What Are the Best Pollinator Candidates for Camellia oleifera: Do Not Forget Hoverflies and Flies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Sites
2.2. Identification of Flower Visitors
2.3. Flower-Visiting Density
2.4. Insect Proportion
2.5. Body Measurements of the Main Pollinator Candidates
2.6. Foraging Behaviours
2.7. Pollen Load Analysis
2.8. Data Analyses
2.8.1. One-Way Analyses of Variance
2.8.2. Correlation Analysis
2.8.3. Principal Component Analysis
2.8.4. Determination of Multiple Attributes
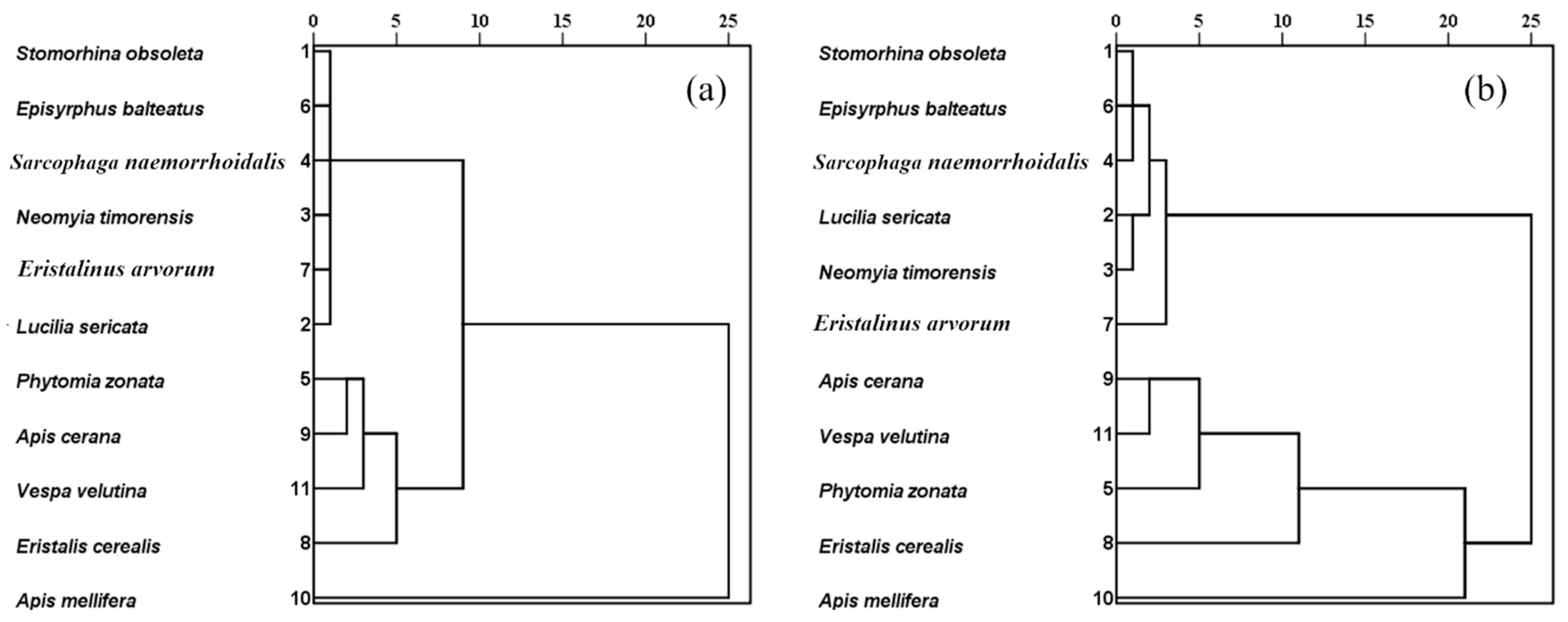
2.8.5. Cluster Analysis
3. Results
3.1. The Diversity and Changes in the Insects Visiting C. oleifera
3.2. Daily Activity of Insects Visiting C. oleifera
3.3. Body Measurements of the Main Visiting Insects
3.4. Determination of the Best Pollinator Candidate
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Rodger, J.G.; Bennett, J.M.; Razanajatovo, M.; Knight, T.M.; Kleunen, M.v.; Ashman, T.; Steets, J.A.; Hui, C.; Arceo-Gómez, G.; Martin, B.; et al. Widespread vulnerability of flowering plant seed production to pollinator declines. Sci. Adv. 2021, 7, eabd3524. [Google Scholar] [CrossRef]
- Whittall, J.B.; Hodges, S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 2007, 447, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Kaczorowski, R.L.; ArceoGómez, G.; O’Neill, E.M.; Hayes, R.A.; Ashman, T. Pollinators contribute to the maintenance of flowering plant diversity. Nature 2021, 597, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.P.; Sytsma, K.J. Complex interactions underlie the correlated evolution of floral traits and their association with pollinators in a clade with diverse pollination systems. Evol. Int. J. Org. Evol. 2021, 75, 1431–1449. [Google Scholar] [CrossRef] [PubMed]
- Basari, N.; Ramli, S.N.; Abdul-Mutalid, N.A.; Shaipulah, N.F.M.; Hashim, N.A. Flowers morphology and nectar concentration determine the preferred food source of stingless bee, Heterotrigona itama. J. Asia-Pac. Entomol. 2021, 24, 232–236. [Google Scholar] [CrossRef]
- Hale, K.R.S.; Valdovinos, F.S.; Martinez, N.D. Mutualism increases diversity, stability, and function of multiplex networks that integrate pollinators into food webs. Nat. Commun. 2020, 11, 2182. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Mashilingi, S.K.; Zhang, H.; Chen, W.; Vaissière, B.E.; Garibaldi, L.A.; An, J. Temporal Trends in Pollination Deficits and Its Potential Impacts on Chinese Agriculture. J. Econ. Entomol. 2021, 114, 1431–1440. [Google Scholar] [CrossRef]
- Manincor, N.D.; Hautekèete, N.; Mazoyer, C.; Moreau, P.; Piquot, Y.; Schatz, B.; Schmitt, E.; Zélazny, M.; Massol, F. How biased is our perception of plant-pollinator networks? A comparison of visit- and pollen-based representations of the same networks. Acta Oecologica 2020, 105, 103551. [Google Scholar] [CrossRef]
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Willcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef]
- Abdur, R.; Shafqat, S.; Mudssar, A.; Hammad, N.T.M. Comparative Efficiency of Native Insect Pollinators in Reproductive Performance of Medicago sativa L. in Pakistan. Insects 2021, 12, 1029. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Zhu, J.; Jiang, Y.; Huang, Y.; Zhou, Z.; Chen, C.; Yu, F. Manganese accumulation and plant physiology behavior of Camellia oleifera in response to different levels of nitrogen fertilization. Ecotoxicol. Environ. Saf. 2019, 184, 109603. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, F.; Yuan, D.; Tan, X.; Niu, G. Comparison of self- and cross-pollination in pollen tube growth, early ovule development and fruit set of Camellia grijsii. Int. J. Agric. Biol. 2019, 21, 819–826. [Google Scholar] [CrossRef]
- Chao, G.; Yuan, D.; Yang, Y.; Wang, B. Pollen Tube Growth and Double Fertilization in Camellia oleifera. J. Am. Soc. Hortic. Sci. 2015, 140, 12–18. [Google Scholar] [CrossRef]
- Li, H.-Y.; Luo, A.-C.; Hao, Y.-J.; Dou, F.-Y.; Kou, R.-M.; Orr, M.C.; Zhu, C.-D.; Huang, D.-Y. Comparison of the pollination efficiency of Apis cerana with wild bees in oil-seed camellia fields. Basic Appl. Ecol. 2021, 56, 250–258. [Google Scholar] [CrossRef]
- Su, R.; Dong, Y.; Dong, K.; He, S. The toxic honey plant Camellia oleifera. J. Apic. Res. 2012, 51, 277–279. [Google Scholar] [CrossRef]
- Huang, D.Y.; Hao, J.S.; Yu, J.F.; Zhang, Y.Z.; Zhu, C.D. Review on Camellia oleifera. Life Sci. Res. 2009, 13, 459–465. [Google Scholar] [CrossRef]
- Wu, T.; Tang, J.; Huang, S.Q. Foraging behavior and pollination efficiency of generalist insects in an understory dioecious shrub Helwingia japonica. Am. J. Bot. 2020, 107, 1274–1282. [Google Scholar] [CrossRef]
- Wei, W.; Wu, H.; Li, X.; Wei, X.; Lu, W.; Zheng, X. Diversity, Daily Activity Patterns, and Pollination Effectiveness of the Insects Visiting Camellia osmantha, C. vietnamensis, and C. oleifera in South China. Insects 2019, 10, 98. [Google Scholar] [CrossRef]
- Sugiura, N. Consistent pollination services to Cypripedium macranthos var. rebunense (Orchidaceae) by Bombus pseudobaicalensis. Plant Species Biol. 2019, 34, 38–42. [Google Scholar] [CrossRef]
- Sutyemez, M. Pollen quality, quantity and fruit set of some self-compatible and self-incompatible cherry cultivars with artificial pollination. Afr. J. Biotechnol. 2013, 10, 3380–3386. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Zhao, Y.; Yang, J. Analysis of the Correlation and Regional Distribution of Plastic Waste Pollution. E3S Web Conf. 2021, 241, 03004. [Google Scholar] [CrossRef]
- Seid, E.; Mohammed, W.; Abebe, T. Genetic Diversity Assessment through Cluster and Principal Component Analysis in Potato (Solanum tuberosum L.) Genotypes for Processing Traits. Int. J. Food Sci. Agric. 2021, 5, 440–447. [Google Scholar] [CrossRef]
- Musabikha, S.; Pratikno, H.; Utama, I.K.A.P. Material Selection for Vertical Axis Tidal Current Turbine using Multiple Attribute Decision Making (MADM). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1158, 012001. [Google Scholar] [CrossRef]
- Qian, R.; Li, H.; Hua, Z.-Y.; Yuan, Y.; Xie, D.-M. Quantitative taxonomic study on agronomic traits of cultivated Gastrodia elata. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2020, 45, 3085–3090. [Google Scholar] [CrossRef]
- Raguso, R.A. Don’t forget the flies: Dipteran diversity and its consequences for floral ecology and evolution. Appl. Entomol. Zool. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Martin, E.A.; Feit, B.; Requier, F.; Friberg, H.; Jonsson, M. Assessing the resilience of biodiversity-driven functions in agroecosystems under environmental change. Adv. Ecol. Res. 2019, 60, 59–123. [Google Scholar] [CrossRef]
- Weise, H.; Auge, H.; Baessler, C.; Bennett, E.M.; Berger, U.; Bohn, F.; Bonn, A.; Borchardt, D.; Brand, F.; Chatzinotas, A. Resilience trinity: Safeguarding ecosystem functioning and services across three different time horizons and decision contexts. Oikos 2020, 129, 445–456. [Google Scholar] [CrossRef]
- Ji, T.; Wei, M.; Li, Z. Some ideas to solve the problem of Camellia oleifera pollination. South China For. Sci. 2018, 46, 22–25+34. [Google Scholar] [CrossRef]
- Rosenfeld, J.S. Functional Redundancy in Ecology and Conservation. Oikos 2002, 98, 156–162. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J. The Long-Proboscid Fly Pollination System in Southern Africa. Ann. Mo. Bot. Gard. 2000, 87, 146–170. [Google Scholar] [CrossRef]
- Miller, T.J.; Raguso, R.A.; Kay, K.M. Novel adaptation to hawkmoth pollinators in Clarkia reduces efficiency, not attraction of diurnal visitors. Ann. Bot. 2014, 113, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Moerkens, R.; Boonen, S.; Wckers, F.L.; Pekas, A. Aphidophagous hoverflies reduce foxglove aphid infestations and improve seed set and fruit yield in sweet pepper. Pest Manag. Sci. 2021, 77, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Rader, R.; Edwards, W.; Westcott, D.A.; Cunningham, S.A.; Howlett, B.G. Pollen transport differs among bees and flies in a human-modified landscape. Divers. Distrib. 2011, 17, 519–529. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Fenster, C.B.; Martén-Rodriguez, S.; Schemske, D.W. Pollination Syndromes and the Evolution of Floral Diversity in Iochroma (Solanaceae). Evolution 2009, 63, 2758–2762. [Google Scholar] [CrossRef]
- Dellinger, A.S. Pollination syndromes in the 21st century: Where do we stand and where may we go? New Phytol. 2020, 228, 1193–1213. [Google Scholar] [CrossRef]
- Ollerton, J.; Alarcón, R.; Waser, N.M.; Price, M.V.; Watts, S.; Cranmer, L.; Hingston, A.; Peter, C.I.; Rotenberry, J. A global test of the pollination syndrome hypothesis. Ann. Bot. 2009, 103, 1471–1480. [Google Scholar] [CrossRef]
- Murúa, M.; Espíndola, A. Pollination syndromes in a specialised plant-pollinator interaction: Does floral morphology predict pollinators in Calceolaria? Plant Biol. 2015, 17, 551–557. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, J.-J.; Huang, C.-G.; Yuan, J.-K.; Zhang, Y.; Luo, S.; Fan, X.-M. Observation on the Morphology and Development of Normal and Pseudopollenof Camellia oleifera. Hans J. Agric. Sci. 2022, 12, 170–177. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, D.-Y.; Li, Y.-M.; Zhang, J.; Hu, G.-X.; Zou, F. Floral morphological and breeding characteristics of the F1 generation of Camellia. J. Fujian Agric. For. Univ. 2020, 49, 440–446. [Google Scholar] [CrossRef]



| No. | Location | Latitude and Longitude | Basic Situation | Sampling Methods |
|---|---|---|---|---|
| 1 | Tianxin District | 28°8′14″ N, 112°59′08″ E | Only small trees, many varieties, well-managed. | Trees in the east, west, south, north, and middle of the areas were selected. |
| 2 | Yuelu District | 28°13′48″ N, 112°55′53″ E | Neat arrangement of trees but low planting density. Predominance of young C. oleifera trees. | One row each in the upper, middle, and lower parts of the terrace was selected; trees in the left, right, and centre of each row were targeted. |
| 3 | Wangcheng District | 28°22′12″ N, 112°49′12″ E | Covers a large area; located far away from urban areas; many tall trees. | Eight plots of 10 m × 10 m were set up; trees in the east, west, south, north, and middle were selected. |
| NO. | Order | Family | Species | Proportion | ||
|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | ||||
| A | Diptera | Calliphoridae | Stomorhina obsoleta | 12.14% | 9.74% | 0.19% |
| B | Diptera | Calliphoridae | Lucilia sericata | 5.00% | 4.62% | 1.81% |
| C | Diptera | Muscidae | Neomyia timorensis | 4.29% | 12.31% | 8.53% |
| D | Diptera | Sarcophaga | Sarcophaga haemorrhoidalis | 11.43% | 4.10% | 0.56% |
| E | Diptera | Syrphidae | Phytomia zonata | 11.43% | 8.72% | 1.72% |
| F | Diptera | Syrphidae | Episyrphus balteatus | 1.43% | 6.67% | 8.72% |
| G | Diptera | Syrphidae | Eristalinus arvorum | 8.57% | 9.74% | 1.39% |
| H | Diptera | Syrphidae | Eristalis cerealis | 2.86% | 3.08% | 5.98% |
| I | Hymenoptera | Apidae | Apis cerana | 2.14% | 5.13% | 3.20% |
| J | Hymenoptera | Apidae | Apis mellifera | 3.57% | 33.33% | 66.06% |
| K | Hymenoptera | Vespidae | Vespa mandarinia | 0.00% | 1.03% | 0.46% |
| L | Hymenoptera | Vespidae | Vespa velutina | 37.14% | 1.54% | 1.39% |
| Categories | Family | Species | Proportion | ||
|---|---|---|---|---|---|
| First Half of December | Second Half of December | Volatility | |||
| Flies | Calliphoridae | Stomorhina obsoleta | 12.14% | 20.60% | 8.46% |
| Lucilia sericata | 5.00% | 1.01% | −3.99% | ||
| Muscidae | Neomyia timorensis | 4.29% | 8.04% | 3.75% | |
| Sarcophagidae | Sarcophaga naemorrhoidalis | 11.43% | 4.52% | −6.91% | |
| Hoverflies | Syrphidae | Phytomia zonata | 11.43% | 15.08% | 3.65% |
| Episyrphus balteatus | 1.43% | 10.05% | 8.62% | ||
| Eristalinus arvorum | 8.57% | 4.52% | −4.05% | ||
| Eristalis cerealis | 2.86% | 3.02% | 0.16% | ||
| Bees | Apidae | Apis cerana | 2.14% | 4.02% | 1.88% |
| Apis mellifera | 3.57% | 22.61% | 19.04% | ||
| Wasps | Vespidae | Vespa velutina | 37.14% | 6.53% | −30.61% |
| Categories | Species | Time Visiting Each Flower | Single Flower Visit Frequency (times/min) | Main Foraging Behaviours | |||
|---|---|---|---|---|---|---|---|
| Shortest | Longest | Lowest | Highest | Average Frequency | |||
| Flies | Stomorhina obsoleta | 9 | 96 | 1 | 5 | 2.17 ± 1.24 c | Spent more than half of their visit on the anthers but touched the stigma less. |
| Lucilia sericata | 16 | 22 | 2 | 4 | 3.00 ± 0.77 abc | ||
| Neomyia timorensis | 8 | 160 | 1 | 5 | 2.25 ± 1.16 c | ||
| Sarcophaga naemorrhoidalis | 8 | 54 | 1 | 6 | 2.69 ± 1.38 bc | ||
| Hoverflies | Phytomia zonata | 3 | 270 | 1 | 5 | 2.71 ± 1.28 bc | More active, and more than half of them touched the stigma. |
| Episyrphus balteatus | 7 | 21 | 1 | 2 | 1.33 ± 0.47 c | Mainly inspected the flowers and stayed for a short time. | |
| Eristalinus arvorum | 5 | 78 | 1 | 6 | 2.71 ± 1.28 bc | Sometimes rested on flowers temporarily without any activity. | |
| Eristalis cerealis | 5 | 34 | 1 | 3 | 2.67 ± 0.60 bc | Took a short time to forage but touched the stigma almost every time. | |
| Bees | Apis cerana | 3 | 46 | 1 | 3 | 2.33 ± 0.58 bc | Visiting time was short, and the enthusiasm for visiting flowers is low. |
| Apis mellifera | 2 | 148 | 1 | 7 | 4.06 ± 1.61 ab | Frequent flower visits; actively collected pollen and made contact with the stigma almost every time. | |
| Wasps | Vespa velutina | 2 | 127 | 1 | 6 | 4.47 ± 1.27 a | Actively visited the flower and touched the stigma almost every time. |
| Categories | Species | Pollen Load (Grain/Individual) | Main Powder-Carrying Position | |||
|---|---|---|---|---|---|---|
| Total Amount of Pollen | Normal Pollen | Pseudopollen | Pseudopollen/Normal Pollen | |||
| Flies | Stomorhina obsoleta | 2320 ± 129.84 d | 1700 ± 169.97 c | 620 ± 220.10 c | 0.38 ± 0.16 ab | Back plate and head |
| Lucilia sericata | 1600 ± 1074.97 d | 1200 ± 1032.80 c | 400 ± 699.21 c | 0.43 ± 0.79 a | ||
| Neomyia timorensis | 2000 ± 608.58 d | 1833 ± 593.17 c | 167 ± 175.68 c | 0.10 ± 0.11 cd | ||
| Sarcophaga naemorrhoidalis | 3188 ± 400.64 d | 2688 ± 512.62 c | 500 ± 238.37 c | 0.21 ± 0.16 abcd | ||
| Hoverflies | Phytomia zonata | 12,667 ± 237.17 c | 11,333 ± 210.82 b | 1500 ± 241.96 b | 0.12 ± 0.09 cd | Body surface and feet |
| Episyrphus balteatus | 733 ± 262.94 d | 600 ± 262.94 c | 133 ± 172.13 c | 0.30 ± 0.42 abc | Back plate and head | |
| Eristalinus arvorum | 3533 ± 688.53 d | 3400 ± 733.67 c | 133 ± 172.13 c | 0.04 ± 0.06 d | Body surface | |
| Eristalis cerealis | 48,800 ± 5391.35 b | 48,200 ± 5202.56 a | 667 ± 699.21 c | 0.01 ± 0.02 d | Villi on the body surface | |
| Bees | Apis cerana | 14,650 ± 1106.80 c | 13,300 ± 948.68 b | 1350 ± 411.64 b | 0.10 ± 0.03 cd | Pollen-carrying legs and villi |
| Apis mellifera | 55,167 ± 6549.81 a | 47,583 ± 6120.09 a | 7500 ± 707.11 a | 0.16 ± 0.02 bcd | ||
| Wasps | Vespa velutina | 13,000 ± 4216.37 c | 11,675 ± 4323.79 b | 1325 ± 373.61 b | 0.13 ± 0.05 cd | Villi on the body surface |
| Categories | Species | Body Length (mm) | Head Width (mm) | Head Length (mm) | Shoulder Length (mm) | Shoulder Width (mm) | Body Surface Characteristics |
|---|---|---|---|---|---|---|---|
| Flies | Stomorhina obsoleta | 6.95 ± 1.17 f | 2.28 ± 0.51 fg | 2.01 ± 0.43 ef | 2.47 ± 0.58 f | 2.17 ± 0.56 h | Body surface bristles |
| Lucilia sericata | 8.99 ± 0.88 e | 3.44 ± 0.33 e | 2.18 ± 0.24 def | 3.62 ± 0.37 de | 3.44 ± 0.42 cd | ||
| Neomyia timorensis | 9.63 ± 0.95 de | 3.58 ± 0.24 de | 2.46 ± 0.60 cde | 3.65 ± 0.41 de | 3.25 ± 0.26 de | ||
| Sarcophaga naemorrhoidalis | 6.60 ± 0.45 f | 2.19 ± 0.35 g | 1.85 ± 0.42 f | 2.66 ± 0.21 f | 2.77 ± 0.36 fg | Bristled, sparse at the back | |
| Hoverflies | Phytomia zonata | 13.49 ± 0.60 b | 4.99 ± 0.18 b | 2.81 ± 0.31 c | 4.82 ± 0.41 b | 5.07 ± 0.36 b | Densely tomentose |
| Episyrphus balteatus | 9.39 ± 0.63 e | 2.57 ± 0.19 f | 2.68 ± 0.41 cd | 2.61 ± 0.14 f | 2.43 ± 0.10 gh | Tomentose on both sides and short hairs on the ventral segment | |
| Eristalinus arvorum | 10.63 ± 1.06 d | 3.88 ± 0.14 cd | 3.57 ± 0.12 b | 3.98 ± 0.27 cd | 3.77 ± 0.38 c | Dorsal plate is tomentose, and the ventral segment is short-haired | |
| Eristalis cerealis | 12.58 ± 1.65 bc | 4.15 ± 0.91 c | 2.76 ± 0.32 c | 4.12 ± 0.56 c | 3.59 ± 1.03 cd | Densely tomentose, and the dorsal plate is particularly dense | |
| Bees | Apis cerana | 12.20 ± 0.73 c | 3.66 ± 0.18 de | 2.91 ± 0.72 c | 3.70 ± 0.31 de | 3.04 ± 0.33 ef | Densely covered with yellow villi, short ventral hairs; pollen-carrying legs |
| Apis mellifera | 13.05 ± 0.65 bc | 3.45 ± 0.24 e | 2.56 ± 0.71 cd | 3.54 ± 0.34 e | 2.97 ± 0.57 ef | ||
| Wasps | Vespa velutina | 20.42 ± 2.48 a | 5.34 ± 0.26 a | 4.33 ± 1.01 a | 6.06 ± 0.47 a | 6.67 ± 0.51 a | Densely tomentose |
| Pollen Load | Body Surface Characteristics | Body Length | Proportion | Visiting Frequency | Visiting Time | |
|---|---|---|---|---|---|---|
| Pollen load | 1.00 | |||||
| Body surface characteristics | 0.74 ** | 1.00 | ||||
| Body length | 0.39 | 0.67 | 1.00 | |||
| Proportion | 0.70 | 0.47 | 0.16 | 1.00 | ||
| Visiting frequency | 0.46 | 0.51 | 0.70 | 0.43 | 1.00 | |
| Visiting time | −0.53 | −0.50 | −0.32 | −0.37 | −0.20 | 1.00 |
| Principal Component | Pollen Load | Body Surface Characteristics | Body Length | Proportion | Visiting Frequency | Eigenvalue | Variance Contribution | Cumulative Contribution Rates |
|---|---|---|---|---|---|---|---|---|
| PC1 | 0.27 | 0.28 | 0.24 | 0.22 | 0.25 | 3.11 | 62.19% | 62.19% |
| PC2 | 0.38 | −0.04 | −0.60 | 0.61 | −0.32 | 1.02 | 20.47% | 82.66% |
| Categories | Species | Pollen Load | Body Surface Characteristics | Body Length | Proportion | Visiting Frequency | PC1 | PC2 | F | Rank |
|---|---|---|---|---|---|---|---|---|---|---|
| Flies | Stomorhina obsoleta | −0.62 | −0.84 | −1.13 | −0.37 | −0.68 | −0.93 | 0.47 | −0.58 | 10 |
| Lucilia sericata | −0.66 | −0.84 | −0.59 | −0.43 | 0.27 | −0.58 | −0.20 | −0.49 | 8 | |
| Neomyia timorensis | −0.64 | −0.84 | −0.43 | −0.03 | −0.59 | −0.67 | 0.22 | −0.45 | 6 | |
| Sarcophaga naemorrhoidalis | −0.58 | −0.84 | −1.22 | −0.46 | −0.09 | −0.81 | 0.30 | −0.53 | 9 | |
| Hoverflies | Phytomia zonata | −0.09 | 0.70 | 0.58 | −0.33 | −0.06 | 0.22 | −0.59 | 0.02 | 5 |
| Episyrphus balteatus | −0.70 | −0.84 | −0.49 | −0.05 | −1.64 | −0.97 | 0.57 | −0.59 | 11 | |
| Eristalinus arvorum | −0.56 | −0.84 | −0.17 | −0.40 | −0.06 | −0.53 | −0.30 | −0.47 | 7 | |
| Eristalis cerealis | 1.78 | 0.70 | 0.34 | −0.23 | −0.11 | 0.68 | 0.34 | 0.60 | 2 | |
| Bees | Apis cerana | 0.02 | 1.47 | 0.24 | −0.35 | −0.50 | 0.27 | −0.26 | 0.14 | 4 |
| Apis mellifera | 2.11 | 1.47 | 0.47 | 2.98 | 1.48 | 2.13 | 1.79 | 2.04 | 1 | |
| Wasps | Vespa velutina | −0.07 | 0.70 | 2.39 | −0.34 | 1.99 | 1.18 | −2.33 | 0.31 | 3 |
| Variables | Importance | Weight |
|---|---|---|
| Pollen load | 5 | 0.3467 |
| Body surface characteristics | 4 | 0.2301 |
| Body length | 3 | 0.1460 |
| Proportion | 2 | 0.0870 |
| Visiting frequency | 1 | 0.0516 |
| Categories | Species | Pollen Load | Body Surface Characteristics | Body Length | Proportion | Visiting Frequency | Total Score | Rank |
|---|---|---|---|---|---|---|---|---|
| Flies | Stomorhina obsoleta | 0.03 | 0.00 | 0.03 | 0.02 | 0.27 | 0.03 | 11 |
| Lucilia sericata | 0.02 | 0.00 | 0.17 | 0.01 | 0.53 | 0.07 | 8 | |
| Neomyia timorensis | 0.02 | 0.00 | 0.22 | 0.12 | 0.29 | 0.07 | 7 | |
| Sarcophaga naemorrhoidalis | 0.05 | 0.00 | 0.00 | 0.00 | 0.43 | 0.05 | 9 | |
| Hoverflies | Phytomia zonata | 0.22 | 0.67 | 0.50 | 0.04 | 0.44 | 0.38 | 5 |
| Episyrphus balteatus | 0.00 | 0.00 | 0.20 | 0.12 | 0.00 | 0.04 | 10 | |
| Eristalinus arvorum | 0.05 | 0.00 | 0.29 | 0.02 | 0.44 | 0.10 | 6 | |
| Eristalis cerealis | 0.88 | 0.67 | 0.43 | 0.07 | 0.42 | 0.65 | 2 | |
| Bees | Apis cerana | 0.26 | 1.00 | 0.41 | 0.03 | 0.32 | 0.46 | 4 |
| Apis mellifera | 1.00 | 1.00 | 0.47 | 1.00 | 0.86 | 0.91 | 1 | |
| Wasps | Vespa velutina | 0.23 | 0.67 | 1.00 | 0.04 | 1.00 | 0.49 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Hu, G.-X.; Zhang, X.-X.; Yuan, J.-K.; Fan, X.-M.; Yuan, D.-Y. What Are the Best Pollinator Candidates for Camellia oleifera: Do Not Forget Hoverflies and Flies. Insects 2022, 13, 539. https://doi.org/10.3390/insects13060539
Yuan B, Hu G-X, Zhang X-X, Yuan J-K, Fan X-M, Yuan D-Y. What Are the Best Pollinator Candidates for Camellia oleifera: Do Not Forget Hoverflies and Flies. Insects. 2022; 13(6):539. https://doi.org/10.3390/insects13060539
Chicago/Turabian StyleYuan, Bin, Guan-Xing Hu, Xiao-Xiao Zhang, Jing-Kun Yuan, Xiao-Ming Fan, and De-Yi Yuan. 2022. "What Are the Best Pollinator Candidates for Camellia oleifera: Do Not Forget Hoverflies and Flies" Insects 13, no. 6: 539. https://doi.org/10.3390/insects13060539
APA StyleYuan, B., Hu, G.-X., Zhang, X.-X., Yuan, J.-K., Fan, X.-M., & Yuan, D.-Y. (2022). What Are the Best Pollinator Candidates for Camellia oleifera: Do Not Forget Hoverflies and Flies. Insects, 13(6), 539. https://doi.org/10.3390/insects13060539






