Deletion of the Serotonin Receptor 7 Gene Changed the Development and Behavior of the Mosquito, Aedes aegypti
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Phylogenetic Analysis
2.3. qPCR
2.4. CRISPR-Cas9-Mediated Knockout of the Ae. aegypti 5-HTR7A
2.5. Evaluation of Stress Response the Mutants
2.6. Statistical Analysis
3. Results
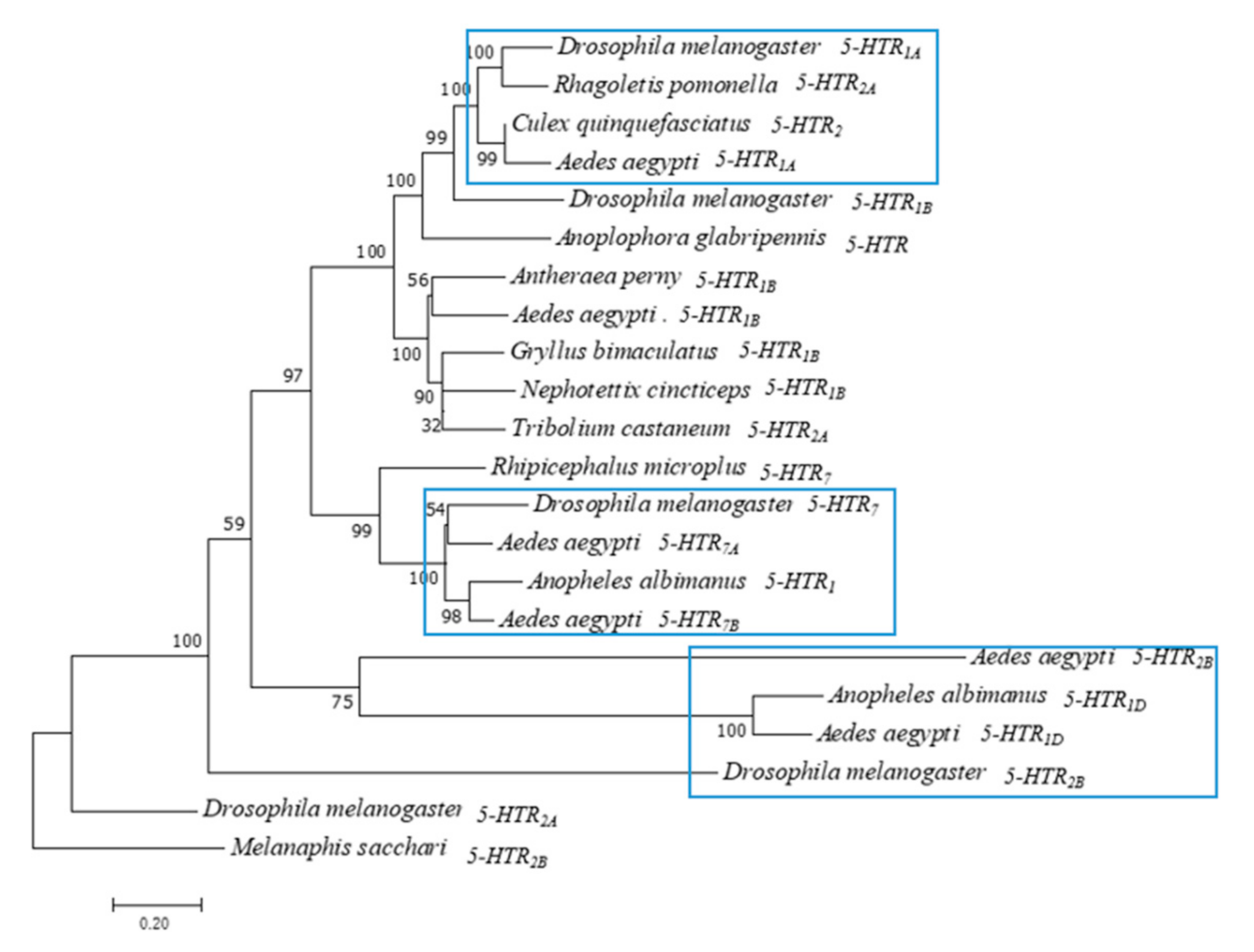
3.1. Phylogenetic Analysis of Ae. aegypti 5-HTR Proteins
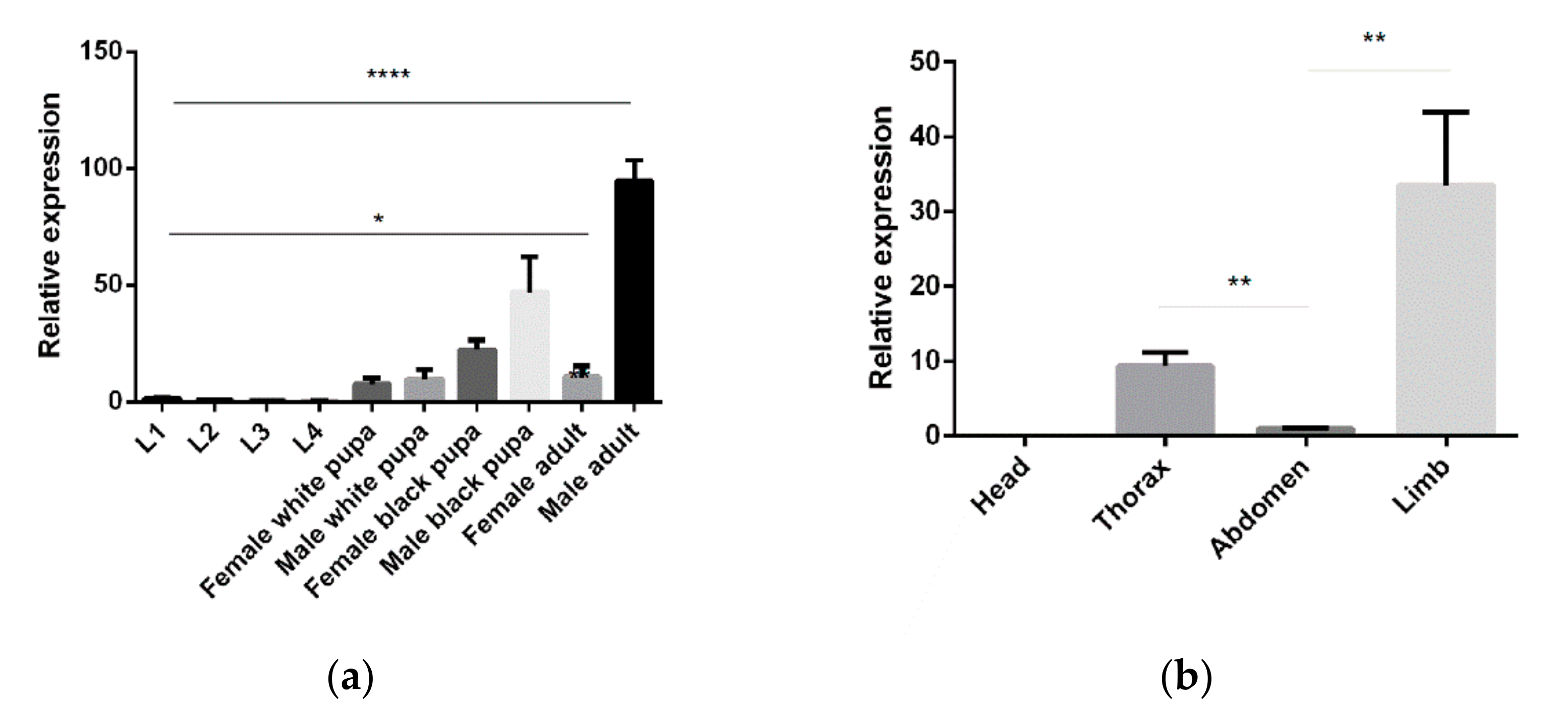
3.2. Temporal and Spatial Expression Profiles of 5-HTR7A Genes
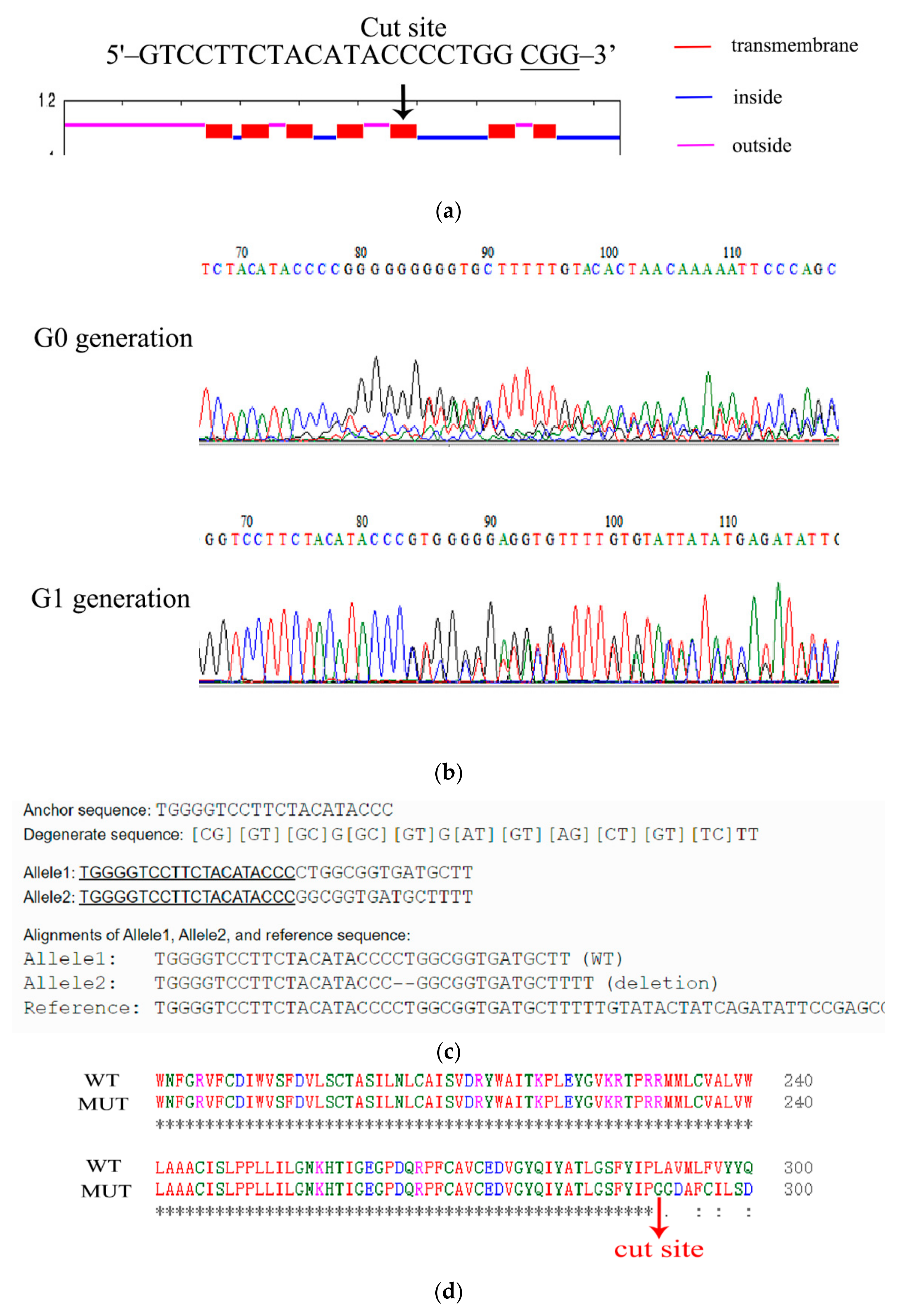
3.3. CRISPR-Cas9-Mediated 5-HTR7A Gene Knockout
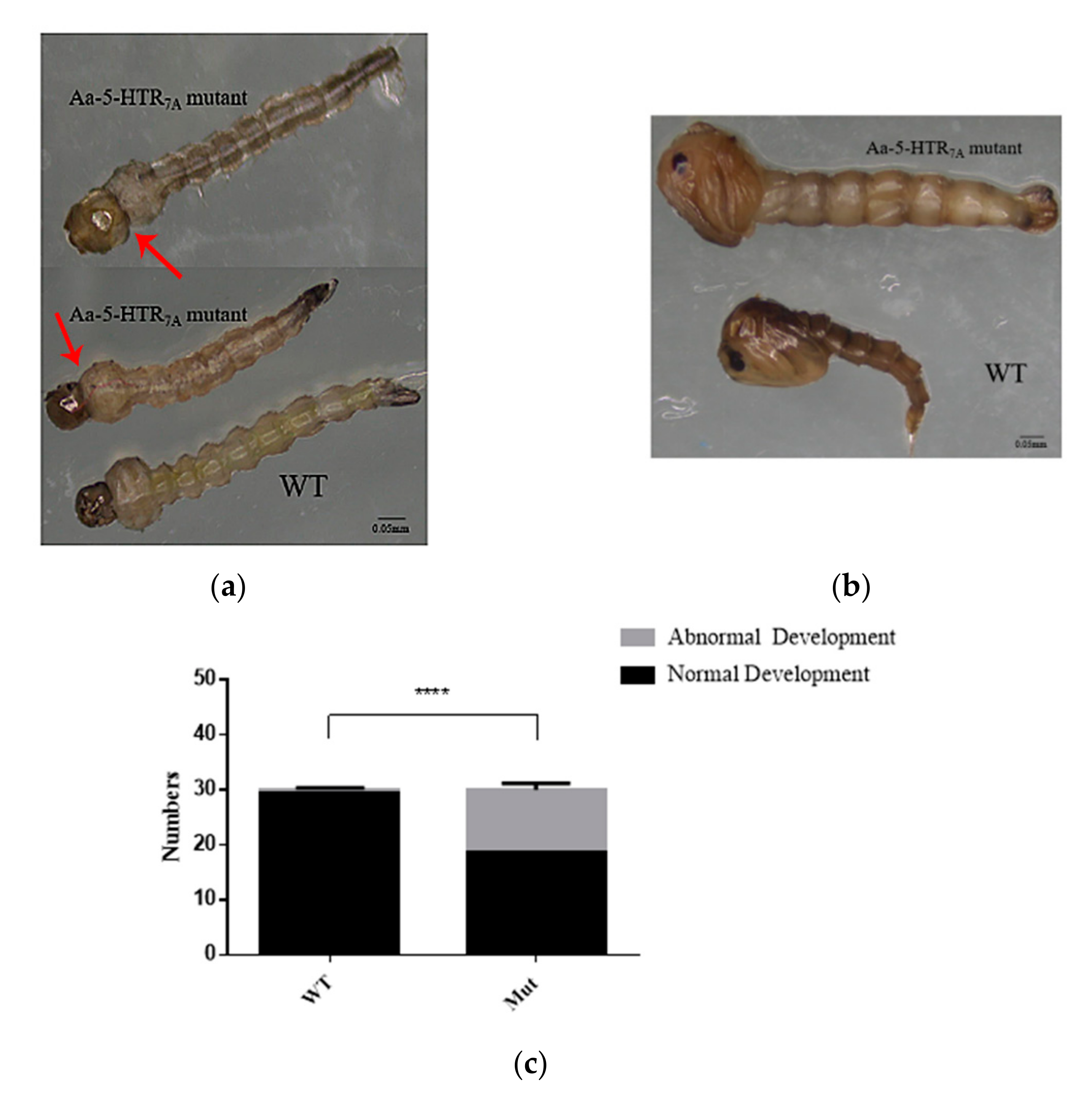
3.4. Morphological Changes in 5-HTR7A Mutant Mosquitoes
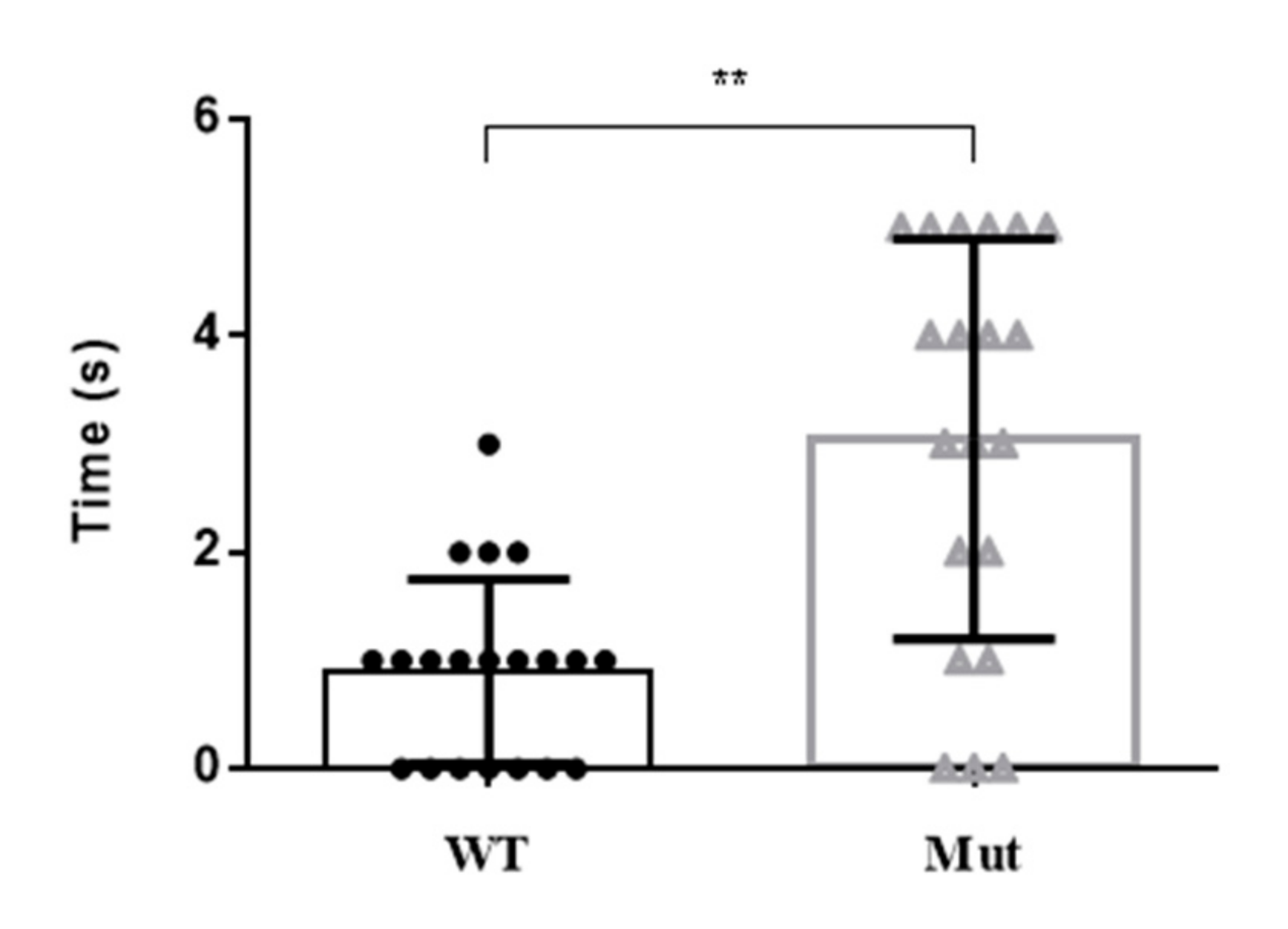
3.5. Stress Response of 5-HTR7A Mutant Mosquitoes
3.6. Effects of 5-HTR7A Mutations on Egg Hatching in Offspring
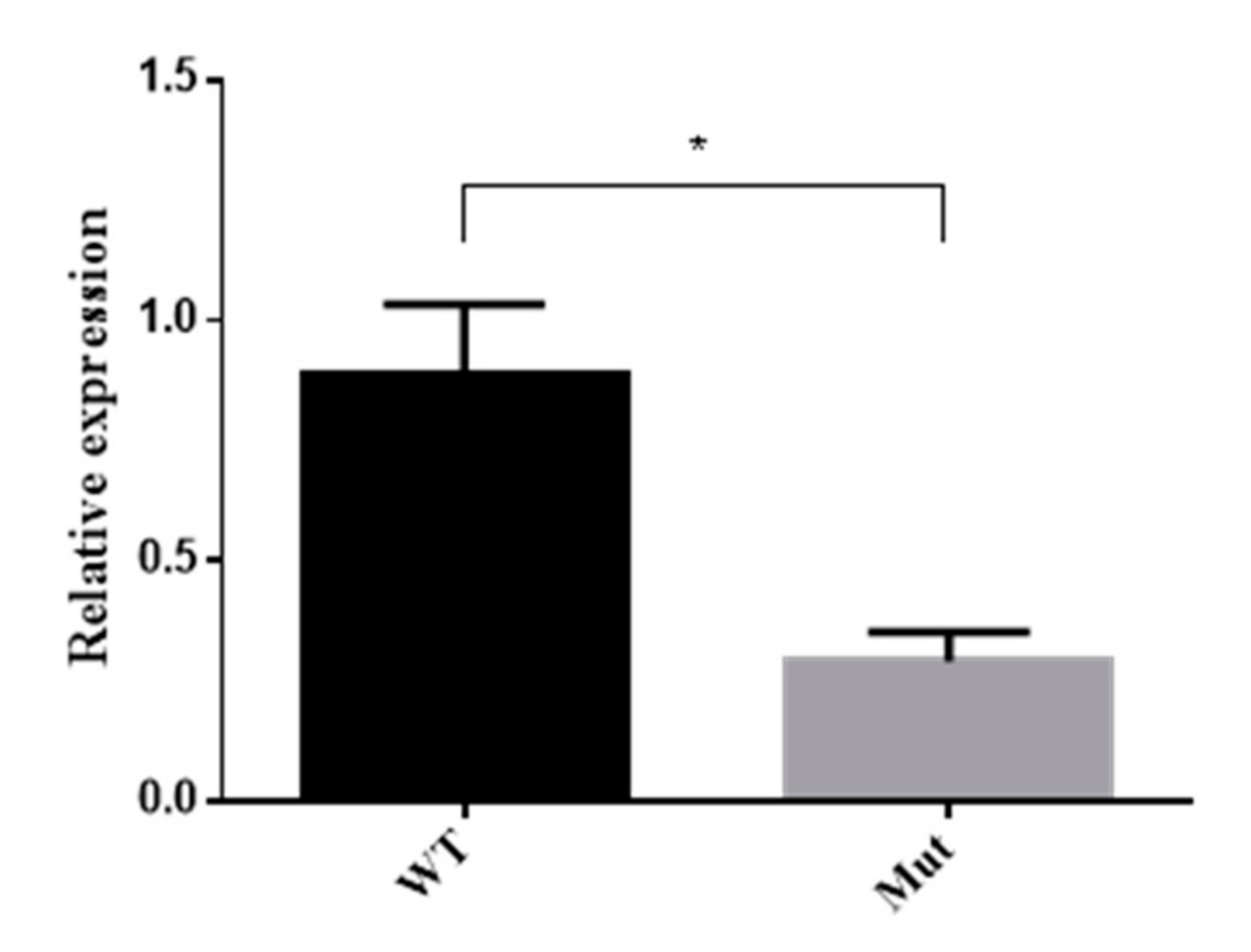
3.7. 5-HTR7A Expression in Mutant Mosquitoes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vleugels, R.; Verlinden, H.; Vanden Broeck, J. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2, e314. [Google Scholar]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Ogawa, S.; Parhar, I.S. Role of serotonin in fish reproduction. Front. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Carlsson, M.A.; Nassel, D.R. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. J. Comp. Neurol. 2015, 523, 1840–1863. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, Z.R.; Nautiyal, K.M.; Ahmari, S.E.; Hen, R. Genetic approaches for understanding the role of serotonin receptors in mood and behavior. Curr. Opin. Neurobiol. 2013, 23, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Sood, S.; Morrison, J.L.; Liu, H.; Horner, R.L. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am. J. Respir. Crit. Care Med. 2005, 172, 1338–1347. [Google Scholar] [CrossRef] [Green Version]
- Halford, J.C.; Harrold, J.A.; Lawton, C.L.; Blundell, J.E. Serotonin (5-HT) drugs: Effects on appetite expression and use for the treatment of obesity. Curr. Drug Targets 2005, 6, 201–213. [Google Scholar] [CrossRef]
- Tierney, A.J. Invertebrate serotonin receptors: A molecular perspective on classification and pharmacology. J. Exp. Biol. 2018, 221 Pt 19, jeb184838. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.X.; Huang, J.; Li, M.Q.; Wu, Y.S.; Xia, R.Y.; Ye, G.Y. Serotonin modulates insect hemocyte phagocytosis via two different serotonin receptors. eLife 2016, 5, e12241. [Google Scholar] [CrossRef] [Green Version]
- French, A.S.; Simcock, K.L.; Rolke, D.; Gartside, S.E.; Blenau, W.; Wright, G.A. The role of serotonin in feeding and gut contractions in the honeybee. J. Insect Physiol. 2014, 61, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Raikhel, A.S. Serotonin signaling regulates insulin-like peptides for growth, reproduction, and metabolism in the disease vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2018, 115, E9822–E9831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Li, Z.; Fan, F.; Huang, Q.; Shao, X.; Song, G. Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP). J. Agric. Food Chem. 2010, 58, 2624–2629. [Google Scholar] [CrossRef] [PubMed]
- Sharan, S.; Hill, C.A. Potential of GPCR-Targeting Insecticides for Control of Arthropod Vectors. In Advances in Agrochemicals: Ion Channels and G Protein-Coupled Receptors (GPCRs) as Targets for Pest Control; American Chemical Society: Washington, DC, USA, 2017; Volume 1265, pp. 55–84. [Google Scholar]
- Lan, J.; Wang, Z.; Chen, Z.; Zhang, L.; Zhao, J.; Guan, Q.; Liao, C.; Liu, N.; Han, Q. Identification of the Aedes aegypti nAChR gene family and molecular target of spinosad. Pest Manag. Sci. 2021, 77, 1633–1641. [Google Scholar] [CrossRef]
- Tolle, M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, M.Z.; Chen, Z.H.; Tang, Y.; Liao, C.H.; Han, Q. Arylalkalamine N-acetyltransferase-1 functions on cuticle pigmentation in the yellow fever mosquito, Aedes aegypti. Insect Sci. 2020, 28, 1591–1600. [Google Scholar] [CrossRef]
- Kistler, K.E.; Vosshall, L.B.; Matthews, B.J. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015, 11, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jaimes-Orduña, J.; Tamez-Guerra, P.; Zavala-García, F.; Pérez-González, O. Identification of predatory and parasitoid insect species associated with Melanaphis sacchari (Hemiptera: Aphididae), a sorghum pest in Nuevo León, Mexico. Fla. Entomol. 2020, 103, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.G. CRISPR-GE: A Convenient Software Toolkit for CRISPR-Based Genome Editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [Green Version]
- Ngai, M.; Shoue, D.A.; Loh, Z.; McDowell, M.A. The pharmacological and functional characterization of the serotonergic system in Anopheles gambiae and Aedes aegypti: Influences on flight and blood-feeding behavior. Sci. Rep. 2019, 9, 4421. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.R.; Taparia, T.; Ignell, R. Regulation of the antennal transcriptome of the dengue vector, Aedes aegypti, during the first gonotrophic cycle. BMC Genom. 2021, 22, 71. [Google Scholar] [CrossRef]
- Siju, K.P.; Hansson, B.S.; Ignell, R. Immunocytochemical localization of serotonin in the central and peripheral chemosensory system of mosquitoes. Arthropod Struct. Dev. 2008, 37, 248–259. [Google Scholar] [CrossRef]
- Hill, S.R.; Ghaninia, M.; Ignell, R. Blood Meal Induced Regulation of Gene Expression in the Maxillary Palps, a Chemosensory Organ of the Mosquito Aedes aegypti. Front. Ecol. Evol. 2019, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Matthews, B.J.; Younger, M.A.; Vosshall, L.B. The ion channel ppk301 controls freshwater egg-laying in the mosquito Aedes aegypti. eLife 2019, 8, e43963. [Google Scholar] [CrossRef]
- Hjorth, S.; Magnusson, T. The 5-HT1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autoreceptors in rat brain in vivo. Naunyn Schmiedeberg’s Arch. Pharmacol. 1988, 338, 463–471. [Google Scholar] [CrossRef]
- Casanovas, J.M.; Lésourd, M.; Artigas, F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. Br. J. Pharmacol. 1997, 122, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Hedlund, P.B.; Kelly, L.; Mazur, C.; Lovenberg, T.; Sutcliffe, J.G.; Bonaventure, P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 2004, 487, 125–132. [Google Scholar] [CrossRef]
- Sprouse, J.; Reynolds, L.; Li, X.; Braselton, J.; Schmidt, A. 8-OH-DPAT as a 5-HT7 agonist: Phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 2004, 46, 52–62. [Google Scholar] [CrossRef]
- Saudou, F.; Boschert, U.; Amlaiky, N.; Plassat, J.L.; Hen, R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992, 11, 7–17. [Google Scholar] [CrossRef]
- Colas, J.F.; Launay, J.M.; Kellermann, O.; Rosay, P.; Maroteaux, L. Drosophila 5-HT2 serotonin receptor: Coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. USA 1995, 92, 5441–5445. [Google Scholar] [CrossRef] [Green Version]
- Vleugels, R.; Lenaerts, C.; Baumann, A.; Vanden Broeck, J.; Verlinden, H. Pharmacological characterization of a 5-HT1-type serotonin receptor in the red flour beetle, Tribolium castaneum. PLoS ONE 2013, 8, e65052. [Google Scholar] [CrossRef] [Green Version]
- Thamm, M.; Rolke, D.; Jordan, N.; Balfanz, S.; Schiffer, C.; Baumann, A.; Blenau, W. Function and distribution of 5-HT2 receptors in the honeybee (Apis mellifera). PLoS ONE 2013, 8, e82407. [Google Scholar] [CrossRef] [Green Version]
- Schlenstedt, J.; Balfanz, S.; Baumann, A.; Blenau, W. Am5-HT7: Molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera). J. Neurochem. 2006, 98, 1985–1998. [Google Scholar] [CrossRef]
- Lee, D.W.; Pietrantonio, P. In vitro expression and pharmacology of the 5-HT7-like receptor present in the mosquito Aedes aegypti tracheolar cells and hindgut-associated nerves. Insect Mol. Biol. 2003, 12, 561–569. [Google Scholar] [CrossRef]
- Thamm, M.; Balfanz, S.; Scheiner, R.; Baumann, A.; Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci. 2010, 67, 2467–2479. [Google Scholar] [CrossRef]
- Troppmann, B.; Balfanz, S.; Baumann, A.; Blenau, W. Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1 receptor. Br. J. Pharmacol. 2010, 159, 1450–1462. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, L.; Wu, Y.; Li, Y.; Chen, X.; Chen, J.; Wang, Q.; Liao, C.; Han, Q. Deletion of the Serotonin Receptor 7 Gene Changed the Development and Behavior of the Mosquito, Aedes aegypti. Insects 2022, 13, 671. https://doi.org/10.3390/insects13080671
Li M, Zhang L, Wu Y, Li Y, Chen X, Chen J, Wang Q, Liao C, Han Q. Deletion of the Serotonin Receptor 7 Gene Changed the Development and Behavior of the Mosquito, Aedes aegypti. Insects. 2022; 13(8):671. https://doi.org/10.3390/insects13080671
Chicago/Turabian StyleLi, Miaozhen, Lei Zhang, Yuchen Wu, Yixun Li, Xin Chen, Jing Chen, Qiuhui Wang, Chenghong Liao, and Qian Han. 2022. "Deletion of the Serotonin Receptor 7 Gene Changed the Development and Behavior of the Mosquito, Aedes aegypti" Insects 13, no. 8: 671. https://doi.org/10.3390/insects13080671





