Differences in the Sublethal Effects of Sulfoxaflor and Acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) Are Related to Its Basic Sensitivity Level
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticide and Reagents
2.3. Toxicity Bioassays
2.4. Sublethal Effects of Acetamiprid and Sulfoxaflor on A. gossypii
2.5. Statistical Analysis
3. Results
3.1. Toxicity of Sulfoxaflor and Acetamiprid against A. gossypii
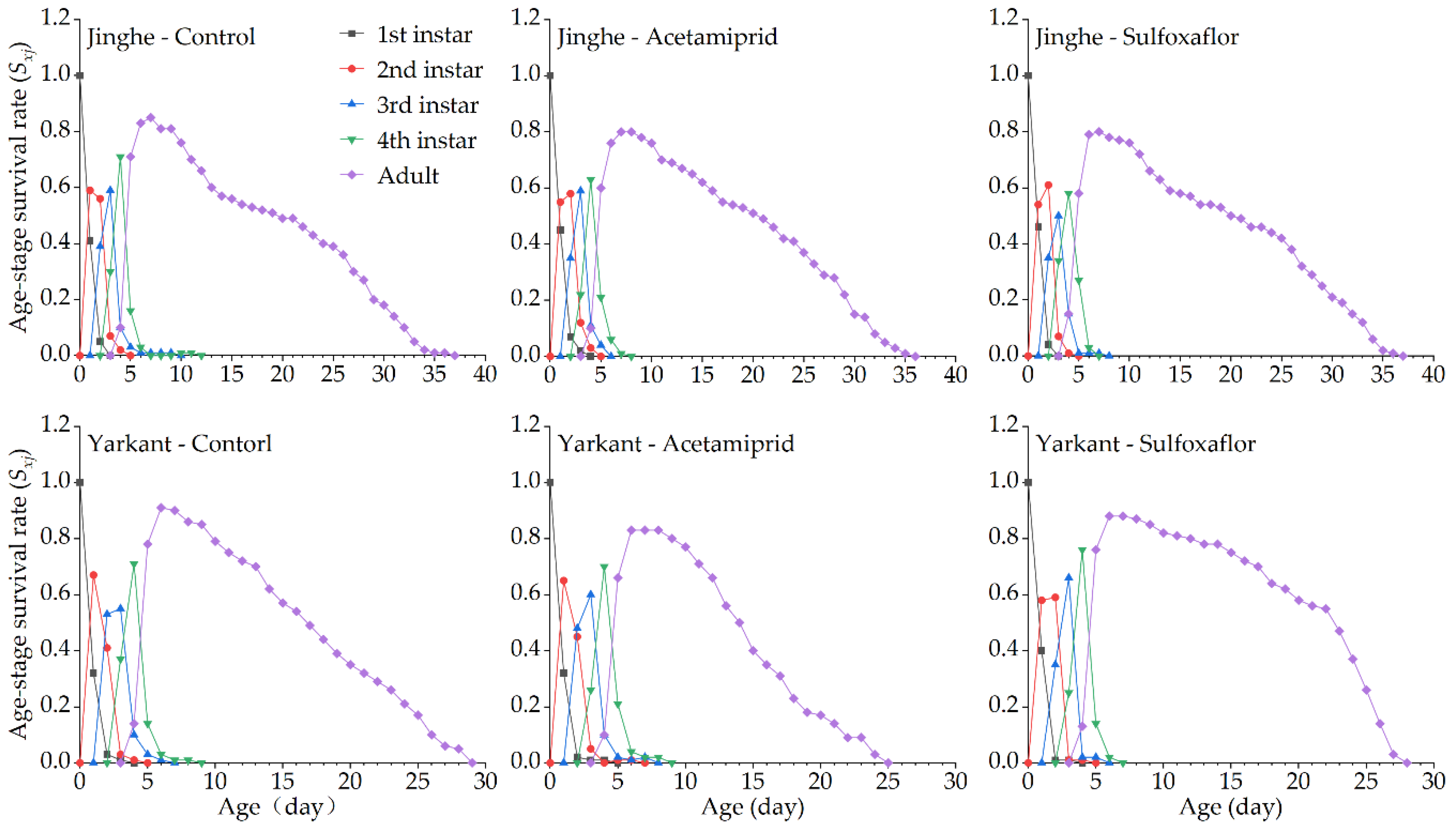
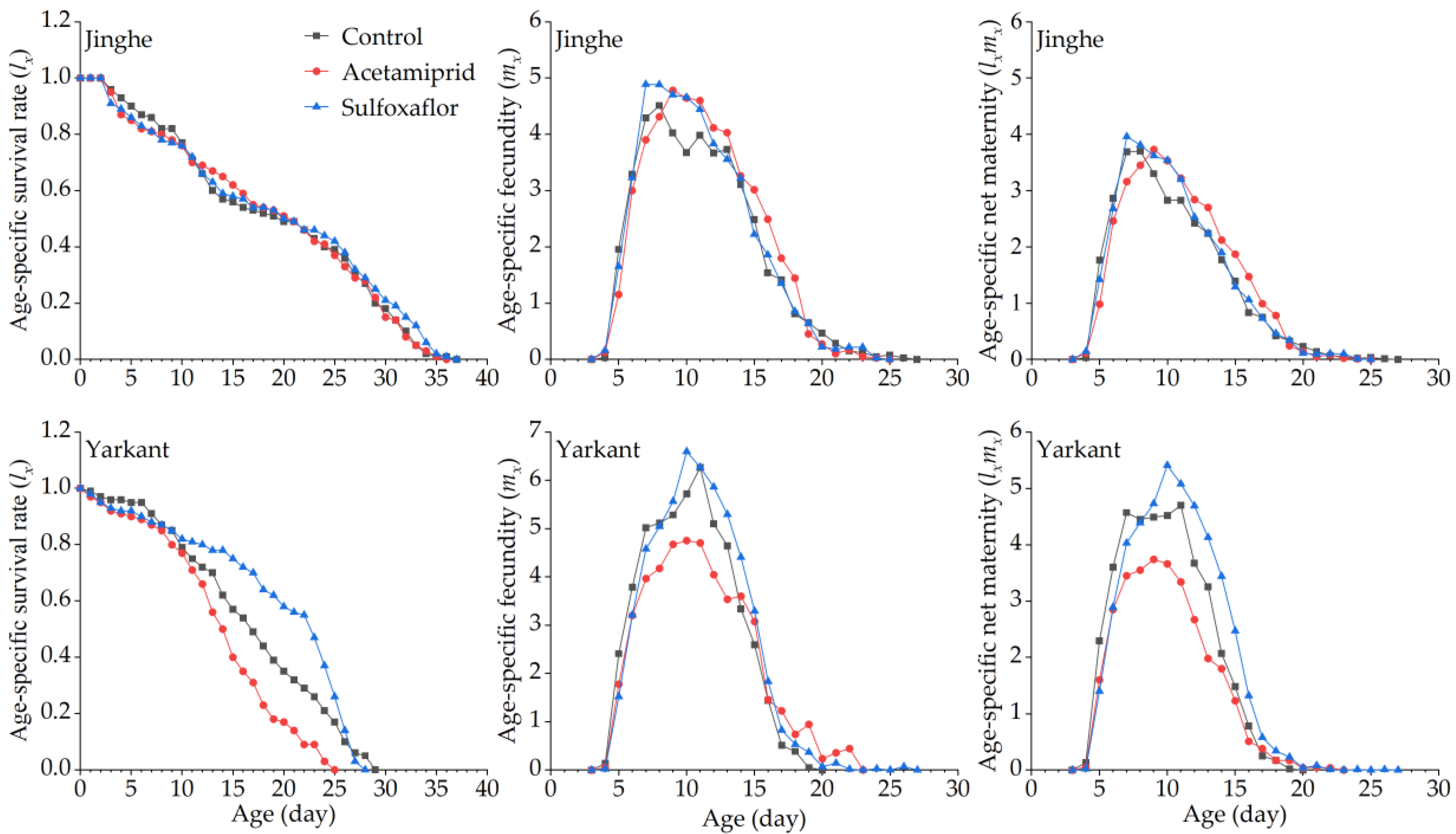
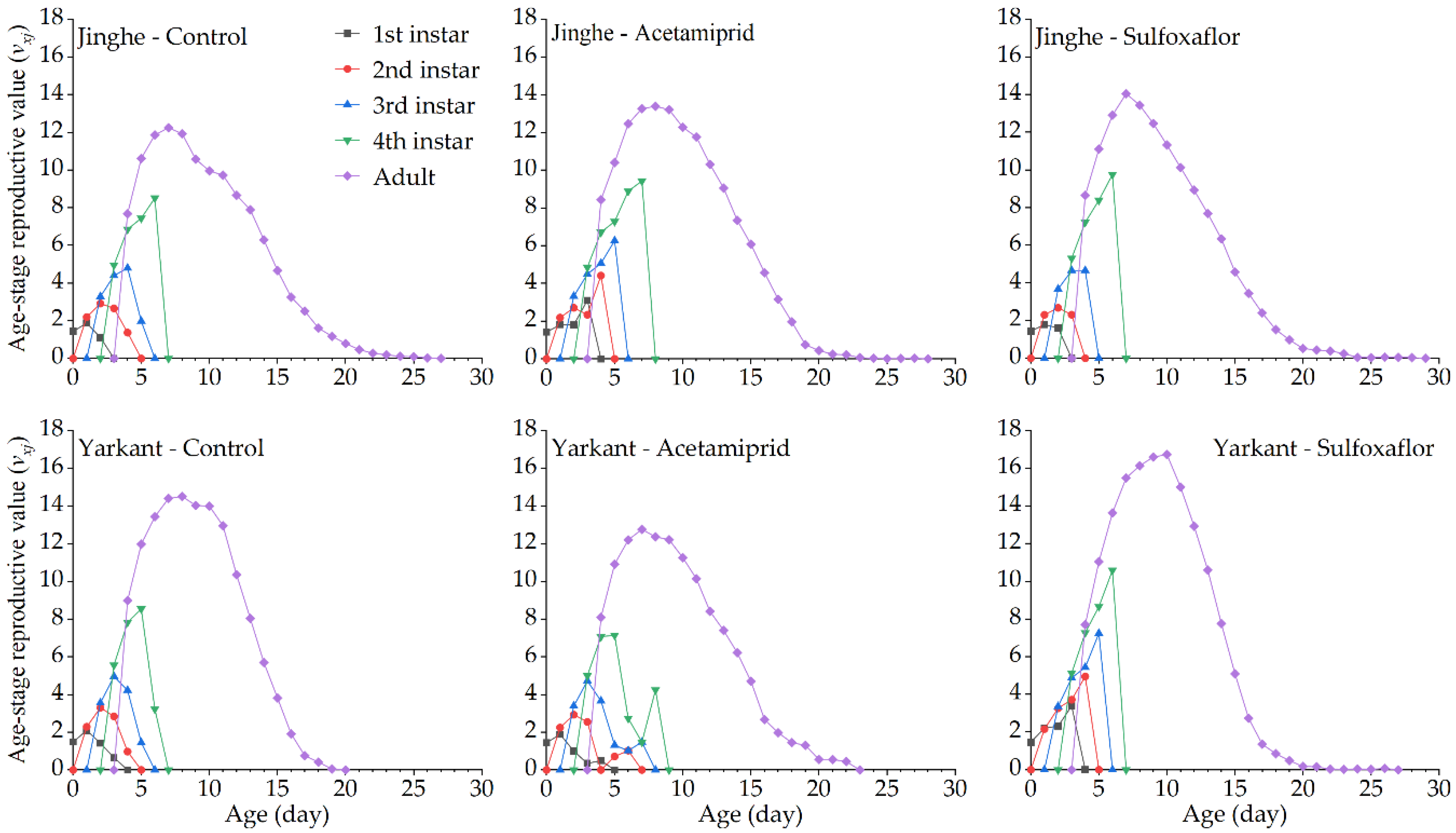
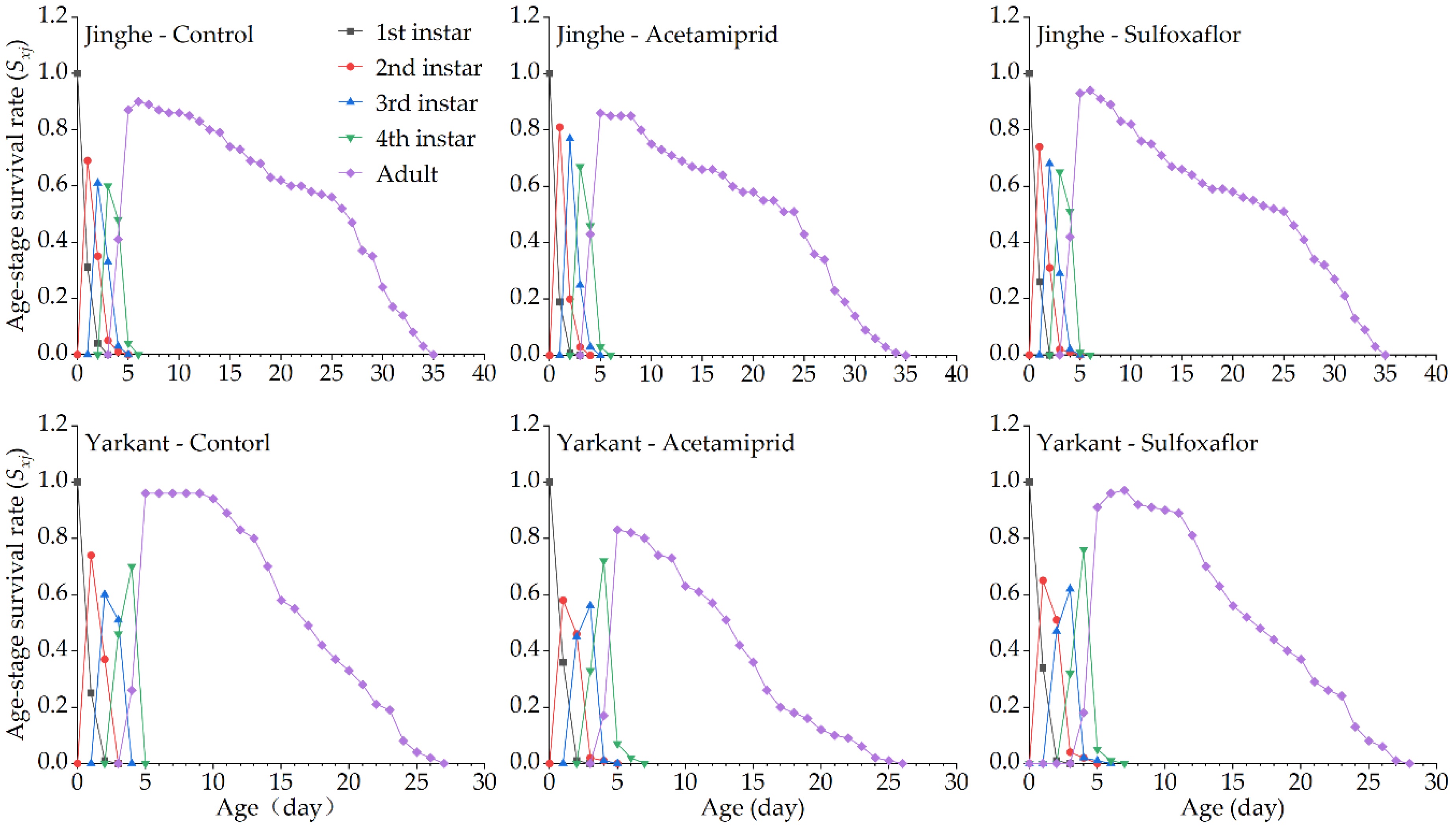
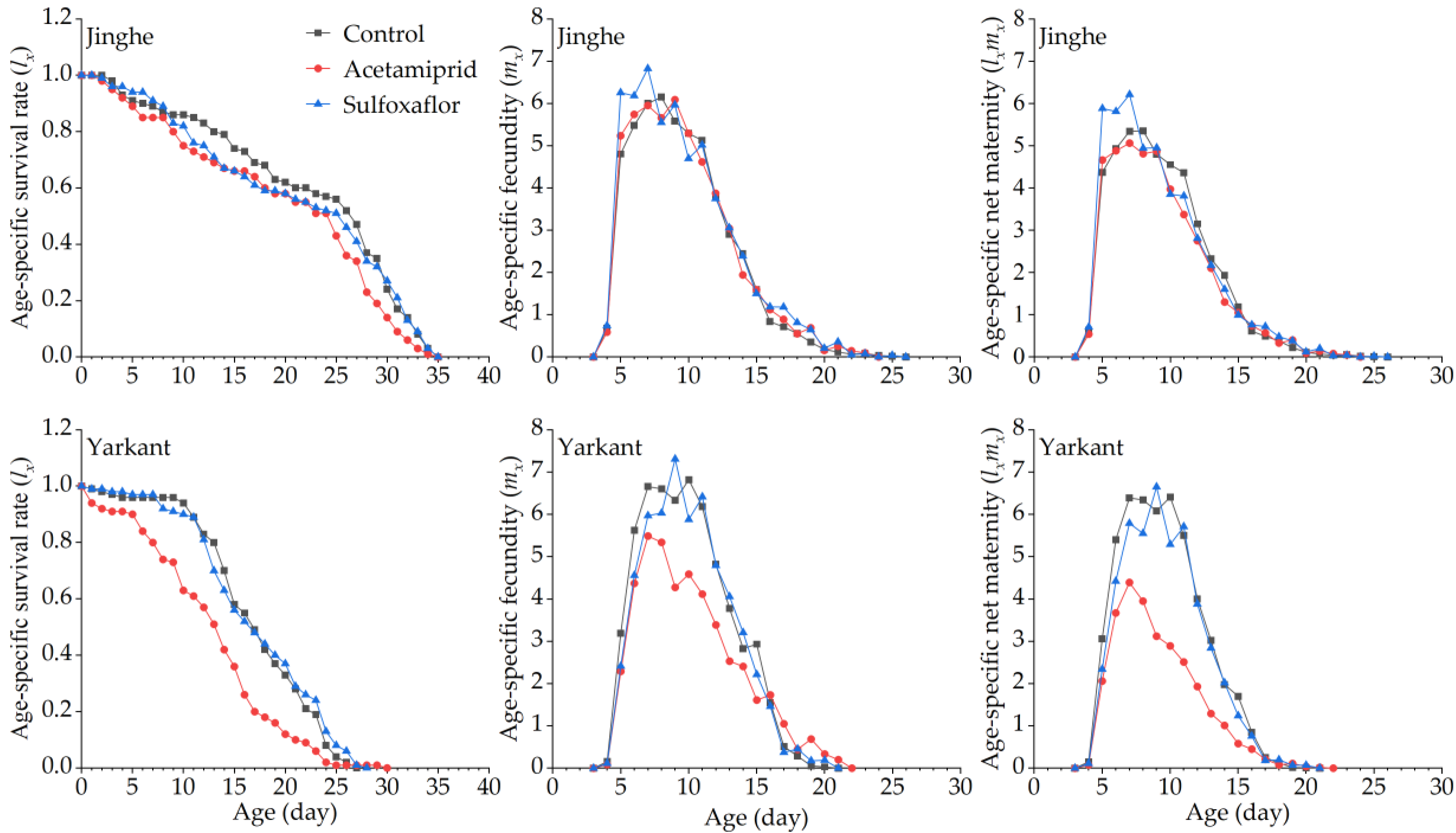
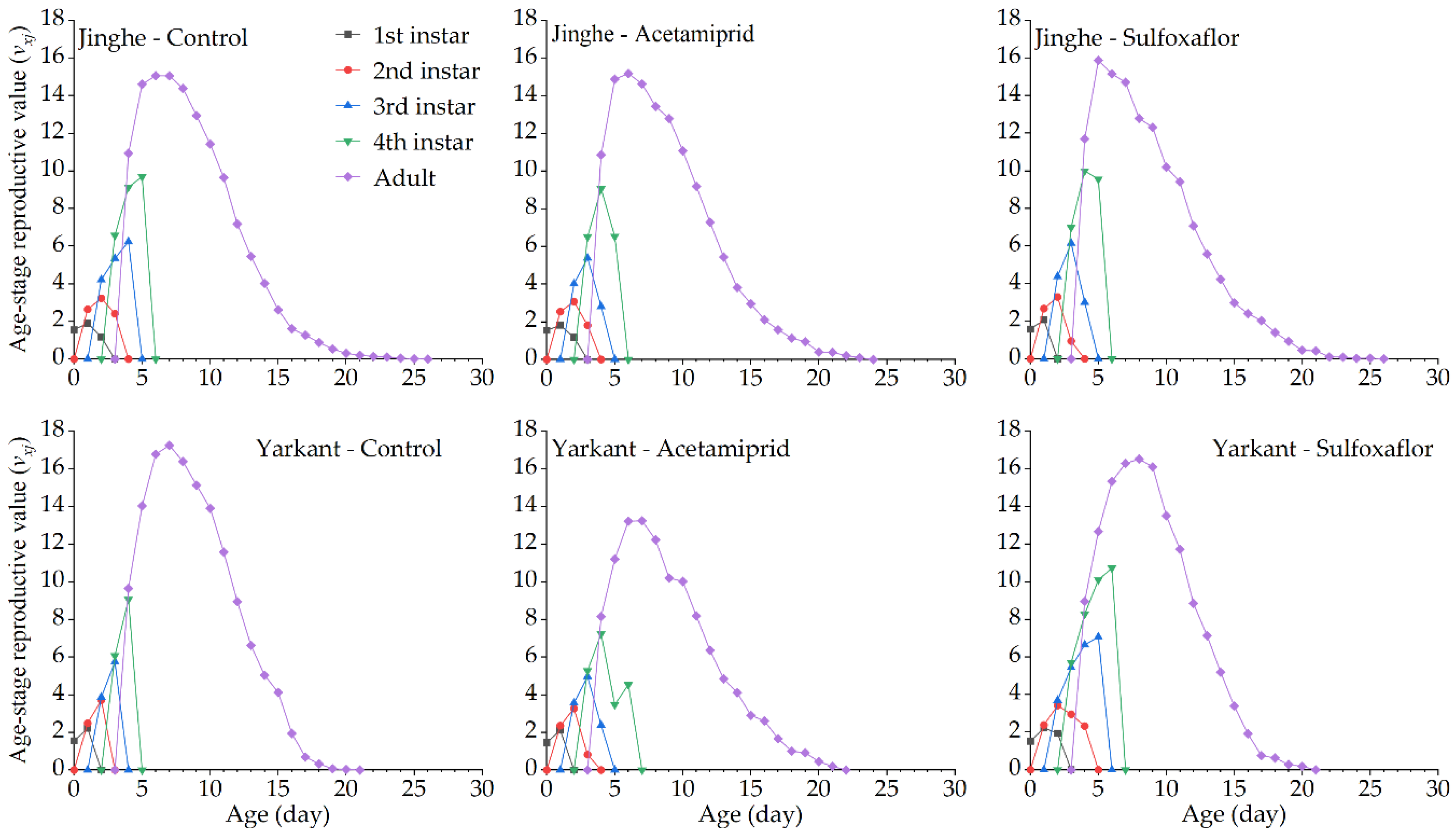
3.2. Sublethal Effects of Acetamiprid on A. Gossypii from Jinghe and Yarkant
3.2.1. Sublethal Effects of Acetamiprid on the F0 Generation
3.2.2. Sublethal Effects of Acetamiprid on the F1 Generation
3.2.3. Sublethal Effects of Acetamiprid on the F2 Generation
3.3. Sublethal Effects of Sulfoxaflor on A. Gossypii from Jinghe and Yarkant
3.3.1. Sublethal Effects of Sulfoxaflor on the F0 Generation
3.3.2. Sublethal Effects of Sulfoxaflor on the F1 Generation
3.3.3. Sublethal Effects of Sulfoxaflor on the F2 Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Hormesis with pesticides. Pest Manag. Sci. 2014, 70, 689. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef]
- Desneux, N.; Ramirez-Romero, R.; Kaiser, L. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 2006, 25, 2675–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceuppens, B.; Eeraerts, M.; Vleugels, T.; Cnops, G.; Roldan-Ruiz, I.; Smagghe, G. Effects of dietary lambda-cyhalothrin exposure on bumblebee survival, reproduction, and foraging behavior in laboratory and greenhouse. J. Pest Sci. 2015, 88, 777–783. [Google Scholar] [CrossRef]
- Ali, E.; Liao, X.; Yang, P.; Mao, K.K.; Zhang, X.L.; Shakeel, M.; Salim, A.M.A.; Wan, H.; Li, J.H. Sublethal Effects of buprofezin on development and reproduction in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Sci. Rep. 2017, 7, 16913. [Google Scholar] [CrossRef]
- Cui, L.; Yuan, H.Z.; Wang, Q.Y.; Wang, Q.Q.; Rui, C.H. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2018, 8, 8915. [Google Scholar] [CrossRef]
- Mohammed, A.A.A.H.; Desneux, N.; Monticelli, L.S.; Fan, Y.J.; Shi, X.Y.; Guedes, R.N.C.; Gao, X.W. Potential for insecticide-mediated shift in ecological dominance between two competing aphid species. Chemosphere 2019, 226, 651–658. [Google Scholar] [CrossRef]
- Brooks, A.C.; Gaskell, P.N.; Maltby, L.L. Sublethal effects and predator-prey interactions: Implications for ecological risk assessment. Environ. Toxicol. Chem. 2009, 28, 2449–2457. [Google Scholar] [CrossRef]
- Santadino, M.; Coviella, C.; Momo, F. Glyphosate sublethal effects on the population dynamics of the earthworm Eisenia fetida (savigny, 1826). Water Air Soil Pollut. 2014, 225, 2207. [Google Scholar] [CrossRef]
- Wang, S.Y.; Qi, Y.F.; Desneux, N.; Shi, X.Y.; Biondi, A.; Gao, X.W. Sublethal and transgenerational effects of short-term and chronic exposures to the neonicotinoid nitenpyram on the cotton aphid Aphis gossypii. J. Pest Sci. 2017, 90, 389–396. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.H.; Li, H.; Chen, C.Y. Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia odoriphaga Yang and Zhang. Pestic. Biochem. Physiol. 2014, 111, 31–37. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Zhao, J.W.; Zheng, Y.; Weng, Q.Y.; Biondi, A.; Desneux, N.; Wu, K.M. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.Y.; Xiao, D.; Li, J.Y.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef]
- Ayyanath, M.M.; Cutler, G.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.W.; Song, D.L. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Sial, M.U.; Zhao, Z.Z.; Zhang, L.; Zhang, Y.N.; Mao, L.G.; Jiang, H.Y. Evaluation of insecticides induced hormesis on the demographic parameters of Myzus persicae and expression changes of metabolic resistance detoxification genes. Sci. Rep. 2018, 8, 16601. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Gao, X.W.; Song, D.L. Imidacloprid-induced hormesis effects on demographic traits of the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 325–337. [Google Scholar] [CrossRef]
- Liao, X.; Ali, E.; Li, W.H.; He, B.Y.; Gong, P.P.; Xu, P.F.; Li, J.H.; Wan, H. Sublethal effects of sulfoxaflor on the development and reproduction of the brown planthopper, Nilaparvata lugens (Stål). Crop Prot. 2019, 118, 6–14. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Tariq, K.; Ali, A.; Gao, X.W.; Song, D.L. Clothianidin-induced sublethal effects and expression changes of vitellogenin and ecdysone receptors genes in the melon aphid Aphis gossypii. Entomol. Gen. 2019, 39, 137–149. [Google Scholar] [CrossRef]
- Lu, Z.B.; Dong, S.; Li, C.; Li, L.L.; Yu, Y.; Yin, S.Y.; Men, X.Y. Sublethal and transgenerational effects of sulfoxaflor on the demography and feeding behaviour of the mirid bug Apolygus lucorum. PLoS ONE 2020, 15, e0232812. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Xiang, M.; Hu, H.M.; An, C.J.; Gao, X.W. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J. Econ. Entomol. 2015, 108, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Fan, J.M.; Chen, J.C.; Gong, Y.J.; Wei, S.J. Sublethal effects of sulfoxaflor on the growth and reproduction of the green peach aphid Myzus persicae. Sci. Agric. Sin. 2017, 50, 496–503. [Google Scholar] [CrossRef]
- Cho, S.R.; Koo, H.N.; Yoon, C.; Kim, G.H. Sublethal effects of flonicamid and thiamethoxam on green peach aphid, Myzus persicae and feeding behavior analysis. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 889–898. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, L.L.; Yang, F.; Li, M.; Liu, X.M.; Lei, C.L.; Si, S.Y. Sublethal effects of thiamethoxam on the demographic parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1750–1754. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.N.; An, J.J.; Park, S.E.; Kim, J.I.; Kim, G.H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Patima, W.; Guo, P.P.; Ma, S.J.; Gao, X.W.; Zhang, L.J.; Zhang, S.; Ma, D.Y. Resistance of different field populations of Aphis gossypii to ten insecticides in Xinjiang. Plant Prot. 2019, 45, 273–278. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.X.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, G.B.; Loso, M.R.; Babcock, J.M.; Hasler, J.M.; Letherer, T.J.; Young, C.D.; Zhu, Y.M.; Casida, J.E.; Sparks, T.C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 2011, 41, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Tang, Q.L.; Xia, J.; Lü, N.N.; Gao, X.W. Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 158, 40–46. [Google Scholar] [CrossRef]
- Liao, X.; Mao, K.K.; Ali, E.; Jin, R.H.; Li, Z.; Li, W.H.; Li, J.H.; Wan, H. Inheritance and fitness costs of sulfoxaflor resistance in Nilaparvata lugens (Stål). Pest Manag. Sci. 2019, 75, 2981–2988. [Google Scholar] [CrossRef]
- Liao, X.; Mao, K.K.; Ali, E.; Zhang, X.L.; Wan, H.; Li, J.H. Temporal variability and resistance correlation of sulfoxaflor susceptibility among chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Prot. 2017, 102, 141–146. [Google Scholar] [CrossRef]
- An, J.J.; Gao, Z.L.; Dang, Z.H.; Yan, X.; Pan, W.L.; Li, Y.F. Development and risk assessment of resistance to sulfoxaflor in cotton aphid (Aphis gossypii). J. Hebei Agric. Univ. 2020, 43, 76–81. [Google Scholar] [CrossRef]
- Li, R.; Liang, P.Z.; Cheng, S.H.; Xue, H.; Guo, T.F.; Lü, N.N.; Liang, P.; Xie, X.P.; Gao, X.W. Determination of resistance and cross-resistance to imidacloprid and sulfoxaflor in field populations of Aphis gossypii in China. J. Plant Prot. 2021, 48, 1104–1113. [Google Scholar] [CrossRef]
- Chen, X.W.; Ma, K.S.; Li, F.; Liang, P.Z.; Liu, Y.; Guo, T.F.; Song, D.L.; Desneux, N.; Gao, X.W. Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 2016, 25, 1841–1848. [Google Scholar] [CrossRef]
- Moores, G.D.; Gao, X.W.; Denholm, I.; Devonshire, A.L. Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii Glover (Homoptera: Aphidida). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Chen, A.Q.; Ma, K.S.; Liang, P.Z.; Liu, Y.; Song, D.L.; Gao, X.W. Both point mutations and low expression levels of the nicotinic acetylcholine receptor β1 subunit are associated with imidacloprid resistance in an Aphis gossypii (Glover) population from a Bt cotton field in China. Pestic. Biochem. Physiol. 2017, 141, 1–8. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, C.R.; Shang, Z.Y.; Yu, Q.T.; Xue, C.B. Insecticide resistance and resistance mechanisms in the melon aphid, Aphis gossypii, in shandong, China. Pestic. Biochem. Physiol. 2021, 172, 104768. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, E.E.; Schleicher, S.; Buchges, A.; Schmidt, J.; Kloppenburg, P.; Salgado, V.L. Desensitization of nicotinic acetylcholine receptors in central nervous system of the stick insect (Carausius morosus) by imidacloprid and sulfoximine insecticides. Insect Biochem. Mol. Biol. 2011, 41, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Desneux, N.; Qu, Y.Y.; Xiao, X.; Khattak, A.M.; Gao, X.W.; Song, D.L. Acetamiprid-induced hormetic effects and vitellogenin gene (Vg) expression in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 259–270. [Google Scholar] [CrossRef]
- Shang, J.; Yao, Y.S.; Zhu, X.Z.; Wang, L.; Li, D.Y.; Zhang, K.X.; Gao, X.K.; Wu, C.C.; Niu, L.; Ji, J.C.; et al. Evaluation of sublethal and transgenerational effects of sulfoxaflor on Aphis gossypii via life table parameters and 16S rRNA sequencing. Pest Manag. Sci. 2021, 77, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Kindlmann, P.; Dixon, A.F.G. Developmental constraints in the evolution of reproductive strategies: Telescoping of generations in parthenogenetic aphids. Funct. Ecol. 1989, 3, 531–537. [Google Scholar] [CrossRef]
- Jin, M.; Du, Z.B.; Wu, Y.Q.; Gong, Z.J.; Jiang, Y.L.; Duan, Y.; Li, T.; Lei, C.L. Sub-lethal effects of four neonicotinoid seed treatments on the demography and feeding behaviour of the wheat aphid Sitobion avenae. Pest Manag. Sci. 2014, 70, 55–59. [Google Scholar] [CrossRef]
- Zhen, C.A.; Miao, L.; Gao, X.W. Sublethal effects of sulfoxaflor on biological characteristics and vitellogenin gene (AlVg) expression in the mirid bug, Apolygus lucorum (Meyer-Dür). Pestic. Biochem. Physiol. 2018, 144, 57–63. [Google Scholar] [CrossRef]
- Valmorbida, I.; Coates, B.S.; Hodgson, E.W.; Ryan, M.; O’Neal, M.E. Evidence of enhanced reproductive performance and lack-of-fitness costs among soybean aphids, Aphis glycines, with varying levels of pyrethroid resistance. Pest Manag. Sci. 2022, 78, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, D.B.; Guedes, R.N.C.; Peternelli, L.A. Developmental rates and population growth of insecticide-resistant and susceptible populations of Sitophilus zeamais. J. Stored Prod. Res. 2005, 41, 271–281. [Google Scholar] [CrossRef]
- Oliveira, E.E.; Guedes, R.N.C.; Totola, M.R.; De Marco, P. Competition between insecticide-susceptible and -resistant populations of the maize weevil, Sitophilus zeamais. Chemosphere 2007, 69, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, Y.; Min, H.; Yu, X.Q.; Li, N.; Rui, C.H.; Gao, X.W. Insecticide resistance monitoring and management demonstration of major insect pests in the main cotton-growing areas of northern China. Acta Entomol. Sin. 2016, 59, 1238–1245. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.F.; Liu, H.Y.; Tian, J.; Shelton, A.M.; Yao, J. Use of safflower as a trap crop for managing the mirid bug, Lygus pratensis Linnaeus (Hemiptera: Miridae), in cotton fields. Pest Manag. Sci. 2021, 77, 1829–1838. [Google Scholar] [CrossRef]
- Wang, W.; Yao, J.; Li, H.B.; Zhang, Y.; Wang, D.; Ma, G.L. Comparative study on conservation of natural enemy in cotton field by different rapes in Xinjiang. Plant Prot. 2011, 37, 142–145. [Google Scholar]

| Insecticide | Region | Slope ± SE a | LC25 mg·L−1 (95%CI) b | LC50 mg·L−1 (95%CI) | RR c | χ2 (df) | p |
|---|---|---|---|---|---|---|---|
| Sulfoxaflor | Yarkant | 1.51 ± 0.12 | 1.22 (0.86–1.63) | 3.42 (2.65–4.35) | 1 | 14.35 (13) | 0.35 |
| Jinghe | 1.02 ± 0.10 | 2.44 (1.47–3.61) | 11.16 (8.03–15.38) | 3.26 | 9.55 (13) | 0.73 | |
| Acetamiprid | Yarkant | 2.24 ± 0.18 | 2.83 (2.19–3.50) | 5.66 (4.63–6.90) | 1 | 15.48 (13) | 0.28 |
| Jinghe | 1.50 ± 0.14 | 12.77 (8.71–16.99) | 35.93 (28.65–43.81) | 6.35 | 13.30 (16) | 0.65 |
| Stages | F1 Generation | F2 Generation | ||||||
|---|---|---|---|---|---|---|---|---|
| Jinghe | Yarkant | Jinghe | Yarkant | |||||
| Control | Acetamiprid | Control | Acetamiprid | Control | Acetamiprid | Control | Acetamiprid | |
| Pre-adult (days) | 5.10 ± 0.07 a | 5.23 ± 0.09 a | 5.01 ± 0.06 a | 5.15 ± 0.08 a | 4.59 ± 0.06 a | 4.53 ± 0.06 a | 4.73 ± 0.05 a | 4.85 ± 0.05 a |
| Adult (days) | 16.51 ± 0.96 a | 17.54 ± 0.90 a | 13.33 ± 0.67 a | 10.87 ± 0.50 b | 20.11 ± 0.83 a | 18.08 ± 0.88 a | 13.31 ± 0.47 a | 9.80 ± 0.55 b |
| APOP (days) | 0.26 ± 0.05 a | 0.40 ± 0.05 a | 0.26 ± 0.05 a | 0.26 ±0.05 a | 0.22 ± 0.05 a | 0.24 ± 0.05 a | 0.34 ± 0.05 a | 0.35 ± 0.05 a |
| TPOP (days) | 5.36 ± 0.07 b | 5.62 ± 0.09 a | 5.29 ± 0.08 a | 5.41 ± 0.08 a | 4.81 ± 0.06 a | 4.77 ± 0.06 a | 5.07 ± 0.04 a | 5.18 ± 0.04 a |
| Oviposition days | 9.71 ± 0.47 a | 10.94 ± 0.42 a | 9.37 ± 0.37 a | 8.85 ± 0.42 a | 11.20 ± 0.34 a | 10.77 ± 0.46 a | 10.29 ± 0.23 a | 7.92 ± 0.41 b |
| Total longevity (days) | 21.61 ± 0.95 a | 22.77 ± 0.89 a | 18.34 ± 0.66 a | 16.02 ± 0.49 b | 24.70 ± 0.83 a | 22.61 ± 0.87 a | 18.04 ± 0.47 a | 14.65 ± 0.55 b |
| Fecundity (offspring/individual) | 36.86 ± 2.05 a | 41.93 ± 1.71 a | 44.44 ± 2.42 a | 36.68 ± 2.21 b | 49.35 ± 1.56 a | 47.45 ± 2.07 a | 53.42 ± 1.29 a | 33.40 ± 1.90 b |
| Parameters | ||||||||
| r (d−1) | 0.363 ± 0.008 a | 0.350 ± 0.008 a | 0.396 ± 0.007 a | 0.363 ± 0.008 b | 0.441 ± 0.006 a | 0.438 ± 0.007 a | 0.436 ± 0.004 a | 0.378 ± 0.008 b |
| λ (d−1) | 1.438 ± 0.011 a | 1.419 ± 0.011 a | 1.486 ± 0.011 a | 1.437 ± 0.012 b | 1.554 ± 0.010 a | 1.549 ± 0.016 a | 1.547 ± 0.007a | 1.459 ± 0.001 b |
| R0 (offspring/individual) | 31.700 ± 2.180 a | 33.960 ± 2.153 a | 40.434 ± 2.532 a | 31.278 ± 2.299 b | 44.912 ± 2.011 a | 41.756 ± 2.378 a | 51.280 ± 1.623 a | 28.388 ± 2.005 b |
| T (days) | 9.522 ± 0.122 b | 10.066 ± 0.130 a | 9.337 ± 0.095 a | 9.493 ± 0.105 a | 8.633 ± 0.081 a | 8.526 ± 0.075 a | 9.025 ± 0.073 a | 8.850 ± 0.098 a |
| GRR (offspring/individual) | 44.336 ± 1.720 a | 47.789 ± 1.261 a | 51.810 ± 1.999 a | 46.969 ± 1.954 a | 53.795 ± 1.225 a | 53.526 ± 1.457 a | 58.341 ± 1.099 a | 44.925 ± 1.970 b |
| Stages | F1 Generation | F2 Generation | ||||||
|---|---|---|---|---|---|---|---|---|
| Jinghe | Yarkant | Jinghe | Yarkant | |||||
| Control | Sulfoxaflor | Control | Sulfoxaflor | Control | Sulfoxaflor | Control | Sulfoxaflor | |
| Pre-adult (days) | 5.10 ± 0.07 a | 5.17 ± 0.08 a | 5.01 ± 0.06 a | 5.04 ± 0.06 a | 4.59 ± 0.06 a | 4.56 ± 0.05 a | 4.73 ± 0.05 b | 4.89 ± 0.05 a |
| Adult (days) | 16.51 ± 0.96 a | 17.57 ± 1.01 a | 13.33 ± 0.67 b | 16.21 ± 0.63 a | 20.11 ± 0.83 a | 18.33 ± 0.96 a | 13.31 ± 0.47 a | 13.01 ± 0.56 a |
| APOP (days) | 0.26 ± 0.05 a | 0.32 ± 0.06 a | 0.26 ± 0.05 a | 0.33 ± 0.06 a | 0.22 ± 0.05 a | 0.21 ± 0.04 a | 0.34 ± 0.05 a | 0.26 ± 0.05 a |
| TPOP (days) | 5.36 ± 0.07 a | 5.48 ± 0.09 a | 5.29 ± 0.08 a | 5.36 ± 0.06 a | 4.81 ± 0.06 a | 4.78 ± 0.05 a | 5.07 ± 0.04 a | 5.14 ± 0.05 a |
| Oviposition days | 9.71 ± 0.47 a | 10.56 ± 0.45 a | 9.37 ± 0.37 b | 11.19 ± 0.33 a | 11.20 ± 0.34 a | 10.64 ± 0.44 a | 10.29 ± 0.23 a | 9.61 ± 0.31 a |
| Total longevity (days) | 21.61 ± 0.95 a | 22.74 ± 1.00 a | 18.34 ± 0.66 b | 21.25 ± 0.62 a | 24.70 ± 0.83 a | 22.89 ± 0.94 a | 18.04 ± 0.47 a | 17.90 ± 0.56 a |
| Fecundity (offspring/individual) | 36.86 ± 2.05 a | 41.19 ± 1.84 a | 44.44 ± 2.42 b | 51.48 ± 1.99 a | 49.35 ± 1.56 a | 49.45 ± 2.12 a | 53.42 ± 1.29 a | 48.58 ± 1.84 b |
| Parameters | ||||||||
| r (d−1) | 0.363 ± 0.008 a | 0.364 ± 0.009 a | 0.396 ± 0.007 a | 0.380 ± 0.006 a | 0.441 ± 0.006 b | 0.462 ± 0.008 a | 0.436 ± 0.004 a | 0.418 ± 0.005 b |
| λ (d−1) | 1.438 ± 0.011 a | 1.440 ± 0.013 a | 1.486 ± 0.011 a | 1.462 ± 0.009 a | 1.554 ± 0.010 b | 1.587 ± 0.013 a | 1.547 ± 0.007a | 1.519 ± 0.007 b |
| R0 (offspring/individual) | 31.700 ± 2.180 a | 33.630 ± 2.202 a | 40.434 ± 2.532 a | 45.294 ± 2.420 a | 44.912 ± 2.011 a | 46.481 ± 2.303 a | 51.280 ± 1.623 a | 47.120 ± 1.965 a |
| T (days) | 9.522 ± 0.122 a | 9.624 ± 0.147 a | 9.337 ± 0.095 b | 10.034 ± 0.073 a | 8.633 ± 0.081 a | 8.312 ± 0.092 b | 9.025 ± 0.073 a | 9.221 ± 0.070 a |
| GRR (offspring/individual) | 44.336 ± 1.720 a | 47.106 ± 1.330 a | 51.810 ± 1.999 a | 55.568 ± 1.380 a | 53.795 ± 1.225 a | 56.472 ± 1.707 a | 58.341 ± 1.099 a | 55.609 ± 1.346 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Huang, Q.; Liu, X.; Liang, G. Differences in the Sublethal Effects of Sulfoxaflor and Acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) Are Related to Its Basic Sensitivity Level. Insects 2022, 13, 498. https://doi.org/10.3390/insects13060498
Wang W, Huang Q, Liu X, Liang G. Differences in the Sublethal Effects of Sulfoxaflor and Acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) Are Related to Its Basic Sensitivity Level. Insects. 2022; 13(6):498. https://doi.org/10.3390/insects13060498
Chicago/Turabian StyleWang, Wei, Qiushi Huang, Xiaoxia Liu, and Gemei Liang. 2022. "Differences in the Sublethal Effects of Sulfoxaflor and Acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) Are Related to Its Basic Sensitivity Level" Insects 13, no. 6: 498. https://doi.org/10.3390/insects13060498





