Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East–Asia Minor 1
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Whitefly Rearing
2.2. Cloning, Sequence Retrieval, and Phylogenetic Analysis
2.3. RT-qPCR Analysis of BtLac2 Expression Patterns
2.4. Functional Analysis of the BtLac2 Gene in B. tabaci by RNAi
2.5. Cuticle Morphology Analysis
2.6. Data Analysis
3. Results
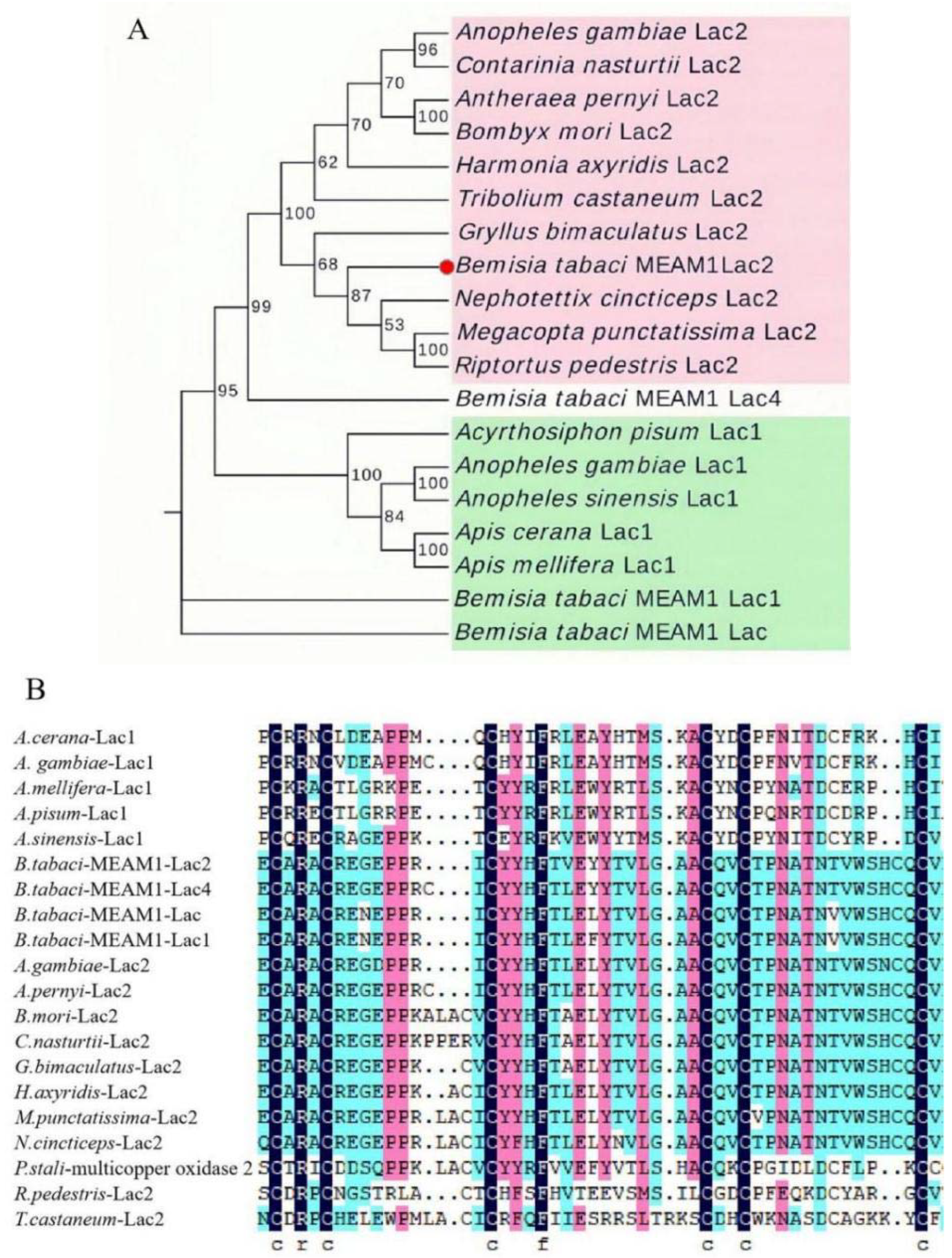
3.1. Characterization of BtLac2 from B. tabaci
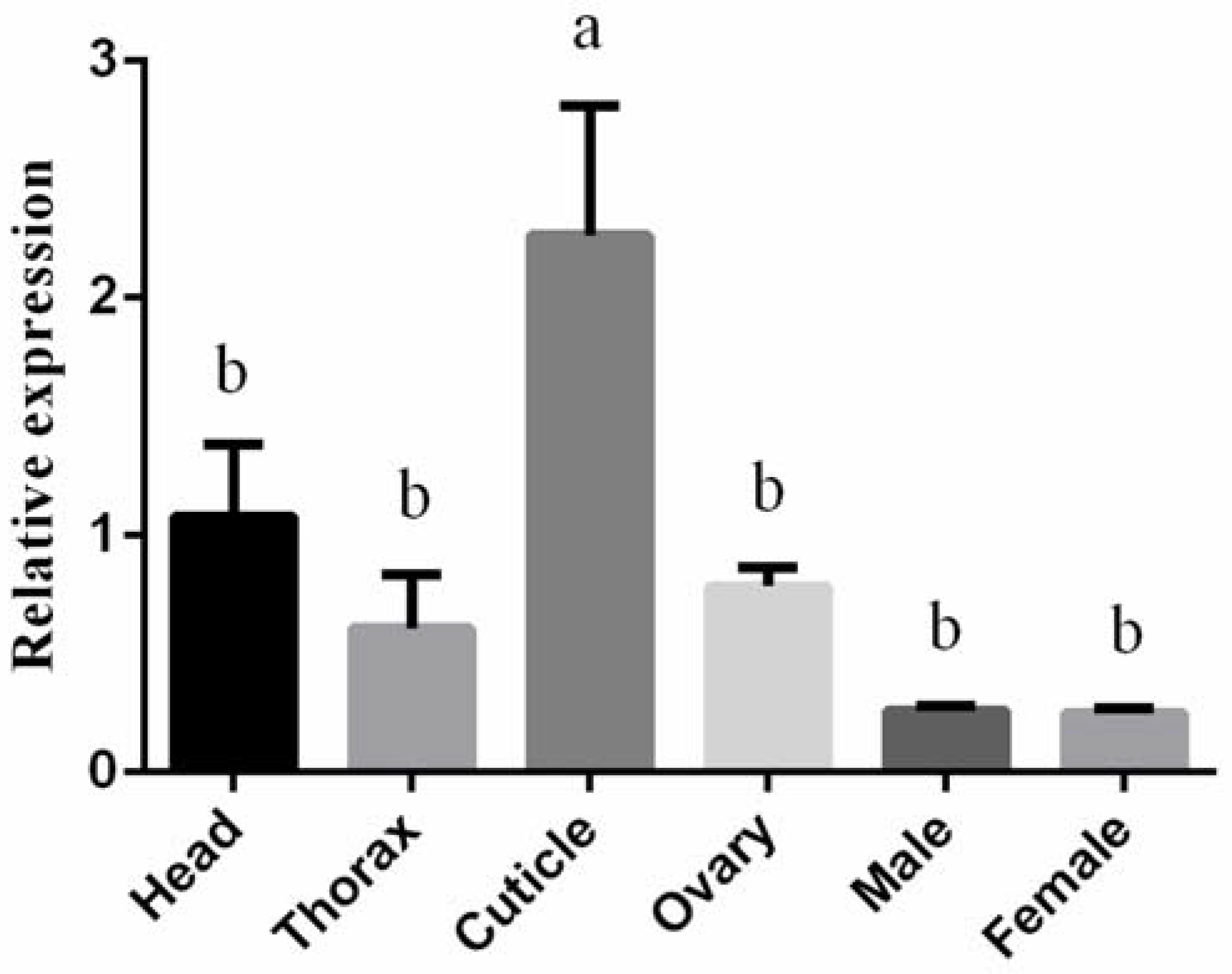
3.2. Expression Profiling of BtLac2
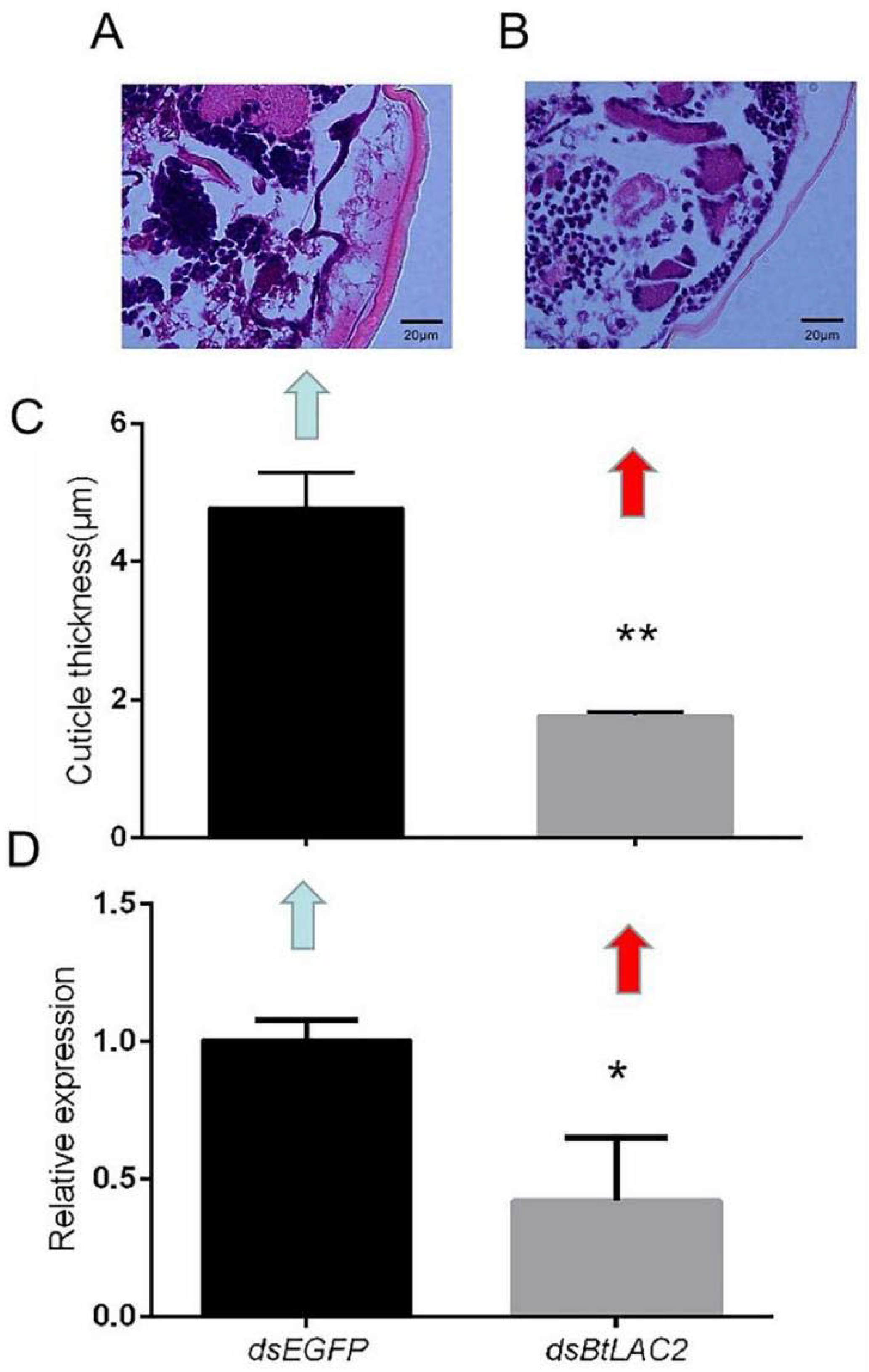
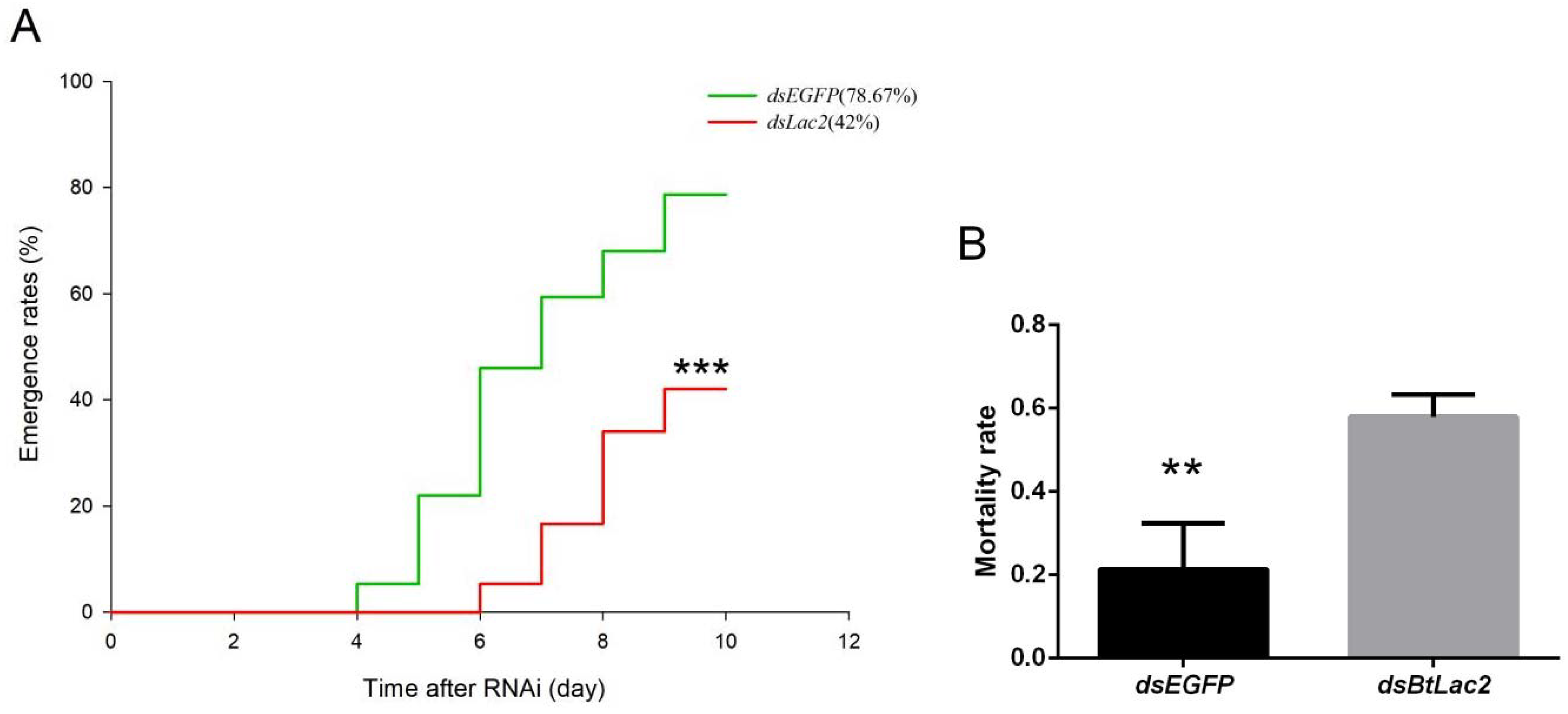
3.3. Low Expression of BtLac2 Results in Nymph Cuticle Sclerotization Defects and Emergence Retardation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, B.R.; Yonekura, M.; Morgan, T.D.; Czapla, T.H.; Hopkins, T.L.; Kramer, K.J. A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989, 19, 611–622. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccase-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.J.; Liu, J.Z.; Hu, K.H.; Zhu, H.X. The level of secreted laccase activity in the edible fungi and their growing cycles are closely related. Curr. Microbiol. 2011, 62, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Wang, H.H.; Xue, C.B. Molecular identification and enzymatic properties of Laccase 2 from the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 2018, 17, 2310–2319. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhou, Y.; Wang, B.; Ding, L.; Wang, Y.; Luo, L.; Zhang, Y.; Kong, W. Genome-wide identification and characterization of laccase gene family in Citrus sinensis. Gene 2019, 689, 114–123. [Google Scholar] [CrossRef]
- Li, L.; Yang, K.; Wang, S.; Lou, Y.; Zhu, C.; Gao, Z. Genome-wide analysis of laccase genes in moso bamboo highlights PeLAC10 involved in lignin biosynthesis and in response to abiotic stresses. Plant Cell Rep. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Olmeda, I.; Casino, P.; Collins, R.E.; Sendra, R.; Callejón, S.; Huesa, J.; Soares, A.S.; Ferrer, S.; Pardo, I. Structural analysis and biochemical properties of laccase enzymes from two Pediococcus species. Microb. Biotechnol. 2021, 14, 1026–1043. [Google Scholar] [CrossRef]
- Palmer, A.E.; Lee, S.K.; Solomon, E.I. Decay of the peroxide intermediate in laccase: Reductive cleavage of the O-O bond. J. Am. Chem. Soc. 2001, 123, 6591–6599. [Google Scholar] [CrossRef]
- Kumar, S.V.; Phale, P.S.; Durani, S.; Wangikar, P.P. Combined sequence and structure analysis of the fungal laccase family. Biotechnol. Bioeng. 2003, 83, 386–394. [Google Scholar] [CrossRef]
- Garavaglia, S.; Cambria, M.T.; Miglio, M.; Ragusa, S.; Iacobazzi, V.; Palmieri, F.; D’Ambrosio, C.; Scaloni, A.; Rizzi, M. The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J. Mol. Biol. 2004, 342, 1519–1531. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittmer, N.T.; Suderman, R.J.; Jiang, H.; Zhu, Y.C.; Gorman, M.J.; Kramer, K.J.; Kanost, M.R. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2004, 34, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Coy, M.R.; Salem, T.Z.; Denton, J.S.; Kovaleva, E.S.; Liu, Z.; Barber, D.S.; Campbell, J.H.; Davis, D.C.; Buchman, G.W.; Boucias, D.G. Phenol-oxidizing laccases from the termite gut. Insect Biochem. Mol. Biol. 2010, 40, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Guo, J.Y.; Chu, D.; Ding, T.B.; Wei, K.K.; Cheng, D.F.; Wan, F.H. Secretory Laccase 1 in Bemisia tabaci MED is involved in whitefly-plant interaction. Sci. Rep. 2017, 7, 3623. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Guo, J.Y.; Chu, D.; Cheng, D.F.; Wan, F.H. Comparison of expression patterns of Laccase-1 gene in Bemisia tabaci MEAM1 and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Acta Entomol. Sin. 2018, 61, 1019–1028. [Google Scholar]
- Arakane, Y.; Muthukrishnan, S.; Beeman, R.W.; Kanost, M.R.; Kramer, K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA 2005, 102, 11337–11342. [Google Scholar] [CrossRef] [Green Version]
- Niu, B.L.; Shen, W.F.; Liu, Y.; Weng, H.B.; He, L.H.; Mu, J.J.; Wu, Z.L.; Jiang, P.; Tao, Y.Z.; Meng, Z.Q. Cloning and RNAi mediated functional characterization of MaLac2 of the pine sawyer, Monochamus alternatus. Insect Mol. Biol. 2008, 17, 303–312. [Google Scholar] [CrossRef]
- Elias-Neto, M.; Soares, M.P.; Simões, Z.L.; Hartfelder, K.; Bitondi, M.M. Developmental characterization, function and regulation of a Laccase 2 encoding gene in the honey bee, Apis mellifera (Hymenoptera, Apinae). Insect Biochem. Mol. Biol. 2010, 40, 241–251. [Google Scholar] [CrossRef]
- Futahashi, R.; Tanaka, K.; Matsuura, Y.; Tanahashi, M.; Kikuchi, Y.; Fukatsu, T. Laccase 2 is required for cuticular pigmentation in stinkbugs. Insect Biochem. Mol. Biol. 2011, 41, 191–196. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Gorman, M.J.; Kanost, M.R. Characterization of endogenous and recombinant forms of Laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2009, 39, 596–606. [Google Scholar] [CrossRef] [Green Version]
- Futahashi, R.; Banno, Y.; Fujiwara, H. Caterpillar color patterns are determined by a two-phase melanin gene prepatterning process: New evidence from tan and Laccase2. Evol. Dev. 2010, 12, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.L.; Mazo-Argas, A.; Brack, B.J.; Reed, R.D. Multiple roles for Laccase 2 in butterfly wing pigmentation, scale development, and cuticle tanning. Evol. Dev. 2020, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhan, X.; Gan, M.; Zhang, D.; Zhang, M.; Zheng, X.; Wu, Y.; Li, Z.Y.; He, A. Laccase 2 is required for sclerotization and pigmentation of Aedes albopictus eggshell. Parasitol. Res. 2013, 112, 1929–1934. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Perring, T.M. The Bemisia tabaci species complex. Crop Prot. 2001, 20, 737. [Google Scholar] [CrossRef]
- Wan, F.H.; Zhang, G.F.; Liu, S.S.; Luo, C.; Chu, D.; Zhang, Y.J.; Zang, L.S.; Jiu, M.; Lü, Z.C.; Cui, X.H.; et al. Invasive mechanism and management strategy of Bemisia tabaci (Gennadius) biotype B: Progress report of 973 program on invasive alien species in China. Chin. Sci. Ser. C Life Sci. 2009, 52, 88–95. [Google Scholar] [CrossRef]
- Peng, Z.K.; Ren, J.; Su, Q.; Zeng, Y.; Tian, L.X.; Wang, S.L.; Wu, Q.J.; Liang, P.; Xie, W.; Zhang, Y.J. Genome-wide identification and analysis of chitinase-like gene family in Bemisia tabaci (Hemiptera: Aleyrodidae). Insects 2021, 12, 254. [Google Scholar] [CrossRef]
- Li, R.; Wen, X.; Wang, S.; Wu, Q.; Yang, N.; Xin, Y.; Pan, H.; Zhou, X.M.; Bai, L.Y.; Xu, B.Y.; et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zheng, Y.; Chao, Z.J.; Chen, H.T.; Zhang, Y.H.; Yin, M.Z.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Yatsu, J.; Asano, T. Cuticle laccase of the silkworm, Bombyx mori: Purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem. Mol. Biol. 2009, 39, 254–262. [Google Scholar] [CrossRef]
- Claus, H.; Filip, Z. The evidence of a laccase-like enzyme activity in a Bacillus sphaericus strain. Microbiol. Res. 1997, 152, 209–216. [Google Scholar] [CrossRef]
- Claus, H.; Faber, G.; König, H. Redox-mediated decolorization of synthetic dyes by fungal laccase. Appl. Microbiol. Biotechnol. 2002, 59, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Yun, Z.; Mo, J. The clone of laccase gene and its potential function in cuticular penetration resistance of Culex pipiens pallens to fenvalerate. Pestic. Biochem. Physiol. 2009, 93, 105–111. [Google Scholar] [CrossRef]
- Amenya, D.A.; Chou, W.; Li, J.; Yan, G.; Gershon, P.D.; James, A.A.; Marinotti, O. Proteomics reveals novel components of the Anopheles gambiae eggshell. J. Insect Physiol. 2010, 56, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.H.; Yan, Z.W.; Hao, Y.J.; Yan, Z.T.; Qiao, L. Suppression of Laccase 2 severely impairs cuticle tanning and pathogen resistance during the pupal metamorphosis of Anopheles sinensis (Diptera: Culicidae). Parasites Vectors 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Julio, A.H.F.; Gigliolli, A.A.S.; Cardoso, K.A.K.; Drosdoski, S.D.; Kulza, R.A.; Seixas, F.A.V.; Ruvolo-Takasusuki, M.C.C.; de Souza, C.G.M.; Lapenta, A.S. Multiple resistance to pirimiphos-methyl and bifenthrin in Tribolium castaneum involves the activity of lipases, esterases, and Laccase 2. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 27–43. [Google Scholar] [CrossRef]
- Horowitz, A.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- He, B.C.; Chu, Y.; Yin, M.; Müllen, K.; An, C.J.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′→3′) | Amplicon (bp) | Remarks |
|---|---|---|---|
| LAC2-1 | F:ATGAAGGTGAAAATGTCACG | 777 | fragment |
| R:GTTTCCTTGTAAGATGGGGC | |||
| LAC2-2 | F:CCCATCTTACAAGGAAACACT | 795 | fragment |
| R:CAGTTCTTGGAGTAAAGCCTT | |||
| LAC2-3 | F:AGGCTTTACTCCAAGAACTGC | 704 | fragment |
| R:TCAATGTAAACTGACCGGG | |||
| q-LAC2 | F:AGTGTCTGCCCAGCTCAACT | 213 | qRT-PCR |
| R:CAATTGCTGCACTCGTTTGT | |||
| q-SDHA | F:GCGACTGATTCTTCTCCTGC | 141 | qRT-PCR |
| R:TGGTGCCAACAGATTAGGTGC | |||
| dsBtLAC2 | F: taatacgactcactatagggGATCGACGACATCCCACCAG | 479 | RNAi |
| R: taatacgactcactatagggATCGAGGGAAACCTTCTGGC | |||
| dsEGFP | F: taatacgactcactatagggCACAAGTTCAGCGTGTCCG | 420 | RNAi |
| R: taatacgactcactatagggTGCCGTTCTTCTGCTTGTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Zhang, Q.; Zhu, W.-Q.; Shi, Y.; Cao, H.-H.; Guo, L.; Chu, D.; Lu, Z.; Liu, T.-X. Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East–Asia Minor 1. Insects 2022, 13, 471. https://doi.org/10.3390/insects13050471
Yang C-H, Zhang Q, Zhu W-Q, Shi Y, Cao H-H, Guo L, Chu D, Lu Z, Liu T-X. Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East–Asia Minor 1. Insects. 2022; 13(5):471. https://doi.org/10.3390/insects13050471
Chicago/Turabian StyleYang, Chun-Hong, Qi Zhang, Wan-Qing Zhu, Yan Shi, He-He Cao, Lei Guo, Dong Chu, Zhaozhi Lu, and Tong-Xian Liu. 2022. "Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East–Asia Minor 1" Insects 13, no. 5: 471. https://doi.org/10.3390/insects13050471
APA StyleYang, C.-H., Zhang, Q., Zhu, W.-Q., Shi, Y., Cao, H.-H., Guo, L., Chu, D., Lu, Z., & Liu, T.-X. (2022). Involvement of Laccase2 in Cuticle Sclerotization of the Whitefly, Bemisia tabaci Middle East–Asia Minor 1. Insects, 13(5), 471. https://doi.org/10.3390/insects13050471






