A.; Bronstein, J.L. Coexistence and competitive exclusion in mutualism. Ecology 2019, 100, e02708. [Google Scholar] [CrossRef]
- Degnan, P.M.; Yu, Y.; Sisneros, N.; Wing, R.A.; Moran, N.A. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl Acad. Sci. USA 2009, 106, 9063–9068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leigh, E.G., Jr. The evolution of mutualism. J. Evol. Biol. 2010, 23, 2507–2528. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, C.R. Interoceanic differences in size and nutrition of coral reef sponge populations. Science 1987, 236, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.J., II; Elliott, K.J.; Giladi, I.; King, J.R.; Bradford, M.A. Field experiments show contradictory short-and long-term myrmecochorous plant impacts on seed-dispersing ants. Ecol. Entomol. 2019, 44, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Pringle, E.G. Integrating plant carbon dynamics with mutualism ecology. New Phytol. 2016, 210, 71–75. [Google Scholar] [CrossRef]
- Harcombe, W.R.; Chacón, J.M.; Adamowicz, E.M.; Chubiz, L.M.; Marx, C.J. Evolution of bidirectional costly mutualism from byproduct consumption. Proc. Natl. Acad. Sci. USA 2018, 115, 12000–12004. [Google Scholar] [CrossRef] [Green Version]
- Sernander, R. Entwurf einer monographie der europäischen myrmecochoren (Draft of a monograph of European myrmekochorie). In Kungliga Svenska Vetenskapsakademiens Handlingar (The Royal Swedish Academy of Sciences Documents); Almqvist & Wiksell: Stockholm, Sweden, 1906; Volume 41, pp. 1–410. [Google Scholar]
- Lengyel, S.; Gove, A.D.; Latimer, A.M.; Majer, J.D.; Dunn, R.R. Ants Sow the Seeds of Global Diversification in Flowering Plants. PLoS ONE 2009, 4, e5480. [Google Scholar] [CrossRef] [Green Version]
- Hughes, L.; Westoby, M.T.; Jurado, E. Convergence of elaiosomes and insect prey: Evidence from ant foraging behaviour and fatty acid composition. Funct. Ecol. 1994, 8, 358–365. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Oliveira, P.S. The Ecology and Evolution of Ant-Plant Interactions; University of Chicago Press, Ltd.: London, UK, 2007. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Ohkawara, K.; Higashi, S.; Ohara, M. Effects of ants, ground beetles and the seed-fall patterns on myrmecochory of Erythronium japonicum Decne.(Liliaceae). Oecologia 1996, 106, 500–506. [Google Scholar] [CrossRef]
- Leal, I.R.; Wirth, R.; Tabarelli, M. Seed dispersal by ants in the semi-arid Caatinga of north-east Brazil. Ann. Bot. 2007, 99, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Ness, J.H.; Morin, D.F.; Giladi, I. Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: Are Aphaenogaster ants keystone mutualists? Oikos 2009, 118, 1793–1804. [Google Scholar] [CrossRef]
- Fischer, R.C.; Ölzant, S.M.; Wanek, W.; Mayer, V. The fate of Corydalis cava elaiosomes within an ant colony of Myrmica rubra: Elaiosomes are preferentially fed to larvae. Insectes Soc. 2005, 52, 55–62. [Google Scholar] [CrossRef]
- Fokuhl, G.; Heinze, J.; Poschlod, P. Colony growth in Myrmica rubra with supplementation of myrmecochorous seeds. Ecol. Res. 2007, 22, 845–847. [Google Scholar] [CrossRef]
- Fokuhl, G.; Heinze, J.; Poschlod, P. An Ant-Plant Mesocosm Experiment Reveals Dispersal Patterns of Myrmecochorous Plants. Forests 2019, 10, 1149. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, R.; Venkatesan, R. Seed elaiosome mediates dispersal by ants and impacts germination in Ricinus communis. Front. Ecol. Evol. 2019, 7, 246. [Google Scholar] [CrossRef] [Green Version]
- Giladi, I. Choosing benefits or partners: A review of the evidence for the evolution of myrmecochory. Oikos 2006, 112, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Gorb, E.; Gorb, S. Seed Dispersal by Ants in a Deciduous Forest Ecosystem: Mechanisms, Strategies, Adaptations; Springer Science & Business Media: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Arnan, X.; Molowny-Horas, R.; Rodrigo, A.; Retana, J. Uncoupling the effects of seed predation and seed dispersal by granivorous ants on plant population dynamics. PLoS ONE 2012, 7, e42869. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.V.; Paolucci, L.N.; Carmo, F.M.; Sperber, C.F.; Campos, R.I. Seed manipulation by ants: Disentangling the effects of ant behaviours on seed germination. Ecol. Entomol. 2018, 43, 712–718. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Paolucci, L.; Solar, R.R.C.; Neves, F.S.; Campos, R.I. Ant removal distance, but not seed manipulation and deposition site increases the establishment of a myrmecochorous plant. Oecologia 2019, 192, 133–142. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Turner, M.G.; Pearson, S.M. Effects of historical land use and forest patch size on myrmecochores and ant communities. Ecol. Appl. 2002, 12, 1364–1377. [Google Scholar] [CrossRef]
- Clark, R.E.; King, J.R. The ant, Aphaenogaster picea, benefits from plant elaiosomes when insect prey is scarce. Environ. Entomol. 2012, 41, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.J.; Pearson, S.M.; Henry, S.; Rossouw, K.; Love, J.P.; Olejniczak, M.J.; Elliott, K.J.; Bradford, M.A. Cryptic indirect effects of exurban edges on a woodland community. Ecosphere 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Bologna, A.; Detrain, C. Larvae promote a decline in the harvesting of myrmecochorous seeds by Myrmica rubra ants. Insectes Soc. 2019, 66, 453–461. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Huttenlocher, H.; Ayasse, M. Myrmecochorous plants use chemical mimicry to cheat seed-dispersing ants. Funct. Ecol. 2010, 24, 545–555. [Google Scholar] [CrossRef]
- Vereecken, N.J.; McNeil, J.N. Cheaters and liars: Chemical mimicry at its finest. Can. J. Zool. 2010, 88, 725–752. [Google Scholar] [CrossRef]
- Heil, M.; Barajas-Barron, A.; Orona-Tamayo, D.; Wielsch, N.; Svatos, A. Partner manipulation stabilises a horizontally transmitted mutualism. Ecol. Lett. 2014, 17, 185–192. [Google Scholar] [CrossRef]
- Lloret, F.; Casanovas, C.; Penuelas, J. Seedling survival of Mediterranean shrubland species in relation to root: Shoot ratio, seed size and water and nitrogen use. Funct. Ecol. 1999, 13, 210–216. [Google Scholar] [CrossRef]
- Ehlers, B.K. Geographic variation for elaiosome–seed size ratio and its allometric relationship in two closely related Corydalis species. Plant Ecol. Divers. 2012, 5, 395–401. [Google Scholar] [CrossRef]
- Mark, S.; Olesen, J.M. Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 1996, 107, 95–101. [Google Scholar] [CrossRef]
- Edwards, W.; Dunlop, M.; Rodgerson, L. The evolution of rewards: Seed dispersal, seed size and elaiosome size. J. Ecol. 2006, 94, 687–694. [Google Scholar] [CrossRef]
- Merienne, H.; Latil, G.; Moretto, P.; Fourcassié, V. Walking kinematics in the polymorphic seed harvester ant Messor barbarus: Influence of body size and load carriage. J. Exp. Biol. 2020, 223, jeb205690. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Westoby, M. Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 1992, 73, 1300–1312. [Google Scholar] [CrossRef]
- Englický, T.; Šerá, B. The Preference of Some Myrmecochorous Plants of Forest Stands by Red Wood Ant (Formica rufa L.)—Experiment on Seeds with Elaiosomes. Russ. J. Ecol. 2018, 49, 577–583. [Google Scholar] [CrossRef]
- Gunther, R.W.; Lanza, J. Variation in attractiveness of Trillium diaspores to a seed-dispersing ant. Am. Midl. Nat. 1989, 122, 321–328. [Google Scholar] [CrossRef]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags und Vertiebsgesellschaft: Tauer, Germany, 2018; 408p. [Google Scholar]
- Fokuhl, G.; Heinze, J.; Poschlod, P. Myrmecochory by small ants–Beneficial effects through elaiosome nutrition and seed dispersal. Acta Oecol. 2012, 38, 71–76. [Google Scholar] [CrossRef]
- Wiezik, M.; Svitok, M.; Wieziková, A.; Dovčiak, M. Shrub encroachment alters composition and diversity of ant communities in abandoned grasslands of western Carpathians. Biodivers. Conserv. 2013, 22, 2305–2320. [Google Scholar] [CrossRef]
- Cushman, J.H.; Lawton, J.H.; Manly, B.F.J. Latitudinal patterns in European ant assemblages: Variation in species richness and body size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Collingwood, C.A. The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 1979, 8, 1–174. [Google Scholar]
- Reifenrath, K.; Becker, C.; Poethke, H.J. Diaspore trait preferences of dispersing ants. J. Chem. Ecol. 2012, 38, 1093–1104. [Google Scholar] [CrossRef]
- Gammans, N.; Bullock, J.M.; Gibbons, H.; Schönrogge, K. Reaction of mutualistic and granivorous ants to Ulex elaiosome chemicals. J. Chem. Ecol. 2006, 32, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Kjellsson, G. Seed fate in a population of Carex pilulifera L. Oecologia 1985, 67, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Anjos, D.V.; Andersen, A.N.; Carvalho, R.L.; Sousa, R.M.; Del-Claro, K. Switching roles from antagonist to mutualist: A harvester ant as a key seed disperser of a myrmecochorous plant. Ecol. Entomol. 2020, 45, 1063–1070. [Google Scholar] [CrossRef]
- Hughes, L.; Westoby, M. Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 1992, 73, 1285–1299. [Google Scholar] [CrossRef]
- Gorb, S.N.; Gorb, E.V. Removal rates of seeds of five myrmecochorous plants by the ant Formica polyctena (Hymenoptera: Formicidae). Oikos 1995, 73, 367–374. [Google Scholar] [CrossRef]
- Türke, M.; Andreas, K.; Gossner, M.M.; Kowalski, E.; Lange, M.; Boch, S.; Socher, S.A.; Prati, D.; Fischer, M.; Weisser, W.W.; et al. Are gastropods, rather than ants, important dispersers of seeds of myrmecochorous forest herbs? Am. Nat. 2012, 179, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weryszko-Chmielewska, E.; Chwil, M. Flowering biology and structure of floral nectaries in Galanthus nivalis L. Acta Soc. Bot. Pol. 2016, 85, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ravigne, V.; Olivieri, I.; Gonzales-Martinez, S.C.; Rousset, F. Selective interactions between short-distance pollen and seed dispersal in self-compatible species. Evolution 2006, 60, 2257–2271. [Google Scholar] [CrossRef] [Green Version]



| G. nivalis | C. cava | A. europaeum | n | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | ||
| Elaiosome only | 0.010 | 0.003 | 0.005 | 0.001 | 0.013 | 0.0008 | 20 |
| Diaspore without Elaiosome | 0.065 | 0.003 | 0.061 | 0.001 | 0.029 | 0.0008 | 20 |
| 1/2 elaiosome + diaspore | 0.073 | 0.003 | 0.07 | 0.001 | 0.039 | 0.0008 | 20 |
| 1/2 diaspore + elaiosome | 0.046 | 0.003 | 0.033 | 0.001 | 0.028 | 0.0008 | 20 |
| Control | 0.084 | 0.003 | 0.075 | 0.001 | 0.057 | 0.0008 | 20 |
| ANOVA F4,95 = | 86.96, p < 0.001 | 601.80, p < 0.001 | 439.41, p < 0.001 | - | |||
| Maximum Number of Ants | Transport of First Diaspore | Seed Remnant | |
|---|---|---|---|
| First ant arrival time | −0.66 | 0.54 | 0.43 |
| Maximum number of ants | - | −0.37 | −0.45 |
| Transport of first diaspore | - | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, P.; Fančovičová, J.; Hlúšková, Z. Seed Dispersal by Ants in Three Early-Flowering Plants. Insects 2022, 13, 386. https://doi.org/10.3390/insects13040386
Prokop P, Fančovičová J, Hlúšková Z. Seed Dispersal by Ants in Three Early-Flowering Plants. Insects. 2022; 13(4):386. https://doi.org/10.3390/insects13040386
Chicago/Turabian StyleProkop, Pavol, Jana Fančovičová, and Zuzana Hlúšková. 2022. "Seed Dispersal by Ants in Three Early-Flowering Plants" Insects 13, no. 4: 386. https://doi.org/10.3390/insects13040386
APA StyleProkop, P., Fančovičová, J., & Hlúšková, Z. (2022). Seed Dispersal by Ants in Three Early-Flowering Plants. Insects, 13(4), 386. https://doi.org/10.3390/insects13040386







