Does Insect Aversion Lead to Increased Household Pesticide Use?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Participants
2.3. Procedure
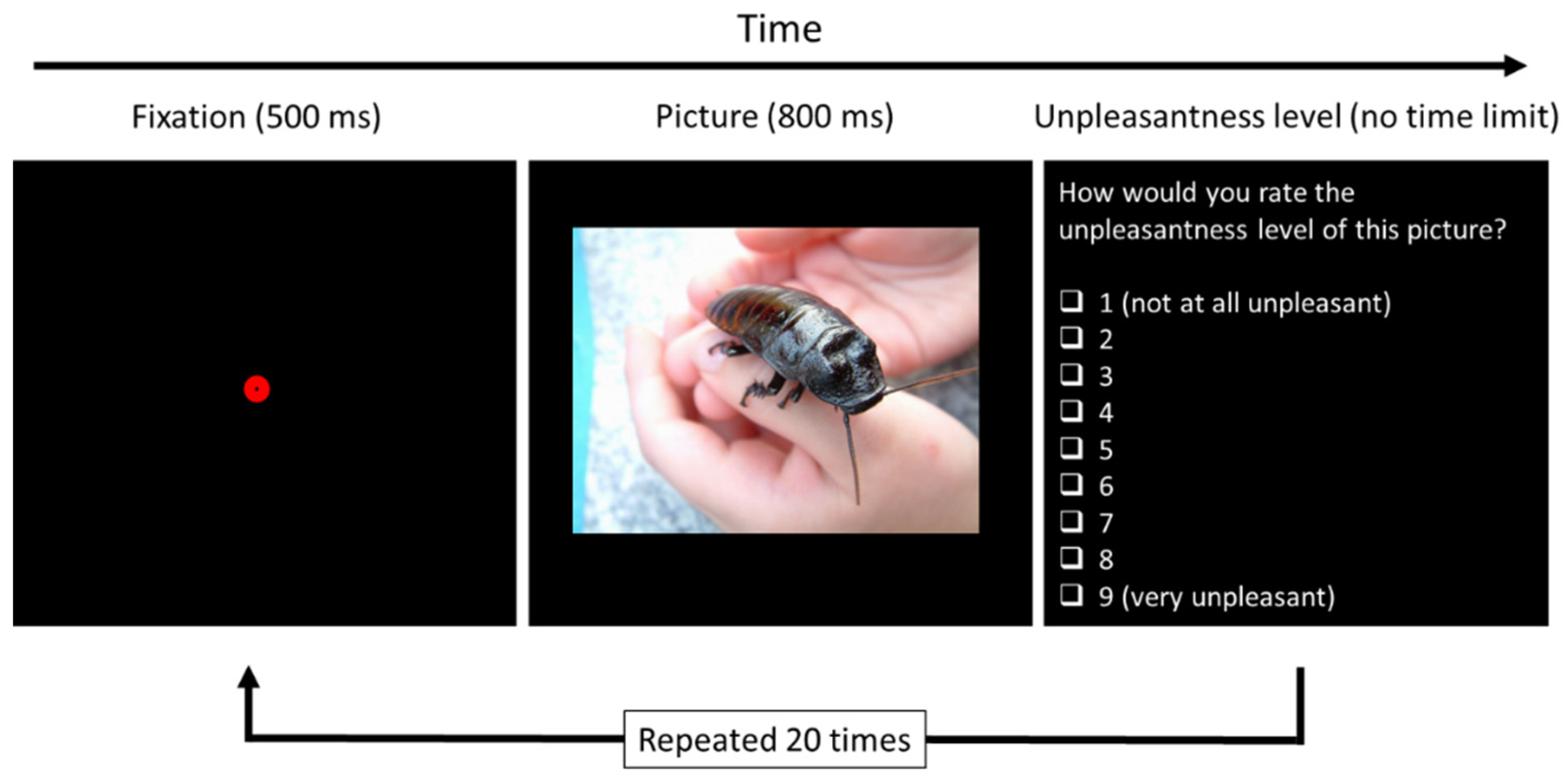
2.4. Computerized Test
2.5. Questionnaire
2.5.1. Questionnaire Section A: Exposure
2.5.2. Questionnaire Section B: Pesticide Use
2.5.3. Questionnaire Section C: Tendency
2.6. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, M.K.; Jacobson, J.B.; McKelvey, W.; Holmes, D.; Fincher, B.; Quantano, A.; Diaz, B.P.; Shabbazz, F.; Shepard, P.; Rundle, A.; et al. Characterization of Residential Pest Control Products Used in Inner City Communities in New York City. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 291–301. [Google Scholar] [CrossRef] [PubMed]
- EPA-Pyrethrins and Pyrethroids. Available online: https://www.epa.gov/ingredients-used-pesticide-products/pyrethrins-and-pyrethroids (accessed on 8 May 2022).
- Power, L.E.; Sudakin, D.L. Pyrethrin and Pyrethroid Exposures in the United States: A Longitudinal Analysis of Incidents Reported to Poison Centers. J. Med. Toxicol. 2007, 3, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlton, E.J.; Moats, H.L.; Feinberg, M.; Shepard, P.; Garfinkel, R.; Whyatt, R.; Evans, D. Pesticide Sales in Low-Income, Minority Neighborhoods. J. Community Health 2004, 29, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.K.; Rundle, A.; Holmes, D.; Reyes, M.; Hoepner, L.A.; Barr, D.B.; Camann, D.E.; Perera, F.P.; Whyatt, R.M. Changes in Pest Infestation Levels, Self-Reported Pesticide Use, and Permethrin Exposure during Pregnancy after the 2000–2001 U.S. Environmental Protection Agency Restriction of Organophosphates. Environ. Health Perspect. 2008, 116, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Rosas, L.G.; Eskenazi, B. Pesticides and Child Neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Mortuza, T.; Chen, C.; White, C.A.; Cummings, B.S.; Muralidhara, S.; Gullick, D.; Bruckner, J. V Toxicokinetics of Deltamethrin: Dosage Dependency, Vehicle Effects, and Low-Dose Age-Equivalent Dosimetry in Rats. Toxicol. Sci. 2018, 162, 327–336. [Google Scholar] [CrossRef]
- Eljarrat, E. Conclusions and Future Trends. In Pyrethroid Insecticides. The Handbook of Environmental Chemistry; Eljarrat, E., Ed.; Springer: Cham, Switzerland, 2020; Volume 92, pp. 305–313. [Google Scholar]
- EPA. USEPA Office of Pesticide Programs’ Re-Evaluation of the FQPA Safety Factor for Pyrethroids: Updated Literature and CAPHRA Program Data Review; 2019. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2008-0331-0084 (accessed on 14 June 2022).
- Viel, J.-F.; Rouget, F.; Warembourg, C.; Monfort, C.; Limon, G.; Cordier, S.; Chevrier, C. Behavioural Disorders in 6-Year-Old Children and Pyrethroid Insecticide Exposure: The PELAGIE Mother-Child Cohort. Occup. Environ. Med. 2017, 74, 275–281. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Household Exposure to Pesticides and Risk of Leukemia in Children and Adolescents: Updated Systematic Review and Meta-Analysis. Int. J. Hyg. Environ. Health 2019, 222, 49–67. [Google Scholar] [CrossRef]
- Perkins, A.; Walters, F.; Sievert, J.; Rhodes, B.; Morrissey, B.; Karr, C.J. Home Use of a Pyrethroid-Containing Pesticide and Facial Paresthesia in a Toddler: A Case Report. Int. J. Environ. Res. Public Health 2016, 13, 829. [Google Scholar] [CrossRef] [Green Version]
- Saillenfait, A.-M.; Ndiaye, D.; Sabaté, J.-P. Pyrethroids: Exposure and Health Effects-an Update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef]
- Chen, S.; Gu, S.; Wang, Y.; Yao, Y.; Wang, G.; Jin, Y.; Wu, Y. Exposure to Pyrethroid Pesticides and the Risk of Childhood Brain Tumors in East China. Environ. Pollut. 2016, 218, 1128–1134. [Google Scholar] [CrossRef]
- Bae, J.-W.; Kwon, W.-S. The Deleterious Toxic Effects of Bifenthrin on Male Fertility. Reprod. Toxicol. 2021, 101, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.-M.; Malard, S. Human Risk Associated with Long-Term Exposure to Pyrethroid Insecticides. In Pyrethroid Insecticides. The Handbook of Environmental Chemistry; Eljarrat, E., Ed.; Springer: Cham, Switzerland, 2020; Volume 92, pp. 259–303. [Google Scholar]
- Bao, W.; Liu, B.; Simonsen, D.W.; Lehmler, H.-J. Association between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern. Med. 2020, 180, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Antoniou, M.N. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, L.E.; do Nascimento, C.M.; Costa, A.R.; Polatto, R.; Papini, S. Persistence of Indoor Permethrin and Estimation of Dermal and Non-Dietary Exposure. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, L.; Mysz, A.; Gibb Snyder, E.; Wyrzykowska-Ceradini, B.; Nardin, J.; Tabor, D.; Starr, J.; Stout, D.; Lemieux, P. Remediating Indoor Pesticide Contamination from Improper Pest Control Treatments: Persistence and Decontamination Studies. J. Hazard. Mater. 2020, 397, 122743. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, L.E.; Costa, A.R.; Polatto, R.; do Nascimento, C.M.; Papini, S. Pyrethroid Concentrations and Persistence Following Indoor Application. Environ. Toxicol. Chem. 2017, 36, 2895–2898. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Huang, F.-J.; Yang, Y.-Q.; Hsieh, C.-J.; Tseng, C.-C.; Yiin, L.-M. Pesticides in Indoor and Outdoor Residential Dust: A Pilot Study in a Rural County of Taiwan. Environ. Sci. Pollut. Res. 2018, 25, 23349–23356. [Google Scholar] [CrossRef]
- Mahler, B.J.; Van Metre, P.C.; Wilson, J.T.; Musgrove, M.; Zaugg, S.D.; Burkhardt, M.R. Fipronil and Its Degradates in Indoor and Outdoor Dust. Environ. Sci. Technol. 2009, 43, 5665–5670. [Google Scholar] [CrossRef]
- Deziel, N.C.; Colt, J.S.; Kent, E.E.; Gunier, R.B.; Reynolds, P.; Booth, B.; Metayer, C.; Ward, M.H. Associations between Self-Reported Pest Treatments and Pesticide Concentrations in Carpet Dust. Environ. Health 2015, 14, 27. [Google Scholar] [CrossRef] [Green Version]
- Stout II, D.M.; Bradham, K.D.; Egeghy, P.P.; Jones, P.A.; Croghan, C.W.; Ashley, P.A.; Pinzer, E.; Friedman, W.; Brinkman, M.C.; Nishioka, M.G.; et al. American Healthy Homes Survey: A National Study of Residential Pesticides Measured from Floor Wipes. Environ. Sci. Technol. 2009, 43, 4294–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glorennec, P.; Serrano, T.; Fravallo, M.; Warembourg, C.; Monfort, C.; Cordier, S.; Viel, J.F.; Le Gléau, F.; Le Bot, B.; Chevrier, C. Determinants of Children’s Exposure to Pyrethroid Insecticides in Western France. Environ. Int. 2017, 104, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Reif, R.; Luo, Y.; Gan, J. Distribution of Pesticides in Dust Particles in Urban Environments. Environ. Pollut. 2016, 214, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Darney, K.; Bodin, L.; Bouchard, M.; Côté, J.; Volatier, J.-L.; Desvignes, V. Aggregate Exposure of the Adult French Population to Pyrethroids. Toxicol. Appl. Pharmacol. 2018, 351, 21–31. [Google Scholar] [CrossRef]
- Bekarian, N.; Payne-Sturges, D.; Edmondson, S.; Chism, B.; Woodruff, T.J. Use of Point-of-Sale Data to Track Usage Patterns of Residential Pesticides: Methodology Development. Environ. Health 2006, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Barr, D.B.; Olsson, A.O.; Wong, L.-Y.; Udunka, S.; Baker, S.E.; Whitehead, R.D.; Magsumbol, M.S.; Williams, B.L.; Needham, L.L. Urinary Concentrations of Metabolites of Pyrethroid Insecticides in the General U.S. Population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 2010, 118, 742–748. [Google Scholar] [CrossRef]
- Babina, K.; Dollard, M.; Pilotto, L.; Edwards, J.W. Environmental Exposure to Organophosphorus and Pyrethroid Pesticides in South Australian Preschool Children: A Cross Sectional Study. Environ. Int. 2012, 48, 109–120. [Google Scholar] [CrossRef]
- Quindroit, P.; Crépet, A.; Brochot, C. Estimating Human Exposure to Pyrethroids’ Mixtures from Biomonitoring Data Using Physiologically Based Pharmacokinetic Modeling. Environ. Res. 2021, 192, 110281. [Google Scholar] [CrossRef]
- Norén, E.; Lindh, C.; Rylander, L.; Glynn, A.; Axelsson, J.; Littorin, M.; Faniband, M.; Larsson, E.; Nielsen, C. Concentrations and Temporal Trends in Pesticide Biomarkers in Urine of Swedish Adolescents, 2000–2017. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 756–767. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, W.; Jacobson, J.B.; Kass, D.; Barr, D.B.; Davis, M.; Calafat, A.M.; Aldous, K.M. Population-Based Biomonitoring of Exposure to Organophosphate and Pyrethroid Pesticides in New York City. Environ. Health Perspect. 2013, 121, 1349–1356. [Google Scholar] [CrossRef]
- Fortin, M.-C.; Bouchard, M.; Carrier, G.; Dumas, P. Biological Monitoring of Exposure to Pyrethrins and Pyrethroids in a Metropolitan Population of the Province of Quebec, Canada. Environ. Res. 2008, 107, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables; Atlanta, GA, USA, 2021. Available online: https://ecologycenter.org/wp-content/uploads/2021/04/FourthReport_UpdatedTables_Volume2_Mar2021-508.pdf (accessed on 14 June 2022).
- Schoelitsz, B.; Meerburg, B.G.; Takken, W. Influence of the Public’s Perception, Attitudes, and Knowledge on the Implementation of Integrated Pest Management for Household Insect Pests. Entomol. Exp. Appl. 2019, 167, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Buchmüller, K.; Bearth, A.; Siegrist, M. Consumers’ Perceptions of Chemical Household Products and the Associated Risks. Food Chem. Toxicol. 2020, 143, 111511. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.N.B.; Nieuwenhuijsen, M.J.; Golding, J. The Use and Disposal of Household Pesticides. Environ. Res. 2005, 97, 109–115. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J.; Grey, C.N.B.; Golding, J. Exposure Misclassification of Household Pesticides and Risk Perception and Behaviour. Ann. Occup. Hyg. 2005, 49, 703–709. [Google Scholar] [CrossRef] [Green Version]
- EPA. Pest Control and Pesticide Safety for Consumers. Available online: https://www.epa.gov/safepestcontrol (accessed on 8 May 2022).
- University of California Pests of Homes, Structures, People and Pets. Available online: http://ipm.ucanr.edu/PMG/menu.house.html (accessed on 8 May 2022).
- Cornell University New York State Integrated Pest Management; Homes and Other Buildings. Available online: https://nysipm.cornell.edu/community/homes-and-other-buildings/ (accessed on 8 May 2022).
- WHO; FAO. International Code of Conduct on Pesticide Management: Guidance on Management of Household Pesticides; World Health Organization; Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2020. [Google Scholar]
- Baldwin, R.W.; Koehler, P.G.; Pereira, R.M.; Oi, F.M. Public Perceptions of Pest Problems. Am. Entomol. 2008, 54, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Frankie, G.W.; Levenson, H. Insect Problems and Insecticide Use: Public Opinion, Information and Behavior. In Perspectives in Urban Entomology; Frankie, G.W., Koehler, C.S., Eds.; Academic Press, Inc.: New York, NY, USA, 1978; pp. 359–399. [Google Scholar]
- Wood, F.E.; Robinson, W.H.; Kraft, S.K.; Zungoli, P.A. Survey of Attitudes and Knowledge of Public Housing Residents toward Cockroaches. Bull. Entomol. Soc. Am. 1981, 27, 9–13. [Google Scholar] [CrossRef]
- Levenson, H.; Frankie, G.W. A Study of Homeowner Attitudes and Practices toward Arthropod Pests and Pesticides in Three U.S. Metropolitan Areas. In Urban Entomology: Interdisciplinary Perspectives; Frankie, G.W., Koehler, C.S., Eds.; Praeger: New York, NY, USA, 1983; pp. 67–106. [Google Scholar]
- Zungoli, P.A.; Robinson, W.H. Feasibility of Establishing an Aesthetic Injury Level for German Cockroach Pest Management Programs. Environ. Entomol. 1984, 13, 1453–1458. [Google Scholar] [CrossRef]
- Hinkle, N.C. Ekbom Syndrome: The Challenge of “Invisible Bug” Infestations. Annu. Rev. Entomol. 2010, 55, 77–94. [Google Scholar] [CrossRef]
- Olkowski, H.; Olkowski, W. Entomophobia in the Urban Ecosystem, Some Observations and Suggestions. Bull. Entomol. Soc. Am. 1976, 22, 313–318. [Google Scholar] [CrossRef]
- Saleh, R.; Bearth, A.; Siegrist, M. “Chemophobia” Today: Consumers’ Knowledge and Perceptions of Chemicals. Risk Anal. 2019, 39, 2668–2682. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, M.; Bearth, A. Chemophobia in Europe and Reasons for Biased Risk Perceptions. Nat. Chem. 2019, 11, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.A.; Barberán, A.; Bertone, M.A.; Menninger, H.L.; Dunn, R.R.; Fierer, N. The Diversity of Arthropods in Homes across the United States as Determined by Environmental DNA Analyses. Mol. Ecol. 2016, 25, 6214–6224. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.; Bertone, M.A.; Savage, A.M.; Bayless, K.M.; Dunn, R.R.; Trautwein, M.D. The Habitats Humans Provide: Factors Affecting the Diversity and Composition of Arthropods in Houses. Sci. Rep. 2017, 7, 15347. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, A.J.; Bascom, K.L.; Wilson, D.T. Gender Role Expectations of Disgust: Men Are Low and Women Are High. Sex Roles 2013, 69, 72–88. [Google Scholar] [CrossRef]
- Hardy, T.N. Entomophobia: The Case for Miss Muffet. Bull. Entomol. Soc. Am. 1988, 34, 64–69. [Google Scholar] [CrossRef]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An Open-Source, Graphical Experiment Builder for the Social Sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Sweet, F.S.T.; Noack, P.; Hauck, T.E.; Weisser, W.W. The relationship between knowing and liking for 91 urban animal species among students. SocArXiv Pap. 2020. [Google Scholar] [CrossRef]
- Polák, J.; Rádlová, S.; Janovcová, M.; Flegr, J.; Landová, E.; Frynta, D. Scary and Nasty Beasts: Self-Reported Fear and Disgust of Common Phobic Animals. Br. J. Psychol. 2020, 111, 297–321. [Google Scholar] [CrossRef]
- de Jong, P.J.; Muris, P. Spider Phobia: Interaction of Disgust and Perceived Likelihood of Involuntary Physical Contact. J. Anxiety Disord. 2002, 16, 51–65. [Google Scholar] [CrossRef]
- Wang, C.; Bischoff, E.; Eiden, A.L.; Zha, C.; Cooper, R.; Graber, J.M. Residents Attitudes and Home Sanitation Predict Presence of German Cockroaches (Blattodea: Ectobiidae) in Apartments for Low-Income Senior Residents. J. Econ. Entomol. 2019, 112, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienes, Z. Understanding Psychology as a Science: An Introduction to Scientific and Statistical Inference; Palgrave Macmillan: New York, NY, USA, 2008; ISBN 1137096055. [Google Scholar]
- JASP Team JASP. JASP Team, 2021; Version 0.15; 2021. [Google Scholar]
- Davey, G.C.L. The “Disgusting” Spider: The Role of Disease and Illness in the Perpetuation of Fear of Spiders. Soc. Anim. 1994, 2, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kellert, S.R. Values and Perceptions of Invertebrates. Conserv. Biol. 1993, 7, 845–855. [Google Scholar] [CrossRef]
- Looy, H.; Wood, J.R. Attitudes toward Invertebrates: Are Educational “Bug Banquets” Effective? J. Environ. Educ. 2006, 37, 37–48. [Google Scholar] [CrossRef]
- Looy, H.; Dunkel, F.V.; Wood, J.R. How Then Shall We Eat? Insect-Eating Attitudes and Sustainable Foodways. Agric. Hum. Values 2014, 31, 131–141. [Google Scholar] [CrossRef]
- Boileau, E.Y.S.; Russell, C. Insect and Human Flourishing in Early Childhood Education: Learning and Crawling Together. In Research Handbook on Childhoodnature; Cutter-Mackenzie-Knowles, A., Malone, K., Barratt Hacking, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1323–1338. ISBN 978-3-319-67286-1. [Google Scholar]
- Ruby, M.B. Vegetarianism. A Blossoming Field of Study. Appetite 2012, 58, 141–150. [Google Scholar] [CrossRef]
- Rothgerber, H. A Comparison of Attitudes toward Meat and Animals among Strict and Semi-Vegetarians. Appetite 2014, 72, 98–105. [Google Scholar] [CrossRef]
- Rust, M.K. Recent Advancements in the Control of Cat Fleas. Insects 2020, 11, 668. [Google Scholar] [CrossRef]
- Bertero, A.; Rivolta, M.; Davanzo, F.; Caloni, F. Suspected Environmental Poisoning by Drugs, Household Products and Pesticides in Domestic Animals. Environ. Toxicol. Pharmacol. 2020, 80, 103471. [Google Scholar] [CrossRef]
- Caloni, F.; Cortinovis, C.; Rivolta, M.; Davanzo, F. Suspected Poisoning of Domestic Animals by Pesticides. Sci. Total Environ. 2016, 539, 331–336. [Google Scholar] [CrossRef]
- Prokop, P.; Tunnicliffe, S.D. Effects of Having Pets at Home on Children’s Attitudes toward Popular and Unpopular Animals. Anthrozoos 2010, 23, 21–35. [Google Scholar] [CrossRef]
- Schlegel, J.; Breuer, G.; Rupf, R. Local Insects as Flagship Species to Promote Nature Conservation? A Survey among Primary School Children on Their Attitudes toward Invertebrates. Anthrozoos 2015, 28, 229–245. [Google Scholar] [CrossRef]
- Soga, M.; Gaston, K.J. Extinction of Experience: The Loss of Human–Nature Interactions. Front. Ecol. Environ. 2016, 14, 94–101. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The Potential of Insects as Food Sources–a Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef]
- Hvenegaard, G. Insect Festivals in North America: Patterns and Purposes. Am. Entomol. 2016, 62, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Fukano, Y.; Soga, M. Why Do so Many Modern People Hate Insects? The Urbanization–Disgust Hypothesis. Sci. Total Environ. 2021, 777, 146229. [Google Scholar] [CrossRef]
- Miller, J.R. Biodiversity Conservation and the Extinction of Experience. Trends Ecol. Evol. 2005, 20, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; White, M.P.; Hunt, A.; Richardson, M.; Pahl, S.; Burt, J. Nature Contact, Nature Connectedness and Associations with Health, Wellbeing and pro-Environmental Behaviours. J. Environ. Psychol. 2020, 68, 101389. [Google Scholar] [CrossRef]

| Questionnaire Section | Question | Reasoning | Possible Responses | Score |
|---|---|---|---|---|
| a (Exposure—the level of indoor exposure to insects) | Type of home | Ground level rooms tend to have a higher diversity of insects than higher floors [1]. | Private house/ground floor | 3 |
| An apartment on the 2nd floor or higher | 0 | |||
| Are there screens on the windows? | Screens prevent insects from entering through windows. | No | 2 | |
| Yes, on some windows | 1 | |||
| Yes, on all windows | 0 | |||
| During spring and summer, how frequently do you see insects inside your home? | The frequency of insect sightings is a major component of a person’s perception of infestation levels. | Very high frequency | 4 | |
| High frequency | 3 | |||
| Medium frequency | 2 | |||
| Low frequency | 1 | |||
| Almost never | 0 | |||
| If you do not use pesticides, what is the reason? * | No need to use pesticides indicates a low infestation level. | No need | −3 | |
| Irrelevant (because there is insecticide use) | 0 | |||
| b (Pesticide use—the extent of pesticide use in the home) | If pesticides are used in your home, who does the extermination? | Households that add professional extermination to HPP use likely have an overall higher level of pesticide use. | Irrelevant (because there is no pesticide use) | 0 |
| Family members | 1 | |||
| Professional exterminator | 1 | |||
| Both | 2 | |||
| If family members do the extermination, what is the frequency of treatments? | Frequency of HPP use directly affects the level of pesticide use. | Irrelevant (or no more than once a year) | 0 | |
| Once in several months | 1 | |||
| Once a month or more frequent | 2 | |||
| If extermination is done by a professional exterminator, what is the frequency of treatments? | Frequency of professional extermination directly affects the level of pesticide use. | Irrelevant (no professional extermination) | 0 | |
| Once every two years | 1 | |||
| Once a year or more frequently | 2 | |||
| Is extermination usually prophylactic (against insects in general) or aimed at specific, existing pests? | General, prophylactic spraying “against insects” tends to be more widespread than responsive treatment of specific targets. | Irrelevant (no extermination) | 0 | |
| Specific | 1 | |||
| General | 3 | |||
| Number of pesticide products currently at home. | A higher number of HPPs indicates more use. | Number of products | 1 point per product | |
| c (Tendency—factors that may affect the family’s predisposition toward using pesticides in their home) | If you do not use pesticides, what is the reason? | Awareness of the toxicity of pesticides and preferring to avoid exposure to them should be negatively correlated with tendency (see discussion). | To avoid exposure to toxic chemicals ** | −6 |
| Irrelevant (because there is pesticide use) | 0 | |||
| Are any of the household members vegetarian? | Vegetarians may be less willing to kill pests (see discussion). | Yes | −1 | |
| No | 1 | |||
| How many pets do you keep at home, and of what kind (only mammals and birds)? | See discussion | None | 2 | |
| Pet/s of only one kind | 1 | |||
| Pets of more than one kind | 0 | |||
| How often do you take nature walks? | See discussion | Often (more than once a month) | 0 | |
| Once every month or two | 1 | |||
| Once every three months or less | 2 |
| Predictor I | Predictor II | Pearson’s r | p |
|---|---|---|---|
| tendency | exposure | −0.168 | 0.164 |
| tendency | insect aversion | 0.034 | 0.779 |
| exposure | Insect aversion | 0.166 | 0.17 |
| Model | Unstandardized ß | Standard Error | Standardized ß | t | p | |
|---|---|---|---|---|---|---|
| 1 | (Intercept) | −0.157 | 0.317 | −0.15 | 0.882 | |
| tendency | 0.467 | 0.111 | 0.453 | 4.188 | <0.001 | |
| 2 | (Intercept) | −3.72 | 1.263 | −2.945 | 0.004 | |
| tendency | 0.539 | 0.101 | 0.522 | 5.32 | <0.001 | |
| exposure | 0.452 | 0.107 | 0.415 | 4.22 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leibovich-Raveh, T.; Gish, M. Does Insect Aversion Lead to Increased Household Pesticide Use? Insects 2022, 13, 555. https://doi.org/10.3390/insects13060555
Leibovich-Raveh T, Gish M. Does Insect Aversion Lead to Increased Household Pesticide Use? Insects. 2022; 13(6):555. https://doi.org/10.3390/insects13060555
Chicago/Turabian StyleLeibovich-Raveh, Tali, and Moshe Gish. 2022. "Does Insect Aversion Lead to Increased Household Pesticide Use?" Insects 13, no. 6: 555. https://doi.org/10.3390/insects13060555
APA StyleLeibovich-Raveh, T., & Gish, M. (2022). Does Insect Aversion Lead to Increased Household Pesticide Use? Insects, 13(6), 555. https://doi.org/10.3390/insects13060555






