Real-Time Feeding Behavior Monitoring by Electrical Penetration Graph Rapidly Reveals Host Plant Susceptibility to Crapemyrtle Bark Scale (Hemiptera: Eriococcidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Plants
2.2. Electrical Penetration Graph Recordings of Acanthococcus Lagerstroemiae Probing on Host Plant Lagerstroemia limii
2.3. EPG-Based Comparison of EPG Parameters among Different Plants
| Electrical Penetration Graph Parameter | Statistical Test |
|---|---|
| GLM-Logistic |
| Welch’s ANOVA |
| Welch’s ANOVA |
| GLM-Logistic |
| Welch’s ANOVA |
| Welch’s ANOVA |
| GLM-Logistic |
| Welch’s ANOVA |
| Welch’s ANOVA |
| GLM-Logistic |
| Welch’s ANOVA |

2.4. Data Processing and Statistical Analysis
3. Results
3.1. Characterization of EPG Waveforms for A. lagerstroemiae Probing on a Validated Host, Crapemyrtle L. limii
3.2. Percentage of A. lagerstroemiae Initiated Stylet Pathway (Waveform C) and Time Spent by A. lagerstroemiae to Initiate and Stay in Stylet Pathway Differed on Different Plant Species
3.3. Percentage of A. lagerstroemiae Accessed Phloem Tissue (Waveform E) and Time Spent by A. lagerstroemiae to Initiate Phloem Phase and Ingest Phloem Sap Differed on Different Plant Species
3.4. Percentage of A. lagerstroemiae Having Xylem Ingestion (Waveform G) Differed among Different Plant Species
4. Discussion
4.1. The First Assessment on the Feeding Behavior of A. lagerstroemiae Shows Clear Similarities and Differences in Characteristics Compared to Other Sap Feeders
4.2. Occurrences of Waveform E and G Detected by EPG Shows Vital Evidence to Unveil Host Plants of A. lagerstroemiae
4.3. Phloem Access and Ingestion Assist in Identifying Plant Resistance to A. lagerstroemiae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merchant, M.E.; Gu, M.; Robbins, J.; Vafaie, E.; Barr, N.; Tripodi, A.D.; Szalanski, A.L. Discovery and Spread of Eriococcus lagerstroemiae Kuwana (Hemiptera: Eriococcidae), a New Invasive Pest of Crape Myrtle, Lagerstroemia spp. Available online: https://bugwoodcloud.org/resource/pdf/ESAPosterDiscovAndSpread2014.pdf (accessed on 21 April 2021).
- Gu, M.; Merchant, M.; Robbins, J.; Hopkins, J. Crape Myrtle Bark Scale: A New Exotic Pest. Available online: https://cdn-ext.agnet.tamu.edu/wp-content/uploads/2018/10/EHT-049-crape-myrtle-bark-scale-a-new-exotic-pest.pdf (accessed on 21 April 2021).
- EDDMapS. Early Detection & Distribution Mapping System. Available online: https://www.eddmaps.org/distribution/viewmap.cfm?sub=80722 (accessed on 21 April 2021).
- US Department of Agriculture, N.A.S.S. 2019 Census of Horticultural Specialties. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/hortic_1_0020_0021,pdf (accessed on 21 April 2021).
- Riddle, T.C.; Mizell, R.F., III. Use of crape myrtle, Lagerstroemia (Myrtales: Lythraceae), cultivars as a pollen source by native and non-native bees (Hymenoptera: Apidae) in Quincy, Florida. Fla. Entomol. 2016, 99, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Lau, P.; Bryant, V.; Ellis, J.D.; Huang, Z.Y.; Sullivan, J.; Schmehl, D.R.; Cabrera, A.R.; Rangel, J. Seasonal variation of pollen collected by honey bees (Apis mellifera) in developed areas across four regions in the United States. PLoS ONE 2019, 14, e0217294. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.; Garcia, J.; Gu, M. Longevity of imidacloprid as a soil treatment for crapemyrtle bark scale on crapemyrtle, 2016–2017. Arthropod Manag. Tests 2018, 43, tsy012. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, Y.; Gu, M.; Vafaie, E.; Merchant, M.; Diaz, R. Crapemyrtle bark scale: A new threat for crapemyrtles, a popular landscape plant in the US. Insects 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwah, P.; Zhang, Y.Y.; Gu, M. Investigating Producers’ Preferences for Crapemyrtle and Their Perceptions Regarding Crapemyrtle Bark Scale. Horticulturae 2021, 7, 146. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Wu, B.; Xie, R.; Knox, G.; Qin, H.; Gu, M. Host suitability for crapemyrtle bark scale (Acanthococcus lagerstroemiae) differed significantly among crapemyrtle species. Insects 2021, 12, 6. [Google Scholar] [CrossRef]
- Xie, R.; Wu, B.; Dou, H.; Liu, C.; Knox, G.; Qin, H.; Gu, M. Feeding preference of crapemyrtle bark scale (Acanthococcus lagerstroemiae) on different species. Insects 2020, 11, 399. [Google Scholar] [CrossRef]
- Gu, M. Alternative Hosts of Crapemyrtle Barkscale. Available online: https://cdn-ext.agnet.tamu.edu/wp-content/uploads/2018/10/EHT-103-alternative-hosts-of-crapemyrtle-bark-scale.pdf (accessed on 21 April 2021).
- Schultz, P.B.; Szalanski, A.L. Hypericum kalmianum (St. Johnswort) confirmed as a new host of the crapemyrtle bark scale in Virginia, USA. J. Agric. Urban Entomol. 2019, 35, 12–14. [Google Scholar] [CrossRef]
- Xie, R.; Wu, B.; Gu, M.; Jones, S.R.; Robbins, J.; Szalanski, A.L.; Qin, H. Confirmation of New Crapemyrtle Bark Scale (Acanthococcus lagerstroemiae) Hosts (Spiraea and Callicarpa) through DNA Barcoding. HortScience 2021, 56, 1549–1551. [Google Scholar] [CrossRef]
- Wu, B.; Xie, R.; Knox, G.W.; Qin, H.; Gu, M. Evaluating Beautyberry and Fig Species as Potential Hosts of Invasive Crapemyrtle Bark Scale in the United States. HortTechnology 2022, 32, 10–15. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Diaz, R. Thermal tolerance and prediction of northern distribution of the Crapemyrtle Bark Scale (Hemiptera: Eriococcidae). Environ. Entomol. 2019, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, A.J.; Vargas, R.; Tapia, D.; Olivares-Donoso, R.; Niemeyer, H.M. Host selection by the generalist aphid Myzus persicae (Hemiptera: Aphididae) and its subspecies specialized on tobacco, after being reared on the same host. Bull. Entomol. Res. 2005, 95, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhou, X.; Pang, B. Influence of five host plants of Aphis gossypii Glover on some population parameters of Hippodamia variegata (Goeze). J. Pest Sci. 2010, 83, 77–83. [Google Scholar] [CrossRef]
- Edwards, O.R. Interspecific and intraspecific variation in the performance of three pest aphid species on five grain legume hosts. Entomol. Exp. Appl. 2001, 100, 21–30. [Google Scholar] [CrossRef]
- Herbert, J.J.; Mizell, R., III; McAuslane, H. Host preference of the crapemyrtle aphid (Hemiptera: Aphididae) and host suitability of crapemyrtle cultivars. Environ. Entomol. 2009, 38, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.; Tjallingii, W.F. Effects of previous plant infestation on sieve element acceptance by two aphids. Entomol. Exp. Appl. 1997, 82, 189–200. [Google Scholar] [CrossRef]
- Tjallingii, W. Electrical nature of recorded signals during stylet penetration by aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- Tjallingii, W. Host plant acceptance by aphids: An EPG analysis. Bulletin OILB SROP 1993, 16, 173–177. [Google Scholar]
- Jiang, Y.; Nombela, G.; Muñiz, M. Analysis by DC–EPG of the resistance to Bemisia tabaci on an Mi-tomato line. Entomol. Exp. Appl. 2001, 99, 295–302. [Google Scholar] [CrossRef]
- Huang, F.; Tjallingii, W.F.; Zhang, P.; Zhang, J.; Lu, Y.; Lin, J. EPG waveform characteristics of solenopsis mealybug stylet penetration on cotton. Entomol. Exp. Appl. 2012, 143, 47–54. [Google Scholar] [CrossRef]
- George, J.; Ammar, E.-D.; Hall, D.G.; Lapointe, S.L. Sclerenchymatous ring as a barrier to phloem feeding by Asian citrus psyllid: Evidence from electrical penetration graph and visualization of stylet pathways. PLoS ONE 2017, 12, e0173520. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Tjallingii, W.F.; Moreno, A.; Fereres, A. Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited Beet yellows virus. J. Virol. 2018, 92, e01076-18. [Google Scholar] [CrossRef] [Green Version]
- Da Silva-Torres, C.S.A.; de Oliveira, M.D.; Torres, J.B. Host selection and establishment of striped mealybug, Ferrisia virgata, on cotton cultivars. Phytoparasitica 2013, 41, 31–40. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; Rahbé, Y.; Tjallingii, W.; Tertuliano, M.; Le Rü, B. Electrically recorded feeding behaviour of cassava mealybug on host and non-host plants. Entomol. Exp. Appl. 1994, 72, 219–232. [Google Scholar] [CrossRef]
- Tjallingii, W.; Esch, T.H. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Bonani, J.; Fereres, A.; Garzo, E.; Miranda, M.; Appezzato-Da-Gloria, B.; Lopes, J.R.S. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 2010, 134, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Spiller, N.; Koenders, L.; Tjallingii, W. Xylem ingestion by aphids–a strategy for maintaining water balance. Entomol. Exp. Appl. 1990, 55, 101–104. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Dufour, S.; Birkemeyer, C.; Birkett, M.; Hardie, J.; Pickett, J. Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J. Chem. Ecol. 2008, 34, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.; Hardie, J. Xylem ingestion by winged aphids. In Proceedings of the 11th International Symposium on Insect-Plant Relationships, Helsingør, Denmark, 4–10 August 2001; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Alvarez, A.; Tjallingii, W.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Ponder, K.; Pritchard, J.; Harrington, R.; Bale, J. Difficulties in location and acceptance of phloem sap combined with reduced concentration of phloem amino acids explain lowered performance of the aphid Rhopalosiphum padi on nitrogen deficient barley (Hordeum vulgare) seedlings. Entomol. Exp. Appl. 2000, 97, 203–210. [Google Scholar] [CrossRef]
- Will, T.; van Bel, A.J. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernays, E.A.; Chapman, R.F. Behavior: The process of host-plant selection. In Host-Plant Selection by Phytophagous Insects; Miller, T.A., van Emden, H.S., Eds.; Chapman & Hall: New York, NY, USA, 1994; Volume 2, pp. 95–165. [Google Scholar]
- Souza, M.; Davis, J. Characterizing host plant resistance to Melanaphis sacchari (Hemiptera: Aphididae) in selected sorghum plant introductions. J. Econ. Entomol. 2021, 114, 959–969. [Google Scholar] [CrossRef]
- Sandanayaka, W.; Jia, Y.; Charles, J. EPG technique as a tool to reveal host plant acceptance by xylem sap-feeding insects. J. Appl. Entomol. 2013, 137, 519–529. [Google Scholar] [CrossRef]
- Cid, M.; Fereres, A. Characterization of the probing and feeding behavior of Planococcus citri (Hemiptera: Pseudococcidae) on grapevine. Ann. Entomol. Soc. Am. 2010, 103, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Ebert, T.A.; Backus, E.A.; Cid, M.; Fereres, A.; Rogers, M.E. A new SAS program for behavioral analysis of electrical penetration graph data. Comput. Electron. Agric. 2015, 116, 80–87. [Google Scholar] [CrossRef]
- Hadley, W. Ggplot2: Elegrant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Mehta, C.R.; Patel, N.R. Exact logistic regression: Theory and examples. Stat. Med. 1995, 14, 2143–2160. [Google Scholar] [CrossRef]
- Souza, M.; Davis, J. Detailed characterization of Melanaphis sacchari (Hemiptera: Aphididae) feeding behavior on different host plants. Environ. Entomol. 2020, 49, 683–691. [Google Scholar] [CrossRef]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [Green Version]
- Games, P.A.; Howell, J.F. Pairwise multiple comparison procedures with unequal n’s and/or variances: A Monte Carlo study. J. Educ. Stat. 1976, 1, 113–125. [Google Scholar]
- Sauder, D.C.; DeMars, C.E. An updated recommendation for multiple comparisons. AMPPS 2019, 2, 26–44. [Google Scholar] [CrossRef]
- Games, P.A.; Keselman, H.; Rogan, J.C. Simultaneous pairwise multiple comparison procedures for means when sample sizes are unequal. Psychol. Bull. 1981, 90, 594. [Google Scholar] [CrossRef]
- Morgan, J.K.; Luzio, G.A.; Ammar, E.-D.; Hunter, W.B.; Hall, D.G.; Shatters, R.G., Jr. Formation of stylet sheaths in āere (in air) from eight species of phytophagous hemipterans from six families (Suborders: Auchenorrhyncha and Sternorrhyncha). PLoS ONE 2013, 8, e62444. [Google Scholar] [CrossRef] [Green Version]
- Rosell, R.C.; Lichty, J.E.; Brown, J.K. Ultrastructure of the mouthparts of adult sweetpotato whitefly, Bemisia tabaci Gennadius (Homoptera: Aleyrodidae). Int. J. Insect Morphol. Embryol. 1995, 24, 297–306. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Behavioral evidence for local reduction of aphid-induced resistance. J. Insect Sci. 2007, 7, 48. [Google Scholar] [CrossRef]
- Ma, R.; Reese, J.C.; Black, W.C., IV; Bramel-Cox, P. Detection of pectinesterase and polygalacturonase from salivary secretions of living greenbugs, Schizaphis graminum (Homoptera: Aphididae). J. Insect Physiol. 1990, 36, 507–512. [Google Scholar] [CrossRef]
- Janssen, J.; Tjallingii, W.; van Lenteren, J. Electrical recording and ultrastructure of stylet penetration by the greenhouse whitefly. Entomol. Exp. Appl. 1989, 52, 69–81. [Google Scholar] [CrossRef]
- Wensler, R.J.; Filshie, B.K. Gustatory sense organs in the food canal of aphids. J. Morphol. 1969, 129, 473–491. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, Y.; Diaz, R. Temperature-dependent development and host range of crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana) (Hemiptera: Eriococcidae). Fla. Entomol. 2019, 102, 181–186. [Google Scholar]
- Mattson, W.J., Jr. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef]
- Douglas, A. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef]
- Schwarzkopf, A.; Rosenberger, D.; Niebergall, M.; Gershenzon, J.; Kunert, G. To feed or not to feed: Plant factors located in the epidermis, mesophyll, and sieve elements influence pea aphid’s ability to feed on legume species. PLoS ONE 2013, 8, e75298. [Google Scholar]
- Calatayud, P.-A.; Rahbé, Y.; Delobel, B.; Khuong-Huu, F.; Tertuliano, M.; Le Rü, B. Influence of secondary compounds in the phloem sap of cassava on expression of antibiosis towards the mealybug Phenacoccus manihoti. Entomol. Exp. Appl. 1994, 72, 47–57. [Google Scholar] [CrossRef]
| EPG Waveform | Waveform Characteristics | Correlations | ||||
|---|---|---|---|---|---|---|
| Voltage Level | Frequency (Hz) | Relative Amplitude (%) z | Activities Assigned for Similar Waveforms in Other Hemipterans | |||
| Min–Max | Median ± SE | Median ± SE | ||||
| C y | Extracellular | 0.98–4.57 | 1.80 ± 0.37 | 12.45 ± 1.13 | Sheath salivation and other intercellular stylet pathway | |
| pd | pd1 | Intracellular | 2.71–6.62 | 4.16 ± 0.48 | 20.12 ± 2.16 | Short cell punctures |
| pd2 | Intracellular | 1.50–3.14 | 2.71 ± 0.16 | 21.48 ± 2.38 | ||
| E1 | Intracellular | 0.49–2.05 | 1.52 ± 0.18 | 21.20 ± 3.52 | Phloem salivation | |
| E2 | Intracellular | 0.68–2.78 | 1.44 ± 0.24 | 21.52 ± 4.55 | Phloem sap ingestion | |
| G | Extracellular | 1.46–3.12 | 1.91 ± 0.20 | 3.00 ± 0.81 | Xylem sap ingestion | |
| Electrical Penetration Graph Parameter | Num df, Denom df | p-Value | |
|---|---|---|---|
| 21.56 | 7, 152.00 | 0.0030 |
| 2.71 | 7, 33.56 | 0.0243 |
| 10.43 | 7, 36.46 | <0.0001 |
| 62.24 | 7, 152 | <0.0001 |
| 4.94 | 4, 14.47 | 0.0102 |
| 2.36 | 4, 15.20 | 0.0992 |
| 61.35 | 7, 152 | <0.0001 |
| 4.81 | 4, 14.61 | 0.0112 |
| 42.78 | 4, 17.09 | <0.0001 |
| 25.32 | 7, 152 | 0.0007 |
| 2.25 | 6, 24.60 | 0.0716 |
| 21.56 | 7, 152.00 | 0.0030 |
| 2.71 | 7, 33.56 | 0.0243 |
| 10.43 | 7, 36.46 | <0.0001 |
| 62.24 | 7, 152 | <0.0001 |
| 4.94 | 4, 14.47 | 0.0102 |
| 2.36 | 4, 15.20 | 0.0992 |
| 61.35 | 7, 152 | <0.0001 |
| 4.81 | 4, 14.61 | 0.0112 |
| 42.78 | 4, 17.09 | <0.0001 |
| 25.32 | 7, 152 | 0.0007 |
| 2.25 | 6, 24.60 | 0.0716 |
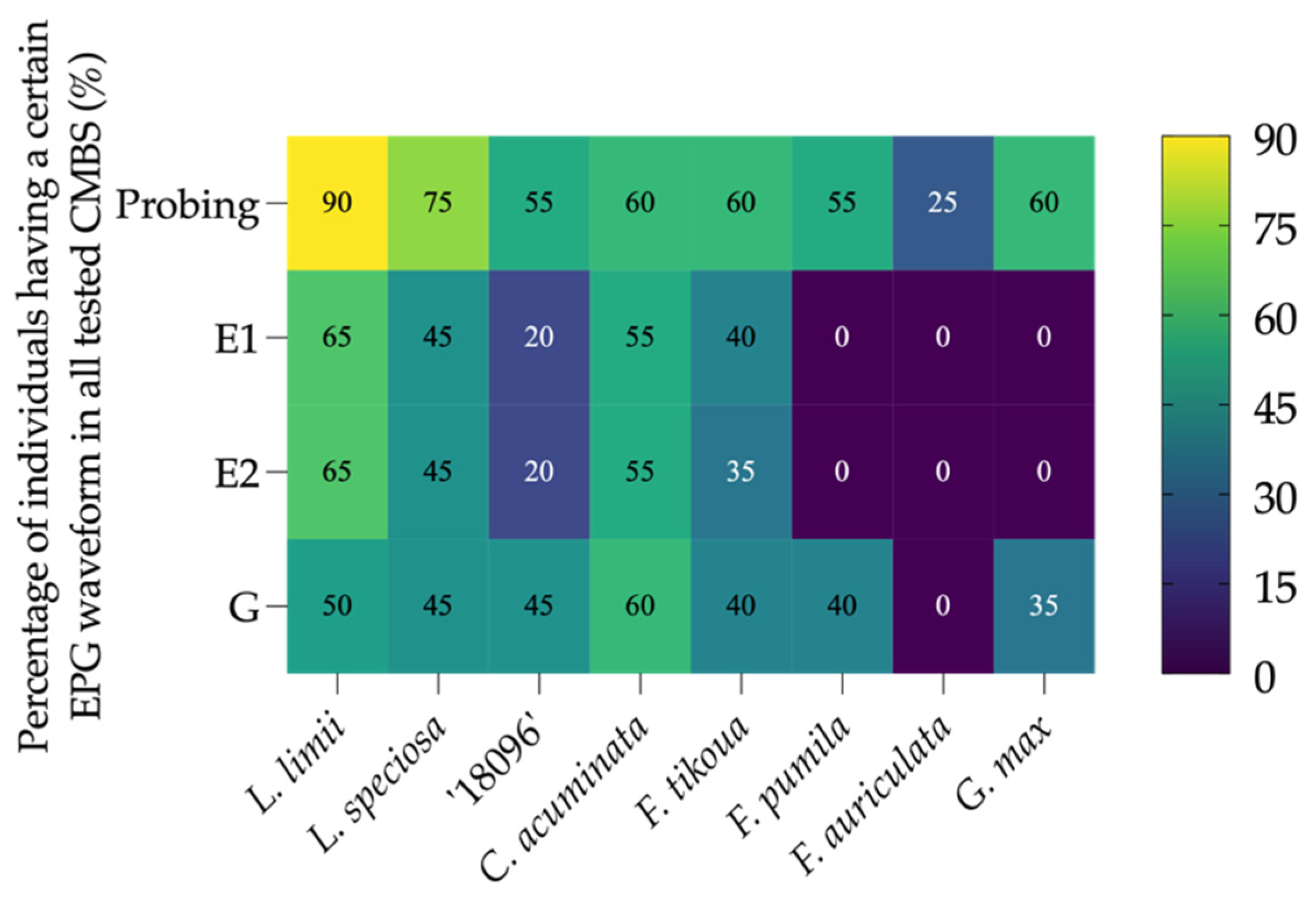
| Electrical Penetration Graph Parameter z | Plant Type | |||||||
|---|---|---|---|---|---|---|---|---|
| Lagerstroemia limii | Lagerstroemia speciosa | Lagerstroemia indica × speciosa ‘18096’ | Callicarpa acuminata | Ficus tikoua | Ficus pumila | Ficus auriculata | Glycine max | |
| 90.00 | 75.00 | 55.00 | 60.00 | 60.00 | 55.00 | 25.00 | 60.00 |
| 238.90 (181.85–313.77) y ab x | 486.85 (361.06–656.32) a | 352.85 (248.90–500.52) ab | 324.00 (231.96–500.52) ab | 257.28 (184.18–359.48) b | 404.52 (285.33–573.44) ab | 397.29 (236.71–666.91) ab | 453.25 (324.49–633.12) ab |
| 483.26 (361.28–646.55) abc | 531.79 (386.70–731.91) bc | 475.07 (327.23–689.29) abc | 502.23 (352.01–716.70) c | 749.61 (525.14–1070.26) abc | 664.18 (457.90–962.62) bc | 1033.65 (595.65–1793.02) a | 795.49 (557.61–1135.30) ab |
| 65.00 | 45.00 | 20.00 | 55.00 | 40.00 | 0.00 | 0.00 | 0.00 |
| 316.23 (253.32–394.51) b | 632.29 (484.28–825.22) a | 454.53 (304.80–677.67) ab | 476.30 (374.15–605.86) b | 473.69 (356.97–628.07) ab | - w | - | - |
| 12.44 (7.32–21.14) a | 30.67 (16.21–58.03) a | 46.80 (17.99–121.80) a | 34.32 (19.28–61.10) a | 21.56 (10.96–42.39) a | - | - | - |
| 65.00 | 45.00 | 20.00 | 55.00 | 35.00 | 0.00 | 0.00 | 0.00 |
| 341.81 (279.40–418.07) b | 666.38 (523.04–849.51) a | 506.57 (352.36–728.99) ab | 514.84 (413.50–640.76) b | 540.86 (411.03–711.70) ab | - | - | - |
| 200.92 (120.18–335.85) a | 64.72 (34.90–120.02) b | 307.66 (226.91–416.82) a | 24.60 (14.07–43.20) c | 26.49 (13.15–53.33) bc | - | - | - |
| 50.00 | 45.00 | 45.00 | 60.00 | 40.00 | 40.00 | 0.00 | 35.00 |
| 158.83 (107.61–234.55) a | 387.55 (232.65–584.44) a | 320.62 (212.72–483.31) a | 375.50 (263.18–535.74) a | 330.35 (227.91–478.98) a | 408.49 (264.32–631.22) a | - | 199.07 (75.87–522.52) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Chun, E.; Xie, R.; Knox, G.W.; Gu, M.; Qin, H. Real-Time Feeding Behavior Monitoring by Electrical Penetration Graph Rapidly Reveals Host Plant Susceptibility to Crapemyrtle Bark Scale (Hemiptera: Eriococcidae). Insects 2022, 13, 495. https://doi.org/10.3390/insects13060495
Wu B, Chun E, Xie R, Knox GW, Gu M, Qin H. Real-Time Feeding Behavior Monitoring by Electrical Penetration Graph Rapidly Reveals Host Plant Susceptibility to Crapemyrtle Bark Scale (Hemiptera: Eriococcidae). Insects. 2022; 13(6):495. https://doi.org/10.3390/insects13060495
Chicago/Turabian StyleWu, Bin, Elizabeth Chun, Runshi Xie, Gary W. Knox, Mengmeng Gu, and Hongmin Qin. 2022. "Real-Time Feeding Behavior Monitoring by Electrical Penetration Graph Rapidly Reveals Host Plant Susceptibility to Crapemyrtle Bark Scale (Hemiptera: Eriococcidae)" Insects 13, no. 6: 495. https://doi.org/10.3390/insects13060495








