Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Crop Cultivation
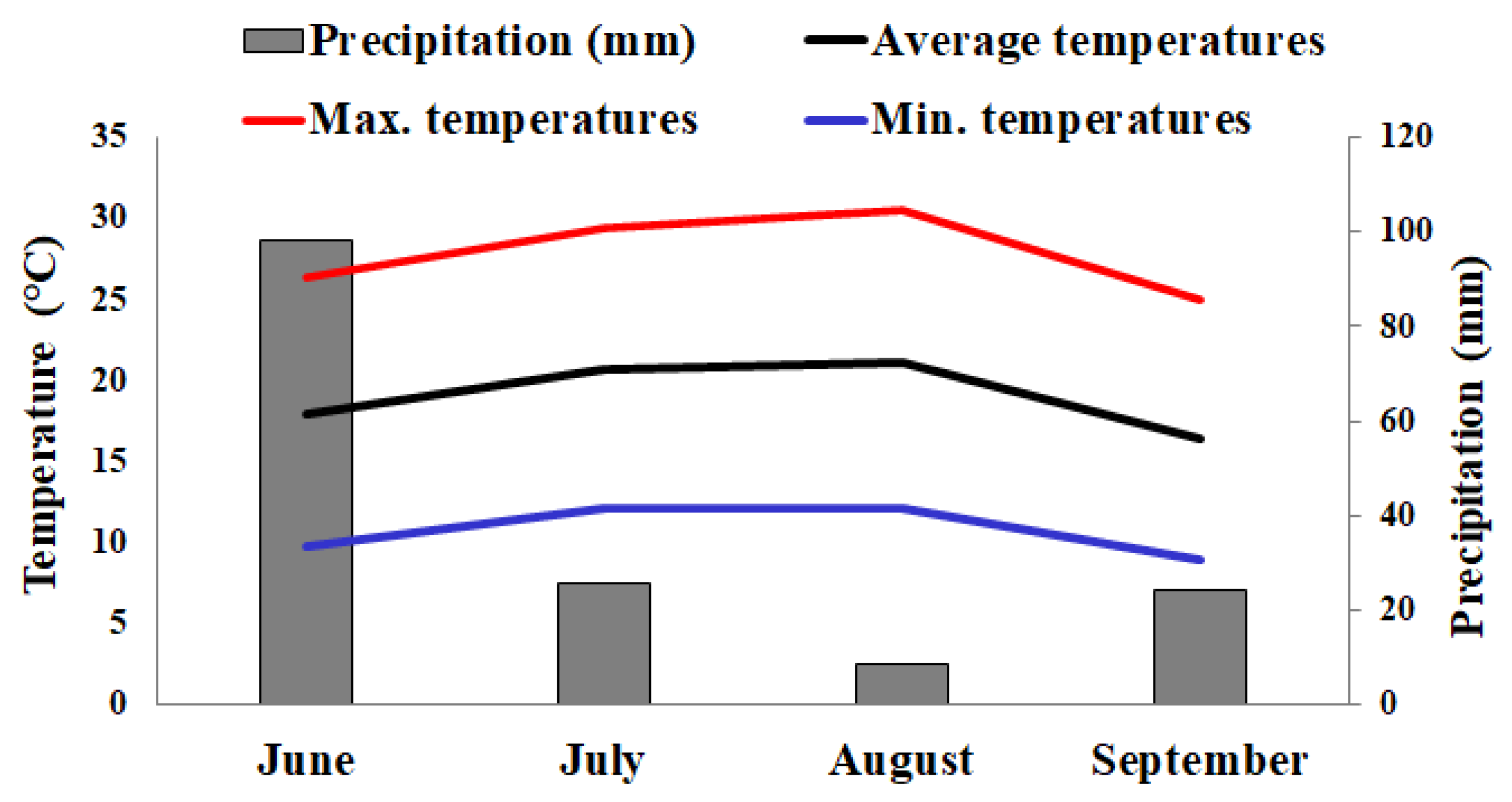
2.2. Meteorological Data
2.3. Experimental Design
2.4. Insect Sampling
2.5. Soil Sampling and Microarthropod Extraction
2.6. Evaluation of Downy Mildew on Tomato Plants
2.7. Agronomic Performance Estimation
2.8. Statistical Analysis
3. Results
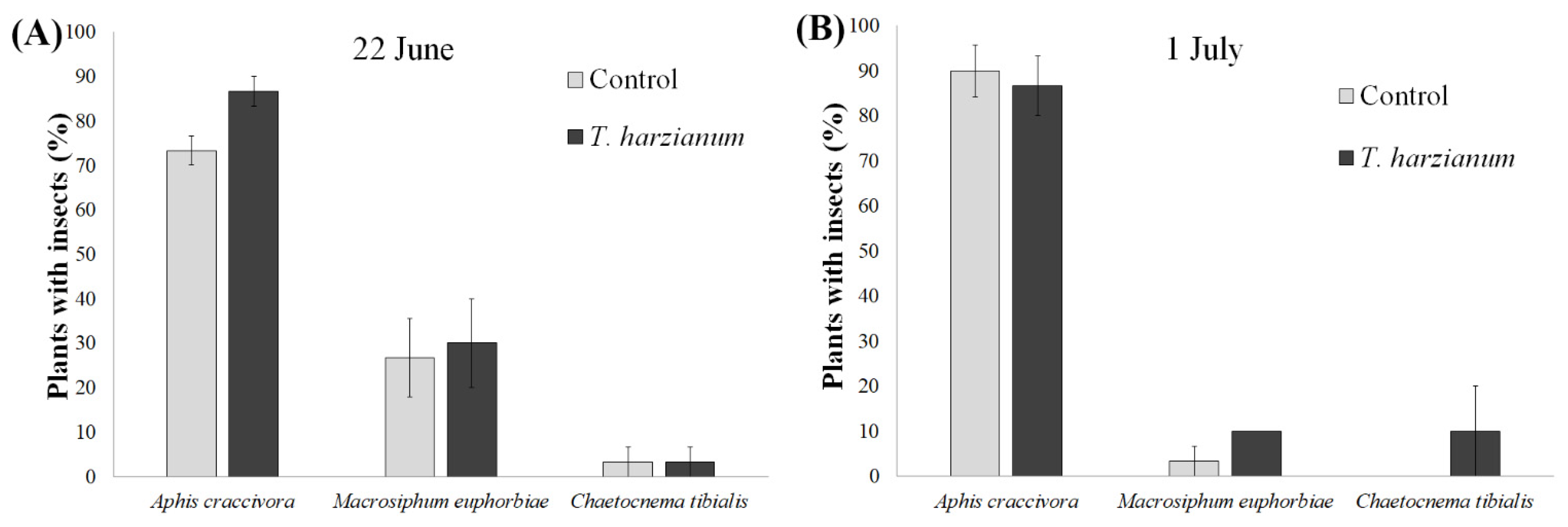
3.1. First Month after Transplantation: Seedling Growth Phase
3.2. Second–Fourth Month after Transplantation: Vegetative Growth, Flowering, Fruit Set, and Fruit Ripening
3.2.1. Piercing-Sucking Herbivores
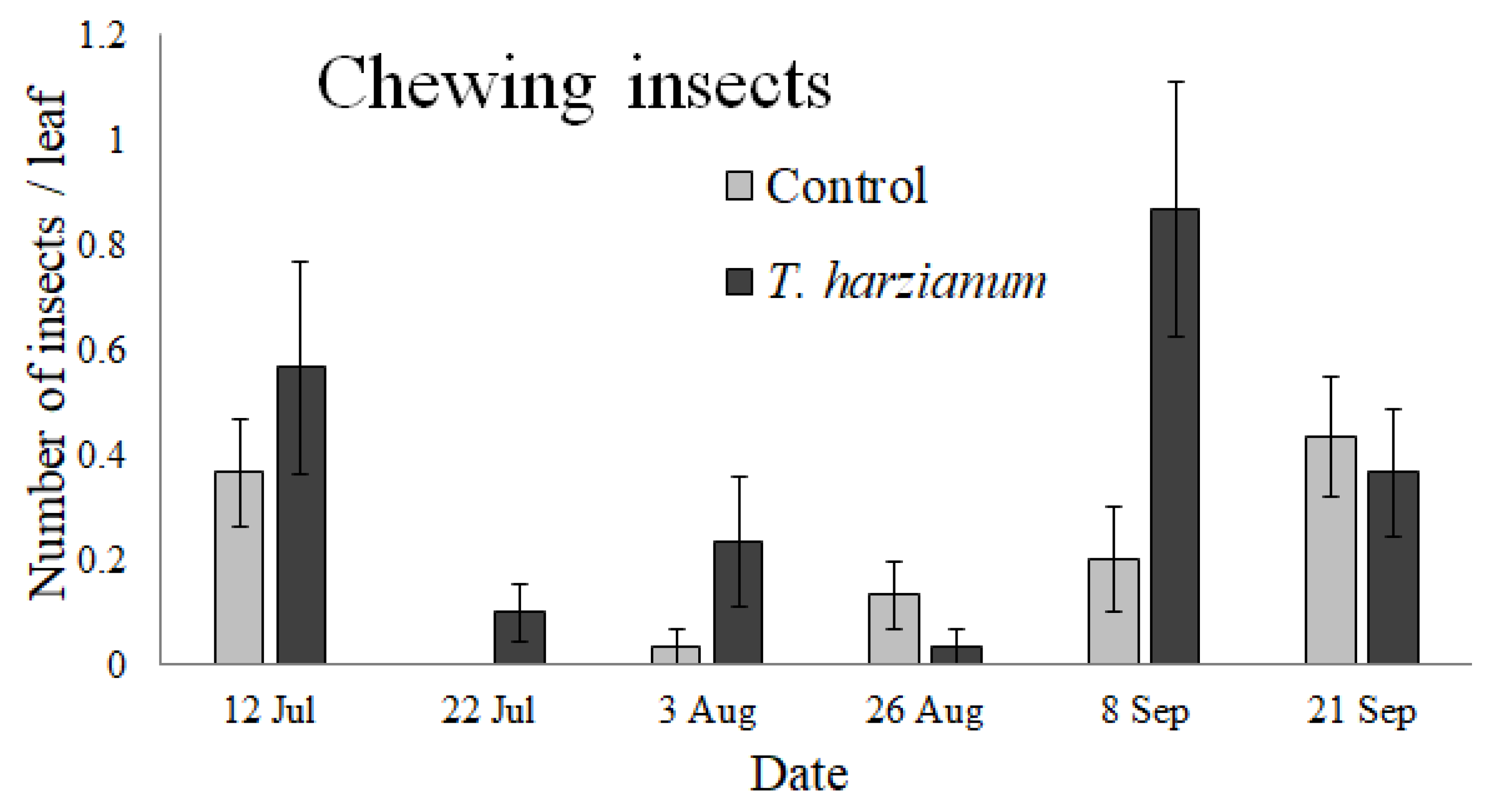
3.2.2. Chewing Insects
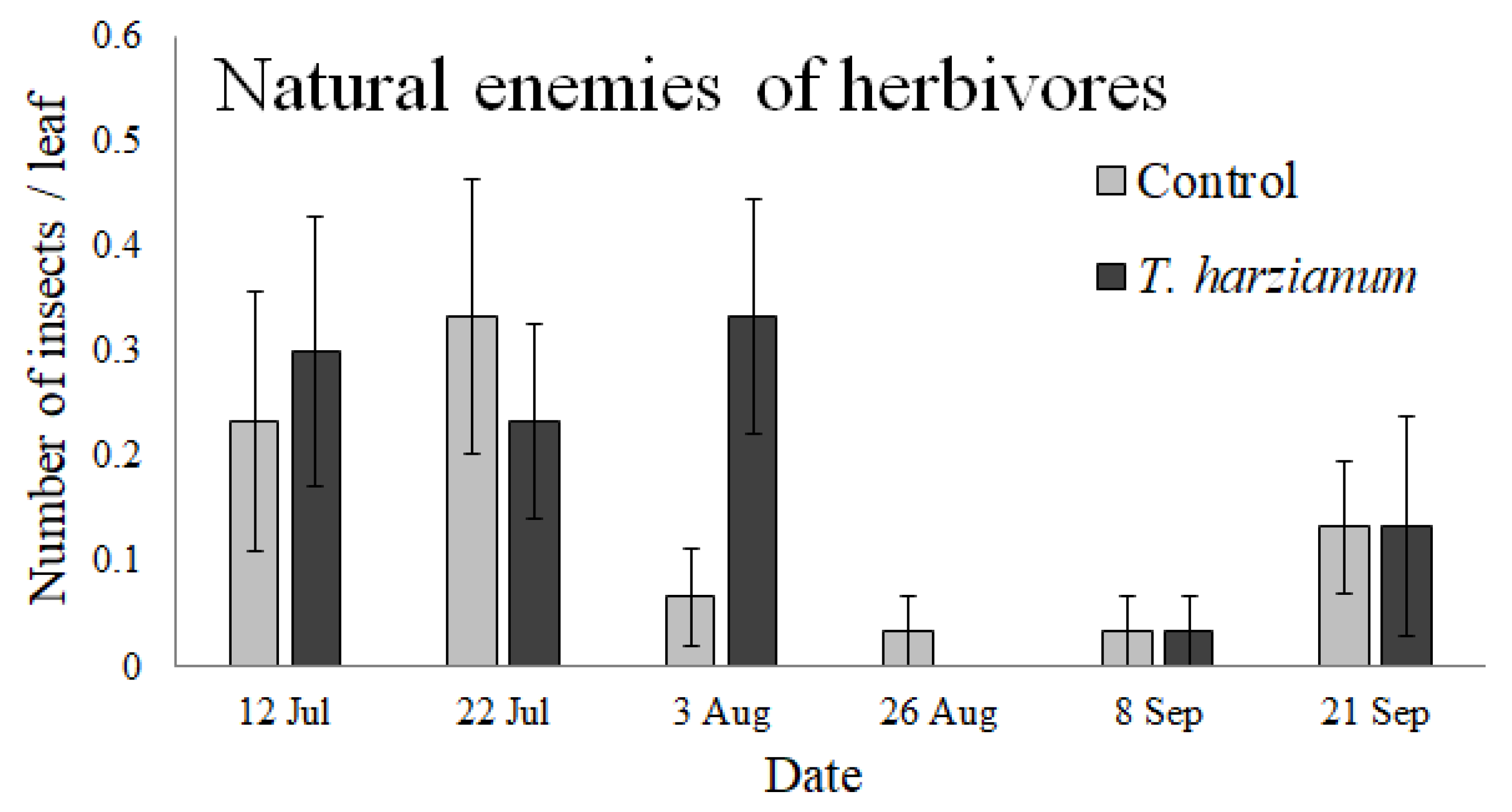
3.2.3. Natural Enemies of Insects
3.2.4. Spider Mites
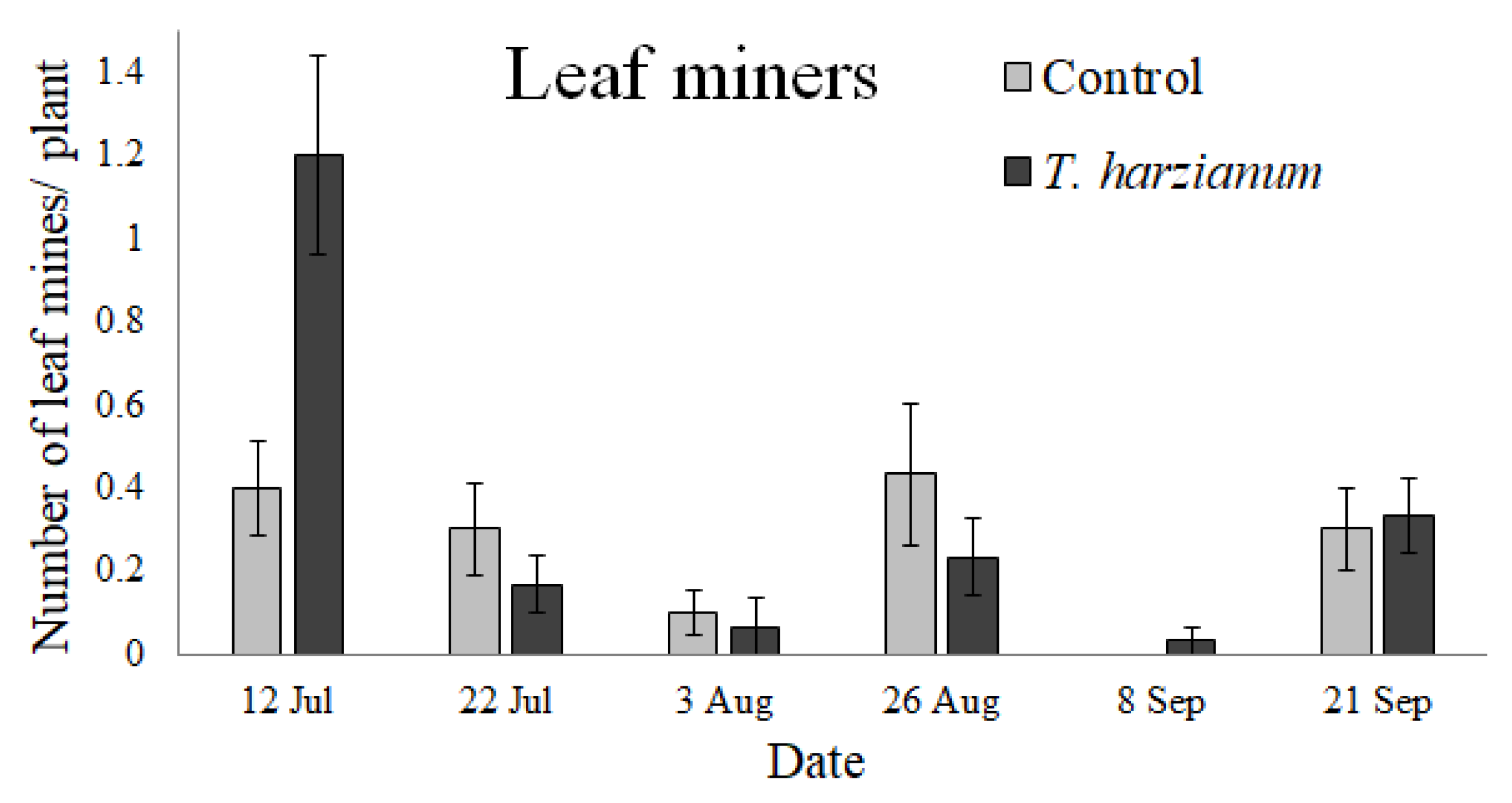
3.2.5. Leaf Miners
3.3. QBSar
3.4. Crop Sampling
3.4.1. Number of Fruit per Plant
3.4.2. Weight, Length, and Width of Marketable Tomato Fruit
3.4.3. Presence/Absence of Downy Mildew
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siracusa, L.; Patanè, C.; Rizzo, V.; Cosentino, S.L.; Ruberto, G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018, 244, 275–283. [Google Scholar] [CrossRef] [PubMed]
- FAO Crop Information. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/tomato/en/ (accessed on 1 March 2022).
- Elia, A.; Conversa, G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012, 40, 64–74. [Google Scholar] [CrossRef]
- Bettini, O. USDA—Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/italy-italian-processed-tomato-overview-2018 (accessed on 3 March 2022).
- I.Stat—Coltivazioni: Ortive. Available online: http://dati.istat.it/Index.aspx?QueryId=33703 (accessed on 7 March 2022).
- Abdul-Baki, A.A.; Teasdale, J.R.; Korcak, R.; Chitwood, D.J.; Huettel, R.N. Fresh-market tomato production in a low-input alternative system using cover-crop mulch. HortScience 1996, 31, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Perring, T.M.; Battaglia, D.; Walling, L.L.; Toma, I.; Fanti, P. Chapter 2—Aphids: Biology, Ecology, and Management. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 15–48. ISBN 978-0-12-802441-6. [Google Scholar]
- Ferreira, J.H.; Matthee, F.N.; Thomas, A.C. Biological control of Eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology 1991, 81, 283–287. [Google Scholar] [CrossRef]
- Walsh, U.F.; Morrissey, J.P.; O’Gara, F. Pseudomonas for biocontrol of phytopathogens: From functional genomics to commercial exploitation. Curr. Opin. Biotechnol. 2001, 12, 289–295. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The Endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of endophytic Beauveria bassiana as a dual biocontrol agent of tomato pathogens and pests. Pathogens 2021, 10, 1242. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J. Trichoderma as Biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; De Masi, L.; De Palma, M.; Lorito, M. The Beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Studholme, D.J.; Harris, B.; Le Cocq, K.; Winsbury, R.; Perera, V.; Ryder, L.; Ward, J.L.; Beale, M.H.; Thornton, C.R.; Grant, M. Investigating the beneficial traits of Trichoderma hamatum GD12 for sustainable agriculture-insights from genomics. Front. Plant Sci. 2013, 4, 258. [Google Scholar] [CrossRef] [Green Version]
- Macías-Rodríguez, L.; Contreras-Cornejo, H.A.; Adame-Garnica, S.G.; Del-Val, E.; Larsen, J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020, 240, 126552. [Google Scholar] [CrossRef]
- Digilio, M.C.; Cascone, P.; Iodice, L.; Guerrieri, E. Interactions between tomato volatile organic compounds and aphid behaviour. J. Plant Interact. 2012, 7, 322–325. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P.; Volpe, M.G.; Bartolini, P.; Moneti, G.; Pieraccini, G.; Di Serio, C.; Maserti, B.; Guerrieri, E.; et al. The association with two different arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Muvea, A.M.; Meyhöfer, R.; Subramanian, S.; Poehling, H.M.; Ekesi, S.; Maniania, N.K. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Appl. Soil Ecol. 2018, 124, 45–53. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.A.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Horwitz, B.A.; Zachow, C.; Berg, G.; Zeilinger, S. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian J. Microbiol. 2012, 52, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Coppola, M.; Cascone, P.; Di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef] [Green Version]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and metabolome reprogramming in tomato plants by Trichoderma harzianum strain T22 primes and enhances defense responses against aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Alınç, T.; Cusumano, A.; Peri, E.; Torta, L.; Colazza, S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sholla, S.M.E.; Kottb, M.R. Bioactivity of Trichoderma (6-Pentyl α-Pyrone) against Tetranychus urticae Koch (Acari: Tetranychidae). Egypt. Acad. J. Biol. Sci. 2017, 10, 29–34. [Google Scholar]
- Battaglia, D.; Bossi, S.; Cascone, P.; Digilio, M.C.; Prieto, J.D.; Fanti, P.; Guerrieri, E.; Iodice, L.; Lingua, G.; Lorito, M.; et al. Tomato below ground-above ground interactions: Trichoderma longibrachiatum affects the performance of Macrosiphum euphorbiae and its natural antagonists. Mol. Plant. Microbe. Interact. 2013, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol. Biochem. 2018, 122, 196–202. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Viveros-Bremauntz, F.; Del-Val, E.; Macías-Rodríguez, L.; López-Carmona, D.A.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Alterations of foliar arthropod communities in a maize agroecosystem induced by the root-associated fungus Trichoderma harzianum. J. Pest Sci. 2020, 94, 363–374. [Google Scholar] [CrossRef]
- Parrilli, M.; Sommaggio, D.; Tassini, C.; Di Marco, S.; Osti, F.; Ferrari, R.; Metruccio, E.; Masetti, A.; Burgio, G. The role of Trichoderma spp. and silica gel in plant defence mechanisms and insect response in vineyard. Bull. Entomol. Res. 2019, 109, 771–780. [Google Scholar] [CrossRef]
- Sotto-Alviola, M.P.; Lit, I.L.; Caasi-Lit, M.T.; Cuevas, V.C. Springtail (Collembola) abundance in Trichoderma- enhanced and conventional cabbage (Brassica oleracea L.) plots in Sariaya, Quezon, Philippines. Philipp. Entomol. 2017, 31, 103–116. [Google Scholar]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Kovach-Orr, C.; Fussmann, G.F. Evolutionary and plastic rescue in multitrophic model communities. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120084. [Google Scholar] [CrossRef] [Green Version]
- Soler, R.; Erb, M.; Kaplan, I. Long distance root-shoot signalling in plant-insect community interactions. Trends Plant Sci. 2013, 18, 149–156. [Google Scholar] [CrossRef]
- Bazghaleh, N.; Prashar, P.; Woo, S.L.; Vandenberg, A. Effects of lentil genotype on the colonization of beneficial Trichoderma species and biocontrol of Aphanomyces root rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R. Temperature differentially influences the capacity of Trichoderma species to induce plant defense responses in tomato against insect pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef] [PubMed]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality Index (QBS-Ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: Https://Www.R-Project.Org/ (accessed on 10 September 2021).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, N.; Pastor, V.; Pastor-Fernández, J.; Flors, V.; Pozo, M.J.; Sánchez-Bel, P. Role and mechanisms of callose priming in mycorrhiza-induced resistance. J. Exp. Bot. 2021, 71, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Pozo de la Hoz, J.; Rivero, J.; Azcón-Aguilar, C.; Urrestarazu, M.; Pozo, M.J. Mycorrhiza-induced resistance against foliar pathogens is uncoupled of nutritional effects under different light intensities. J. Fungi 2021, 7, 402. [Google Scholar] [CrossRef]
- Vitti, A.; Sofo, A.; Scopa, A.; Nuzzaci, M. Sustainable agricultural practices in disease defence of traditional crops in southern Italy: The case study of tomato cherry protected by Trichoderma harzianum T-22 against cucumber mosaic virus (CMV) BT. In The Sustainability of Agro-Food and Natural Res; Vastola, A., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–143. ISBN 978-3-319-16357-4. [Google Scholar]
- Basińska-Barczak, A.; Błaszczyk, L.; Szentner, K. Plant cell wall changes in common wheat roots as a result of their interaction with beneficial fungi of Trichoderma. Cells 2020, 9, 2319. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2319. [Google Scholar] [CrossRef]
- Ponzio, C.; Gols, R.; Pieterse, C.M.J.; Dicke, M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 2013, 27, 587–598. [Google Scholar] [CrossRef]
- Walling, L.L. The Myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Reimer-Michalski, E.-M.; Conrath, U. Innate immune memory in plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [Green Version]
- Bürger, M.; Chory, J. Stressed out about hormones: How plants orchestrate immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Salas-Marina, M.A.; Silva-Flores, M.A.; Uresti-Rivera, E.E.; Castro-Longoria, E.; Herrera-Estrella, A.; Casas-Flores, S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin- dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungus Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Islam, M.S.; Subbiah, V.K.; Siddiquee, S. Efficacy of entomopathogenic Trichoderma isolates against sugarcane woolly aphid, Ceratovacuna lanigera Zehntner (Hemiptera: Aphididae). Horticulturae 2022, 8, 2. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Chiusano, M.L.; Colantuono, C.; Lorito, M.; Pennacchio, F.; Rao, R.; Woo, S.L.; Guerrieri, E.; Digilio, M.C. Trichoderma harzianum enhances tomato indirect defense against aphids. Insect Sci. 2017, 24, 1025–1033. [Google Scholar] [CrossRef]
- Guerrieri, E.; Lingua, G.; Digilio, M.C.; Massa, N.; Berta, G. Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol. Entomol. 2004, 29, 753–756. [Google Scholar] [CrossRef]
- Prieto, J.D.; Castañé, C.; Calvet, C.; Camprubi, A.; Battaglia, D.; Trotta, V.; Fanti, P. Tomato belowground–aboveground interactions: Rhizophagus irregularis affects foraging behavior and life history traits of the predator Macrolophus pygmaeus (Hemiptera: Miridae). Arthropod-Plant Interact. 2017, 11, 15–22. [Google Scholar] [CrossRef]
- Colella, T.; Candido, V.; Campanelli, G.; Camele, I.; Battaglia, D. Effect of irrigation regimes and artificial mycorrhization on insect pest infestations and yield in tomato crop. Phytoparasitica 2014, 42, 235–246. [Google Scholar] [CrossRef]
- Loivamäki, M.; Mumm, R.; Dicke, M.; Schnitzler, J.-P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17430–17435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivelli, A.; Trotta, V.; Toma, I.; Fanti, P.; Battaglia, D. Relation between plant water status and Macrosiphum euphorbiae ( Hemiptera: Aphididae ) population dynamics on three cultivars of tomato. Eur. J. Entomol. 2013, 110, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Heinen, R.; Biere, A.; Harvey, J.A.; Bezemer, T.M. Effects of soil organisms on aboveground plant-insect interactions in the field: Patterns, mechanisms and the role of methodology. Front. Ecol. Evol. 2018, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccavo, V.; Forlano, P.; Mang, S.M.; Fanti, P.; Nuzzaci, M.; Battaglia, D.; Trotta, V. Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects 2022, 13, 418. https://doi.org/10.3390/insects13050418
Caccavo V, Forlano P, Mang SM, Fanti P, Nuzzaci M, Battaglia D, Trotta V. Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects. 2022; 13(5):418. https://doi.org/10.3390/insects13050418
Chicago/Turabian StyleCaccavo, Vittoria, Pierluigi Forlano, Stefania Mirela Mang, Paolo Fanti, Maria Nuzzaci, Donatella Battaglia, and Vincenzo Trotta. 2022. "Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field" Insects 13, no. 5: 418. https://doi.org/10.3390/insects13050418
APA StyleCaccavo, V., Forlano, P., Mang, S. M., Fanti, P., Nuzzaci, M., Battaglia, D., & Trotta, V. (2022). Effects of Trichoderma harzianum Strain T22 on the Arthropod Community Associated with Tomato Plants and on the Crop Performance in an Experimental Field. Insects, 13(5), 418. https://doi.org/10.3390/insects13050418





