Evolution of the Colocasiomyia gigantea Species Group (Diptera: Drosophilidae): Phylogeny, Biogeography and Shift of Host Use
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Markers, Taxon Sampling and DNA Sequencing
2.2. Data Partitioning and Model Selection
2.3. Phylogenetic Reconstruction
2.4. Biogeographic Analyses
2.5. Reconstruction of the Evolution of Host Use and Body Size
3. Results
3.1. DNA Sequencing, Model Selection and Data Partitioning
3.1.1. DNA Sequencing
3.1.2. Model Selection and Data Partitioning
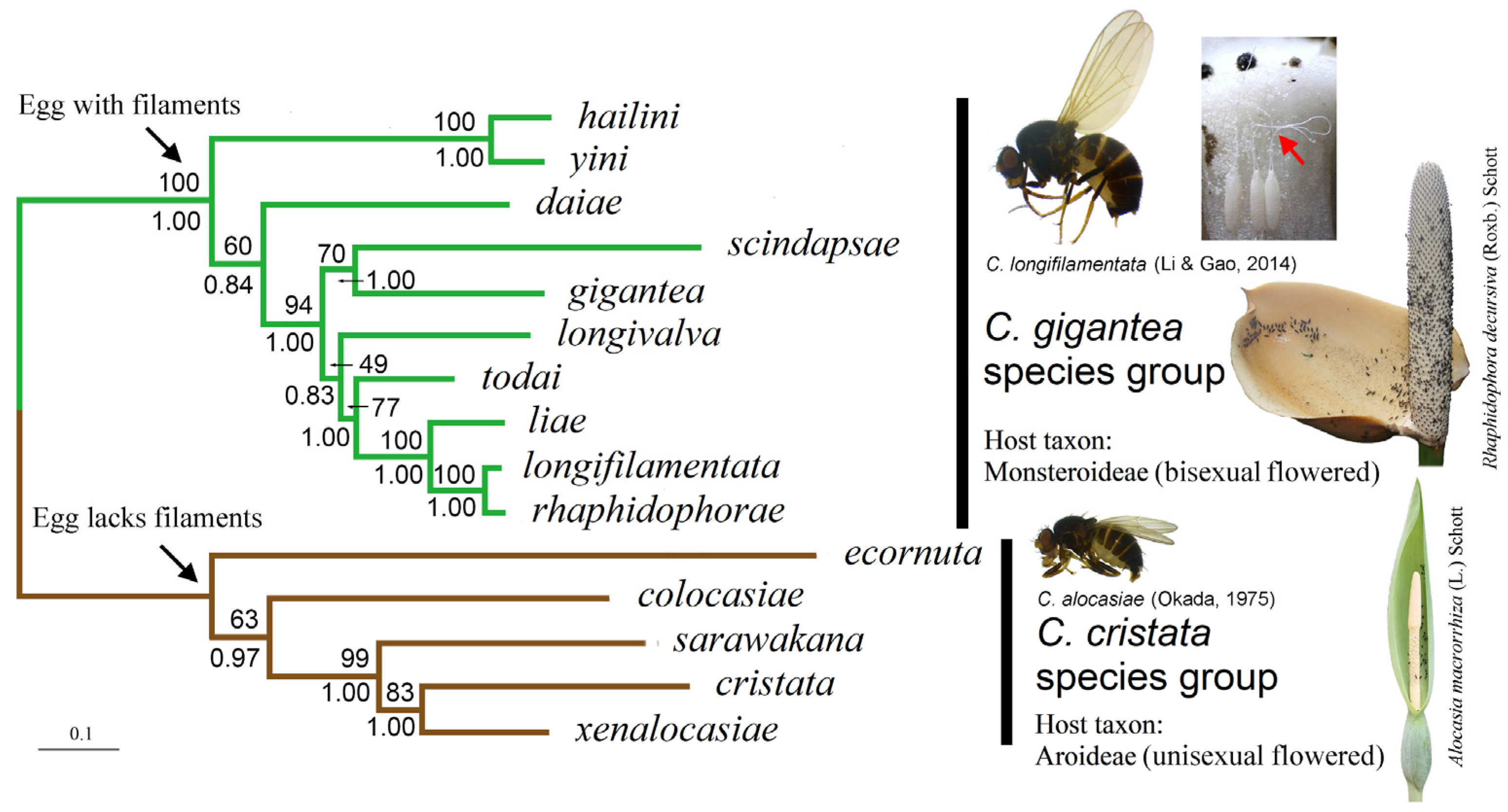
3.2. Phylogenetic Relationship in the C. gigantea Group
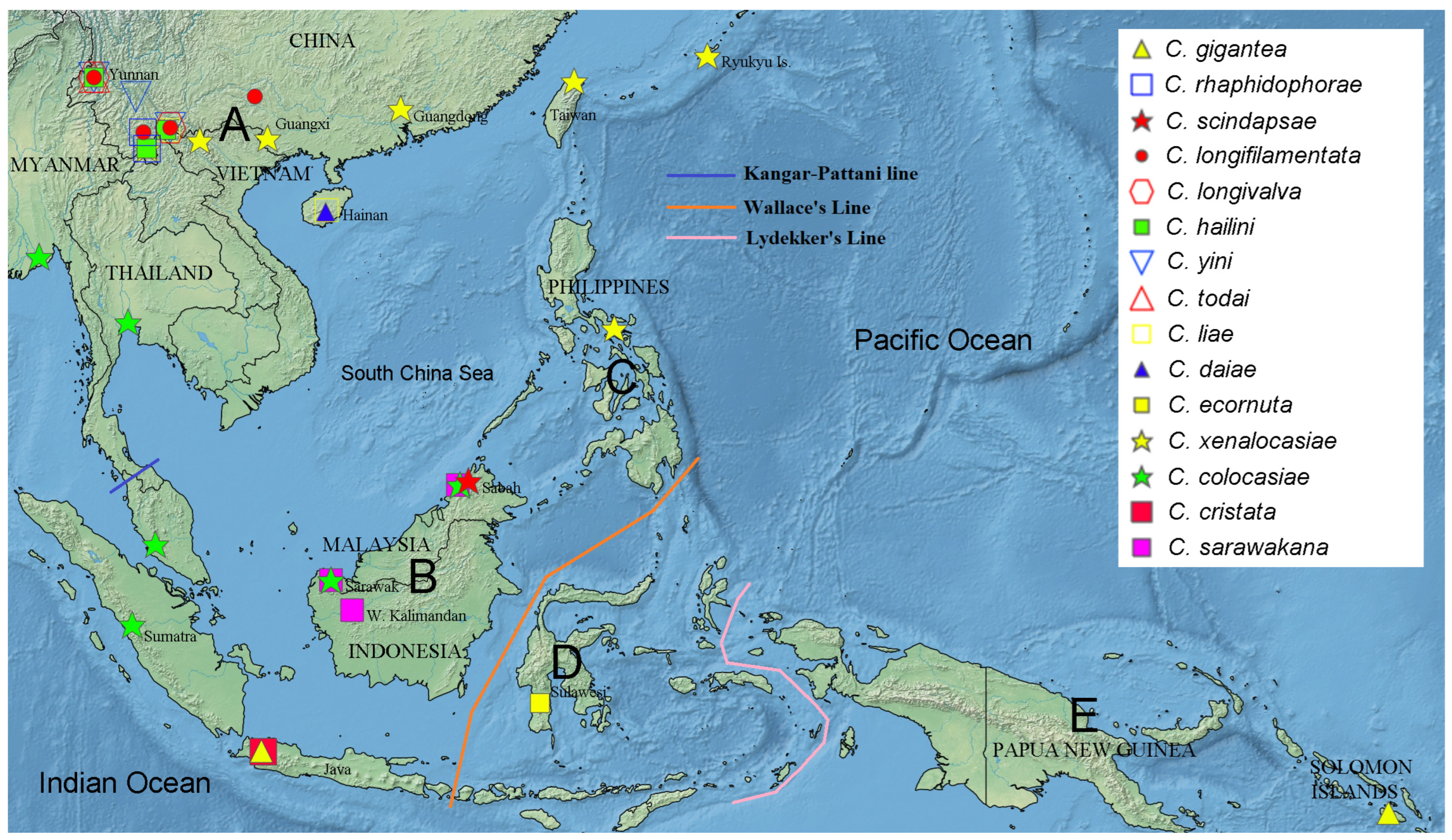
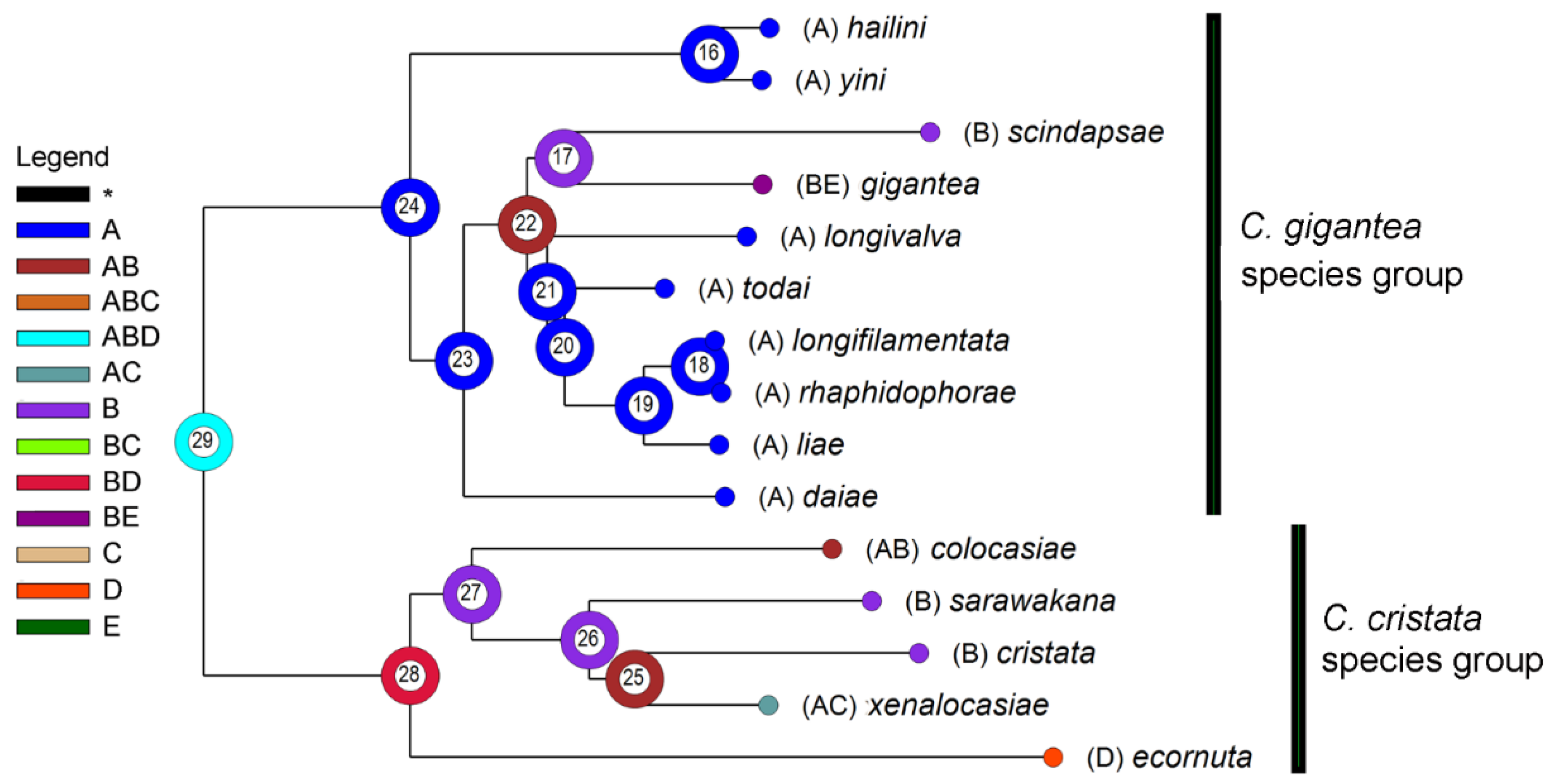
3.3. Biogeography
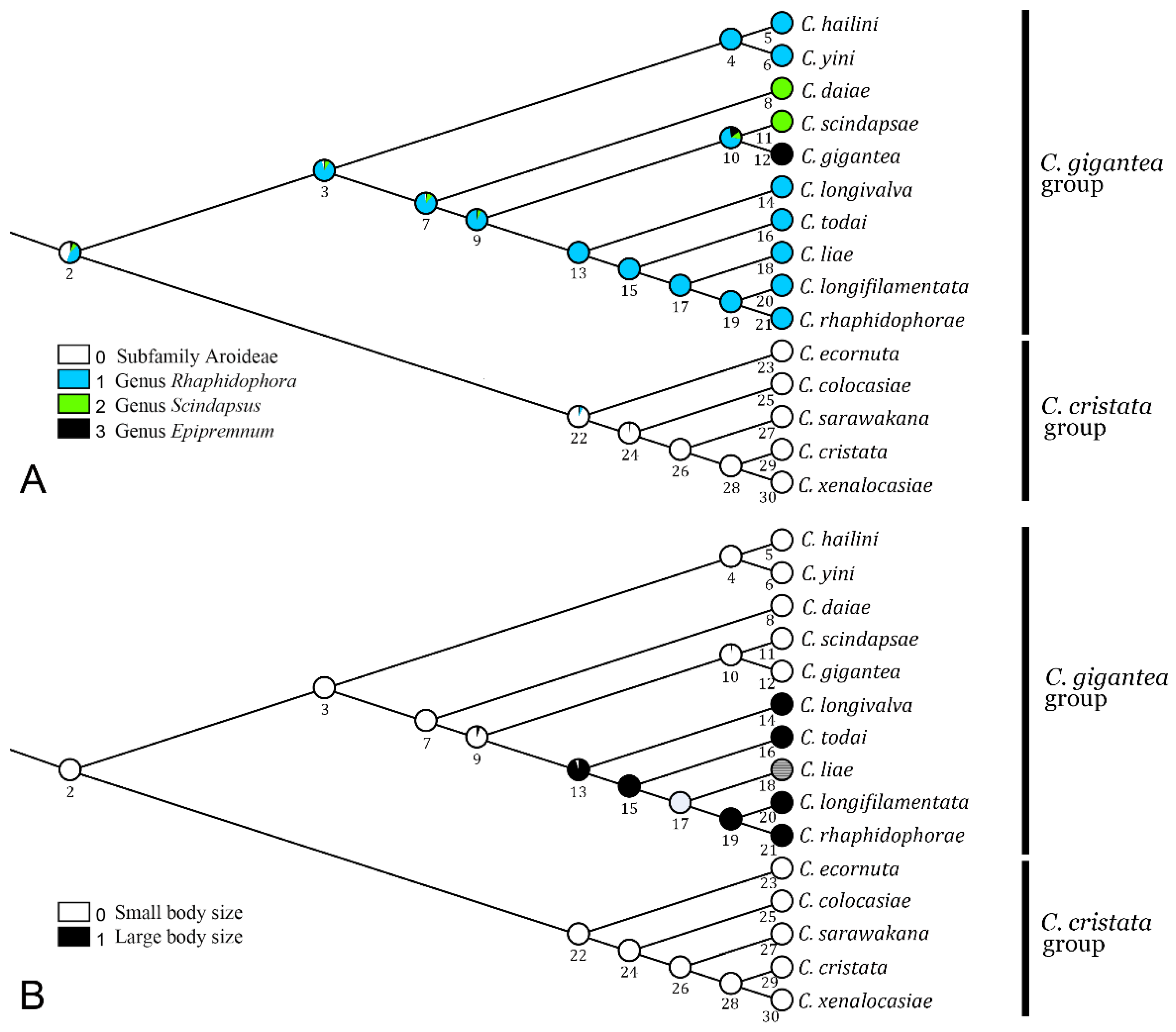
3.4. Ancestral Host Use and Evolution of Body Size
4. Discussion
4.1. Phylogeny of the C. gigantea Species Group
4.1.1. Monophyly of the Gigantea Group and Its Relationship to the Cristata Group
4.1.2. Phylogenetic Position of the C. hailini-C. yini Lineage
4.1.3. The C. scindapsae-C. gigantea Pair and C. daiae
4.1.4. The C. longivalva-C. todai-C. liae-C. longifilamentata-C. rhaphidophorae Pentad
4.2. Biogeography, Major Host Shift, Diversification and Body-Size Evolution
4.3. Adaptation of the C. gigantea Group to Monsteroid Host Plants
4.4. The Potential of the C. gigantea Group in Evolutionary/Developmental Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Host Subfamily | Character Status | |||
|---|---|---|---|---|
| Aroideae | Monsteroideae | |||
| Host tribe/clade/genus [27,53] | Genera: Homalomena Schott (Philodendron clade) and Aglaonema Schott (tribe Aglaonemateae) | Tribe: Schismatoglottideae (genera: Schismatoglottis and Piptospatha) | Colocasia clade; genera Colocasia, Alocasia, Leucocasia and Steudnera | Rhaphidophora clade (Rhaphidophora, Scindapsus, Epipremnum) |
| Corresponding species group in Colocasiomyia | C. toshiokai group (6 known species) [45] | C. baechlii group (2 known + 37 undescribed species) [54] | C. cristata group (21 known + 14 undescribed species) [6] | C. gigantea group (10 known species) [4] |
| Flower sexuality [27,53,55] | Unisexual | Bisexual | ||
| Spadix zonation [27,53,55] | Female/male | Female/sterile or bare/male | Female/sterile/male | No zonation |
| Spadix appendix [27,53,55] | Absent | Absent | Well developed | Absent |
| Spathe shape [27,53,55] | Without floral chamber (Aglomena) or varied (Homalomena) | Without floral chamber | With floral chamber | Boat-shaped (without floral chamber) |
| Species Group | Species | Thorax Length (ThL) | Code | |
|---|---|---|---|---|
| n (♂/♀) | Range (♂/♀; in mm) | |||
| C. gigantea group | hailini Li and Gao, 2014 [5] | 11/10 | (0.76–0.94)/(0.89–1.22) | A |
| yini Li and Gao, 2014 [5] | 6/5 | (0.90–1.12)/(0.93–1.20) | A | |
| daiae Xue and Gao, 2022 [9] | 6/5 | (0.90–1.07)/(0.86–1.24) | A | |
| scindapsae Fartyal and Toda, 2013 [4] | 10/10 | (0.88–1.03)/(0.85–1.24) | A | |
| gigantea Okada, 1987 [11] | n/a | n/a | A | |
| longivalva Li and Gao, 2014 [5] | 4/8 | (1.18–1.42)/(1.26–1.47) | B | |
| todai Jiao and Gao, 2020 [8] | 6/5 | (1.56–1.72)/(1.49–1.73) | B | |
| liae Jiao and Gao, 2014 [8] | 1/1 | 1.10/0.93 | ? | |
| longifilamentata Li and Gao, 2014 [5] | 7/5 | (1.17–1.37)/(1.33–1.47) | B | |
| rhaphidophorae Gao and Toda, 2013 [4] | 7/13 | (1.30–1.48)/(1.18–1.57) | B | |
| C. cristata group | ecornuta Toda and Takano, 2021 [6] | 1 (?) | ca. 0.80 | A |
| colocasiae (Duda, 1924) [6] | 1 (?) | ca. 0.94 | A | |
| sarawakana Toda and Yafuso, 2021 [6] | 1 (?) | ca. 0.86 | A | |
| cristata der Meijere, 1914 [6] | 1 (?) | ca. 1.05 | A | |
| xenalocasiae (Okada, 1980) * | 10/10 | (0.65–0.80)/(0.66–0.92) | A | |
References
- Okada, T. Synhospitalic evolution of the genus Drosophilella Duda (Diptera, Drosophilidae), with description of a new Species from Okinawa and Taiwan. Kontyû 1980, 48, 218–225. [Google Scholar]
- Grimaldi, D. Systematics of the genus Colocasiomyia de Meijere (Diptera, Drosophilidae)—Cladistics, a new generic synonym, new records, and a new species from Nepal. Entomol. Scand. 1991, 22, 417–426. [Google Scholar] [CrossRef]
- Sultana, F.; Hu, Y.G.; Toda, M.J.; Takenaka, K.; Yafuso, M. Phylogeny and classification of Colocasiomyia (Diptera, Drosophilidae), and its evolution of pollination mutualism with aroid plants. Syst. Entomol. 2006, 31, 684–702. [Google Scholar] [CrossRef]
- Fartyal, R.S.; Gao, J.J.; Toda, M.J.; Hu, Y.G.; Takano, K.T.; Suwito, A.; Katoh, T.; Takigahira, T.; Yin, J.T. Colocasiomyia (Diptera: Drosophilidae) revised phylogenetically, with a new species group having peculiar lifecycles on monsteroid (Araceae) host plants. Syst. Entomol. 2013, 38, 763–782. [Google Scholar] [CrossRef]
- Li, N.N.; Toda, M.J.; Fu, Z.; Chen, J.M.; Li, S.H.; Gao, J.J. Taxonomy of the Colocasiomyia gigantea species group (Diptera, Drosophilidae), with descriptions of four new species from Yunnan, China. Zookeys 2014, 406, 41–64. [Google Scholar] [CrossRef] [Green Version]
- Takano, K.T.; Gao, J.J.; Hu, Y.G.; Li, N.N.; Yafuso, M.; Suwito, A.; Repin, R.; Pungga, R.A.S.; Meleng, P.A.; Kaliang, C.H.; et al. Phylogeny, taxonomy and flower-breeding ecology of the Colocasiomyia cristata species group (Diptera: Drosophilidae), with descriptions of ten new species. Zootaxa 2021, 5079, 1–70. [Google Scholar] [CrossRef]
- Cusimano, N.; Bogner, J.; Mayo, S.J.; Boyce, P.C.; Wong, S.Y.; Hesse, M.; Hetterscheid, W.L.; Keating, R.C.; French, J.C. Relationships within the Araceae: Comparison of morphological patterns with molecular phylogenies. Am. J. Bot. 2011, 98, 654–668. [Google Scholar] [CrossRef]
- Jiao, R.J.; Bai, L.H.; Gao, J.J. Descriptions of two new species of the genus Colocasiomyia (Diptera, Drosophilidae) breeding on Rhaphidophora host plants in Yunnan, China. Zookeys 2020, 968, 127–141. [Google Scholar] [CrossRef]
- Xue, X.C.; Jiao, R.J.; Yu, L.I.; Gao, J.J. A new species of the Colocasiomyia gigantea species group (Diptera, Drosophilidae) breeding on inflorescences of Scindapsus maclurei (Araceae). Zootaxa 2022, 5120, 586–594. [Google Scholar] [CrossRef]
- Okada, T. Taximetrical analysis of costal chaetotaxy of the genus Drosophilella Duda, with Description of a new species from Sri Lanka (Diptera, Drosophilidae). Proc. Jpn. Soc. Syst. Zool. 1986, 34, 53–59. [Google Scholar]
- Okada, T. Further notes on the genus Drosophilella Duda with descriptions of two new species from Indonesia (Diptera, Drosophilidae). Proc. Jpn. Soc. Syst. Zool. 1987, 36, 38–45. [Google Scholar]
- Finet, C.; Kassner, V.A.; Carvalho, A.B.; Chung, H.; Al, E. DrosoPhyla: Genomic resources for drosophilid phylogeny and systematics. Genome Biol. Evol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Toda, M.J.; Li, N.N.; Zhang, Y.P.; Gao, J.J. A new genus of anthophilous drosophilids, Impatiophila (Diptera, Drosophilidae): Morphology, DNA barcoding and molecular phylogeny, with descriptions of thirty-nine new species. Zootaxa 2016, 4120, 1–100. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Brandley, M.C.; Schmitz, A.; Reeder, T.W. Partitioned Bayesian analyses, partition choice, and the phylogenetic relationships of scincid lizards. Syst. Biol. 2005, 54, 373–390. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Xie, D.; Suchard, M.A.; Drummond, A.J.; Baele, G. Posterior summarization in Bayesian phylogenetics Using Tracer 1.7. Syst. Biol. 2013, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2019, 37, 604–606. [Google Scholar] [CrossRef]
- van Steenis, C.G.G.J. The delimitation of Malesia and its main plant geographical divisions. Flora Males. 1950, 1, 120–125. [Google Scholar]
- Lohman, D.J.; de Bruyn, M.; Page, T.; von Rintelen, K.; Hall, R.; Ng, P.K.L.; Shih, H.-T.; Carvalho, G.R.; von Rintelen, T. Biogeography of the Indo-Australian Archipelago. Ann. Rev. Ecol. Evol. Syst. 2011, 42, 205–226. [Google Scholar] [CrossRef] [Green Version]
- Maddison, W.P.M. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. Available online: http://www.mesquiteproject.org (accessed on 21 May 2022).
- Zhang, W.X.; Toda, M.J. A new species-subgroup of the Drosophila immigrans species-group (Diptera, Drosophilidae), with description of two new species from China and revision of taxonomic terminology. Jpn. J. Entomol. Scand. 1992, 60, 839–850. [Google Scholar]
- McQueen, E.W.; Afkhami, M.; Atallah, J.; Belote, J.M.; Gompel, N.; Heifetz, Y.; Kamimura, Y.; Kornhauser, S.C.; Masly, J.P.; O’Grady, P.; et al. A standardized nomenclature and atlas of the female terminalia of Drosophila melanogaster. Fly 2022, 16, 128–151. [Google Scholar] [CrossRef] [PubMed]
- Boyce, P. Plate 377. Rhaphidophora glauca. Curtis’s Bot. Mag. 1999, 16, 273–280. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Bui, H.Q.; Ha, M.T.; Le, X.D.; Nguyen, C.S.; Boyce, P.C. Rhaphidophora sonlaensis (Araceae), a new species from northern Vietnam. Ann. Bot. Fenn. 2017, 54, 111–115. [Google Scholar] [CrossRef]
- Cole, T.C.H.; Wong, S.Y.; Li, H. Araceae Phylogeny Poster (Chinese). Available online: https://www.researchgate.net/publication/338718929 (accessed on 21 May 2022).
- Bogner, J.; Boyce, P.C. Scindapsus lucens (Araceae: Monsteroideae), a new species related to Scindapsus pictus. Kew Bull. 1994, 49, 789–792. [Google Scholar] [CrossRef]
- Boyce, P. A review of Epipremnum (Araceae) in Cultivation. Aroideana 2004, 27, 205–211. [Google Scholar]
- Hinton, H.E. Plastron respiration in the eggs of Drosophila and other flies. Nature 1959, 184, 280–281. [Google Scholar] [CrossRef]
- Hinton, H.E. Respiratory systems of insect egg shells. Annu. Rev. Entomol. 1969, 14, 343–368. [Google Scholar] [CrossRef] [Green Version]
- Sober, E. The Principle of Parsimony. Br. J. Philos. Sci. 1981, 32, 145–156. [Google Scholar] [CrossRef]
- Grimaldi, D.A. Relicts in the Drosophilidae. In Zoogeography of Caribbean Insects; Liebherr, J.K., Ed.; Cornell University Press: Ithaca, NY, USA, 1988. [Google Scholar]
- Hennig, W. Die Acalyptratae des Baltischen Bernsteins und ihre Bedeutung für die Erforschung der phylogenetischen Entwicklung dieser Dipterengruppe. Stuttg. Beiträge Nat. 1965, 145, 1–215. [Google Scholar]
- Powell, J.R.; DeSalle, R. Drosophila molecular phylogenies and their uses. In Evolutionary Biology; Hecht, M.K., MacIntyre, R.J., Clegg, M.T., Eds.; Plenum Press: New York, NY, USA, 1995. [Google Scholar]
- Schiestl, F.P.; Dotterl, S. The evolution of floral scent and olfactory preferences in pollinators: Coevolution or pre-existing bias? Evolution 2012, 66, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Y.Y.; Qu, X.J.; Ma, H.; Hu, Y.; Li, H.T.; Yi, T.S.; Li, D.Z. Phylotranscriptomic analyses reveal multiple whole-genome duplication events, the history of diversification and adaptations in the Araceae. Ann. Bot. 2022. [Google Scholar] [CrossRef] [PubMed]
- Whittall, J.B.; Hodges, S.A. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 2007, 447, 706–709. [Google Scholar] [CrossRef]
- Hodges, S.A.; Whittall, J.B. One-sided evolution or two? A reply to Ennos. Heredity 2008, 100, 541–542. [Google Scholar] [CrossRef]
- Johnson, S.D.; Anderson, B. Coevolution between Food-Rewarding Flowers and Their Pollinators. Evol. Educ. Outreach 2010, 3, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Prieto, D.; Cascante-Marín, A. Pollination by nitidulid beetles in the hemi-epiphytic aroid Monstera lentii (Araceae: Monsteroideae). Flora 2017, 231, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.R.; Prieto, D. A new Costa Rican species of Drosophila visiting inflorescences of the hemi-epiphytic climber Monstera lentii (Araceae). Rev. Bras. Entomol. 2018, 62, 225–231. [Google Scholar] [CrossRef]
- Takenaka, K.; Yin, J.T.; Wen, S.Y.; Toda, M.J. Pollination mutualism between a new species of the genus Colocasiomyia de Meijere (Diptera: Drosophilidae) and Steudnera colocasiifolia (Araceae) in Yunnan, China. Entomol. Sci. 2006, 9, 79–91. [Google Scholar] [CrossRef]
- Takano, K.T.; Repin, R.; Mohamed, M.B.; Toda, M.J. Pollination mutualism between Alocasia macrorrhizos (Araceae) and two taxonomically undescribed Colocasiomyia species (Diptera: Drosophilidae) in Sabah, Borneo. Plant Biol. 2012, 14, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Toda, M.J.; Takano, K.T.; Yafuso, M.; Suwito, A.; Wong, S.Y.; Shang, S.Q.; Gao, J.J. A review of taxonomy and flower-breeding ecology of the Colocasiomyia toshiokai species group (Diptera: Drosophilidae), with description of a new species from Indonesia. Eur. J. Entomol. 2019, 116, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Barraclough, T.G.; Nee, S.; Harvey, P.H. Sister-group analysis in identifying correlates of diversification—Comment. Evol. Ecol. 1998, 12, 751–754. [Google Scholar]
- Throckmorton, L.H. The Problem of Phylogeny in the Genus Drosophila; The University of Texas Press: Austin, TX, USA, 1962; Volume 6205, pp. 207–343. [Google Scholar]
- Throckmorton, L.H. The Relationships of the Endemic Hawaiian Drosophilidae; The University of Texas Press: Austin, TX, USA, 1966; Volume 6615, pp. 335–396. [Google Scholar]
- David, J.R.; Yassin, A.; Rasamizafi, L.A.; Ravaomanarivo, L.H.R.; Debat, V. Scratching for food: An original feeding behavior in an African flower breeding Drosophila. Fly 2011, 5, 285–290. [Google Scholar] [CrossRef]
- Berg, C.A. The Drosophila shell game: Patterning genes and morphological change. Trends Genet. 2005, 21, 346–355. [Google Scholar] [CrossRef]
- Osterfield, M.; Berg, C.A.; Shvartsman, S.Y. Epithelial patterning, morphogenesis, and evolution: Drosophila eggshell as a model. Dev. Cell 2017, 41, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.G.; Merrihew, G.E.; MacCoss, M.J.; Berg, C.A. Proteomics analysis identifies orthologs of human chitinase-like proteins as inducers of tube morphogenesis defects in Drosophila melanogaster. Genetics 2017, 206, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhu, G.; Boyce, P.; Murata, J.; Hetterscheid, W.L.A.; Bogner, J.; Jacobsen, N. The Flora of China. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 21 May 2022).
- Toda, M.J.; Lakim, M.B. Genus Colocasiomyia (Drosophilidae: Diptera) in Sabah, Bornean Malaysia: High species diversity and use of host aroid inflorescences. Entomol. Sci. 2011, 14, 262–270. [Google Scholar] [CrossRef]
- Chartier, M.; Gibernau, M.; Renner, S.S. The evolution of pollinator-plant interaction types in the Araceae. Evolution 2014, 68, 1533–1543. [Google Scholar] [CrossRef]
| Species Group | Species | Voucher #/Source of Sequence or Collection Site * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| COI | COII | 28S | ATPsyn-Alpha | ATPsyn-Gamma | alphaTub84B | Hsc70cb | EF-2 | ||
| cristata | colocasiae | #01274/a | #01272/a | #01272/a | #01272/a | #01272/a | #01272/a | #01274/a | #01272/a |
| cristata | #01254/a | #01254/a | #01254/a | #01254/a | #01254/a | #0125/a | #01254/a | #01254/a | |
| sarawakana | #01265/a | #01232/a | #01233/a | #01265/a | #01265/a | n/a | #01233/a | #01264/a | |
| ecornuta | #01267/a | #01267/a | #01267/a | #01267/a | #01267/a | #01267/a | #01267/a | #01267/a | |
| xenalocasiae | #01352/b | #01352/a | #01352/a | #01627/a | #01357/a | #01358/a | #01627/a | #01628/a | |
| gigantea | gigantea | #01442/a | #01442/a | #01442/a | #01442/a | #01442/a | #01442/a | #01442/a | #01442/a |
| hailini | #01517/b | #01448/a | #01517/a | #01448/a | #01448/a | #01448/a | #01448/a | #01448/a | |
| longifilamentata | #01252/a | #01133/a | #01134/a | #01134/a | #01134/a | #01134/a | #01134/a | #01134/a | |
| longivalva | #01722/a | #01722/a | #01722/a | #01722/a | #01722/a | #01722/a | #01722/a | #01722/a | |
| scindapsae | #01236/b | #01236/a | #01236/a | #01186/a | #01186/a | #01186/a | #01186/a | #01186/a | |
| yini | #00160/b | #01302/e | #01302/e | #01302/e | #01302/e | #01302/e | #01302/e | #01302/e | |
| rhaphidophorae | #01137/b | #01137/f | #01137/f | #01137/f | #01137/f | #01137/f | #01137/f | #01137/f | |
| liae | #10486/c | #12411/g | #12411/g | #12411/g | #12411/g | #12411/g | #12411/g | #12412/g | |
| todai | #10130/c | #09241/h | #09243/h | #09241/h | #09241/h | #09243/h | #09241/h | #09243/h | |
| daiae | #10566/d | #10566/i | #10566/i | #10566/i | #10566/i | #10566/i | #10566/i | #10566/i | |
| Species Group | Species | Geographical Distribution a | Host Use b | Body Size c | |
|---|---|---|---|---|---|
| C. gigantea group | hailini Li and Gao, 2014 | A | 1 | A | |
| yini Li and Gao, 2014 | A | 1 | A | ||
| daiae Xue and Gao, 2022 | A | 2 | B | ||
| scindapsae Fartyal and Toda, 2013 | B | 2 | A | ||
| gigantea Okada, 1987 | BE | 3 | A | ||
| longivalva Li and Gao, 2014 | A | 1 | B | ||
| todai Jiao and Gao, 2020 | A | 1 | B | ||
| liae Li and Gao, 2020 | A | 1 | ? | ||
| longifilamentata Li and Gao, 2014 | A | 1 | B | ||
| rhaphidophorae Gao and Toda, 2013 | A | 1 | A | ||
| C. cristata group | ecornuta Toda and Takano, 2021 | D | 0 | A | |
| colocasiae (Duda 1924) | AB | 0 | A | ||
| sarawakana Toda and Yafuso, 2021 | B | 0 | A | ||
| cristata de Meijere, 1914 | B | 0 | A | ||
| xenalocasiae (Okada 1980) | AC | 0 | A | ||
| Partitioning Strategy (Composition) a | -lnL | |||
|---|---|---|---|---|
| ESS b | Mean | 95% HPD c Lower | 95% HPD Upper | |
| P1 (all sequence-concatenated) | 4788 | 17,485.032 | 17,493.729 | 17,476.583 |
| P2a (mt, nu) | 2853 | 17,142.185 | 17,152.597 | 17,133.047 |
| P2b (all-PCG, 28S) | 4035 | 17,422.944 | 17,432.948 | 17,413.465 |
| P3a (mt, nu-PCG, 28S) | 2802 | 17,069.504 | 17,097.743 | 17,059.122 |
| P3b (all-PCG-CP1+2, all-PCG-CP3, 28S) | 3700 | 16,728.193 | 16,379.127 | 16,717.484 |
| P4 (all-PCG-CP1, all-PCG-CP2, all-PCG-CP3, 28S) | 2196 | 16,594.812 | 16,607.046 | 16,583.448 |
| P5 (mt-CP1+, mt-CP3, nu-PCG-CP1+2, nu-PCG-CP3, 28S) | 1856 | 16,126.526 | 16,138.411 | 16,115.309 |
| P7a (mt, and 6 partitions each for a nu gene) | 3467 | 17,381.246 | 17,391.044 | 17,372.103 |
| P7b (mt-CP1, mt-CP2, mt-CP3, nu-PCG-CP1, nu-PCG-CP2, nu-PCG-CP3, 28S) | 3114 | 16,784.351 | 16,793.752 | 16,775.353 |
| P8 (8 partitions each for a gene locus) | 3204 | 17,349.354 | 17,359.400 | 17,340.058 |
| Strategy 2 | Strategy 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P8 | P7b | P7a | P5 | P4 | P3b | P3a | P2b | P2a | |
| P1 | 271.365 | 1401.362 | 207.572 | 2717.012 | 1780.440 | −32,913.674 | −31,390.284 | −34,430.744 | −28,850.346 |
| P2a | −414.338 | 715.688 | −478.122 | 2031.318 | 1094.746 | −34,285.062 | −32,761.672 | −35,802.132 | |
| P2b | 147.180 | 1277.186 | 83.369 | 2592.836 | 1656.264 | −33,162.026 | −31,638.636 | ||
| P3a | −505.700 | 624.306 | −569.484 | 1939.956 | 1003.384 | −34,467.786 | |||
| P3b | −1242.322 | −112.316 | −1306.106 | 1203.334 | 266.762 | ||||
| P4 | −1509.084 | −379.078 | −1572.868 | 936.572 | |||||
| P5 | −2445.656 | −1315.650 | −2509.440 | ||||||
| P7a | 63.784 | 1193.790 | |||||||
| P7b | −1130.006 | ||||||||
| Node | Most Likely State | Event | Event Route * | Probability | ||
|---|---|---|---|---|---|---|
| Dispersal | Vicariance | Extinction | ||||
| 16 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 17 | B | 1 | 0 | 0 | B→B^B→BE^B→B|BE | 1.0000 |
| 18 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 19 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 20 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 21 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 22 | AB | 0 | 1 | 0 | AB→A|B | 1.0000 |
| 23 | A | 1 | 0 | 0 | A→A^A→AB^A→A|AB | 1.0000 |
| 24 | A | 0 | 0 | 0 | A→A^A→A|A | 1.0000 |
| 25 | AB | 1 | 1 | 0 | AB→ABC→B|AC | 0.3333 |
| 26 | B | 1 | 0 | 0 | B→B^B→AB^B→B|AB | 0.3333 |
| 27 | B | 1 | 0 | 0 | B→B^B→AB^B→AB|B | 1.0000 |
| 28 | BD | 0 | 1 | 0 | BD→D|B | 1.0000 |
| 29 | ABD | 0 | 1 | 0 | ABD→BD|A | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Li, N.-N.; Yang, L.-K.; Li, J.-L.; Gao, J.-J. Evolution of the Colocasiomyia gigantea Species Group (Diptera: Drosophilidae): Phylogeny, Biogeography and Shift of Host Use. Insects 2022, 13, 647. https://doi.org/10.3390/insects13070647
Xiao L, Li N-N, Yang L-K, Li J-L, Gao J-J. Evolution of the Colocasiomyia gigantea Species Group (Diptera: Drosophilidae): Phylogeny, Biogeography and Shift of Host Use. Insects. 2022; 13(7):647. https://doi.org/10.3390/insects13070647
Chicago/Turabian StyleXiao, Ling, Nan-Nan Li, Long-Kun Yang, Jia-Ling Li, and Jian-Jun Gao. 2022. "Evolution of the Colocasiomyia gigantea Species Group (Diptera: Drosophilidae): Phylogeny, Biogeography and Shift of Host Use" Insects 13, no. 7: 647. https://doi.org/10.3390/insects13070647





