Effect of Insulin Receptor on Juvenile Hormone Signal and Fecundity in Spodoptera litura (F.)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Bovine Insulin Microinjection
2.3. RNA Isolation and Quantitative Real-Time-Polymerase Chain Reaction (qRT-PCR)
2.4. Sample Collection for Different Tissue Expression Analysis
2.5. RNA Interference
2.6. Determination of Protein and Triglyceride (TAG)
2.7. JH- III Determination
2.8. Western Blotting
2.9. Ovary Dissection and Microscopy
2.10. Statistical Analysis
3. Results
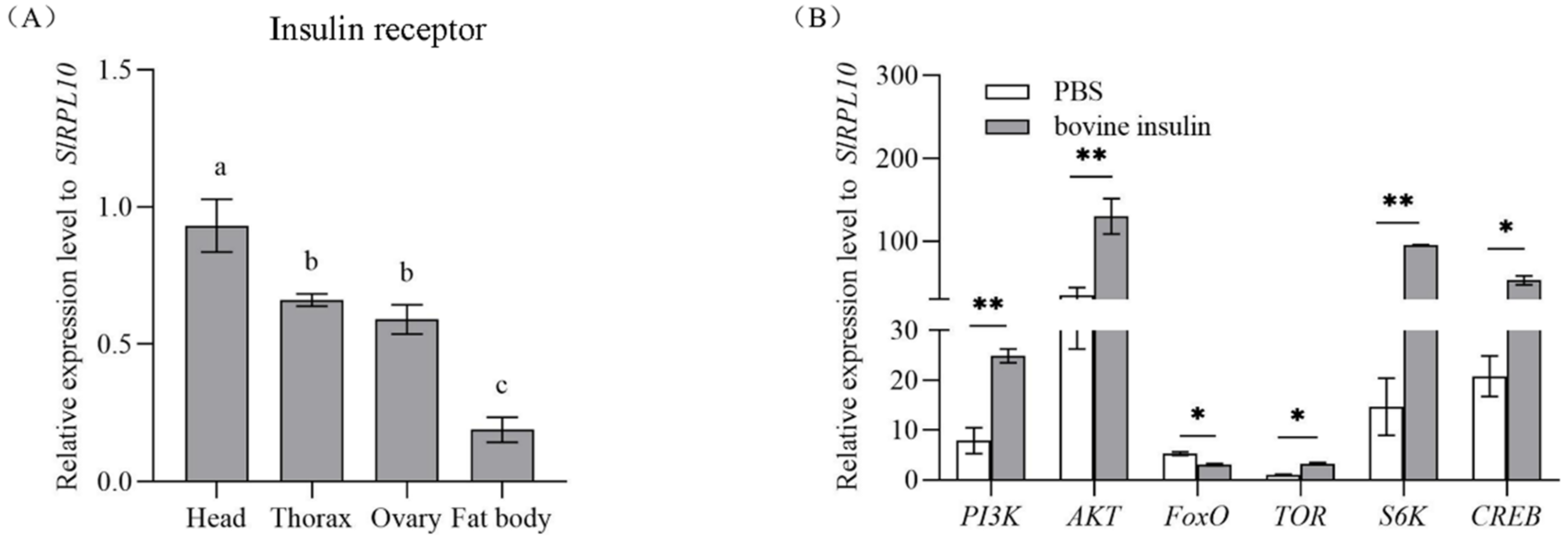
3.1. Tissue-Specific Expression of SlInR
3.2. Changes in Insulin Pathway Gene Expression after Bovine Insulin Application
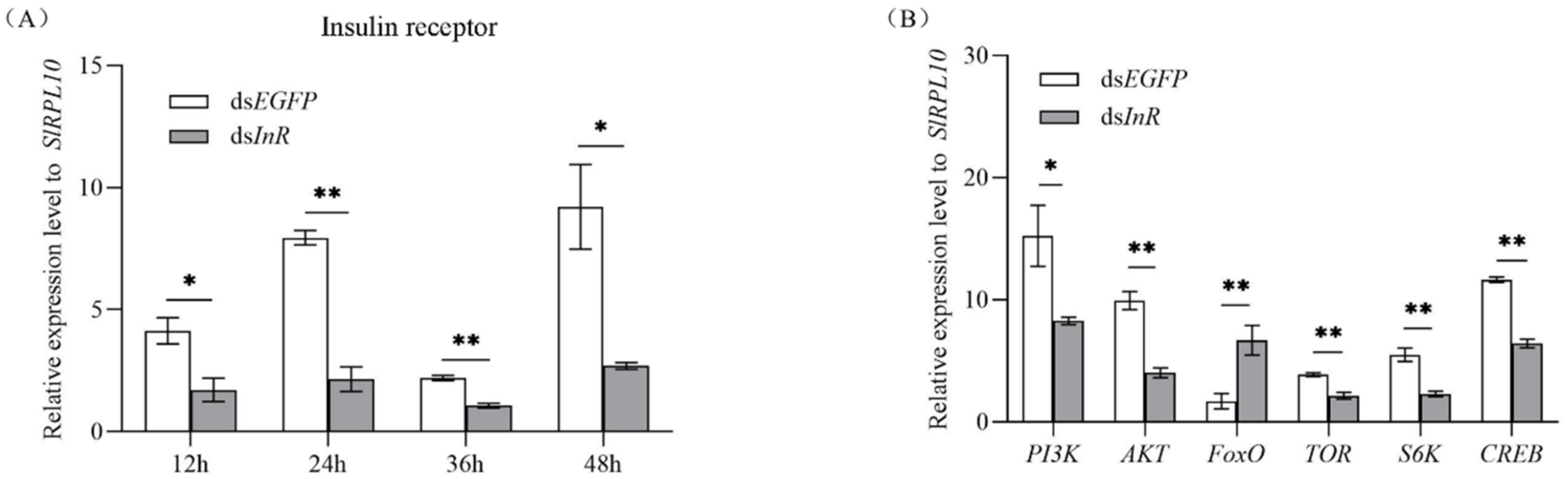
3.3. Effect of SlInR Silence on Insulin Pathway Gene expression
3.4. Effect of SlInR Knockdown on Protein and Triglyceride (TAG) Metabolism
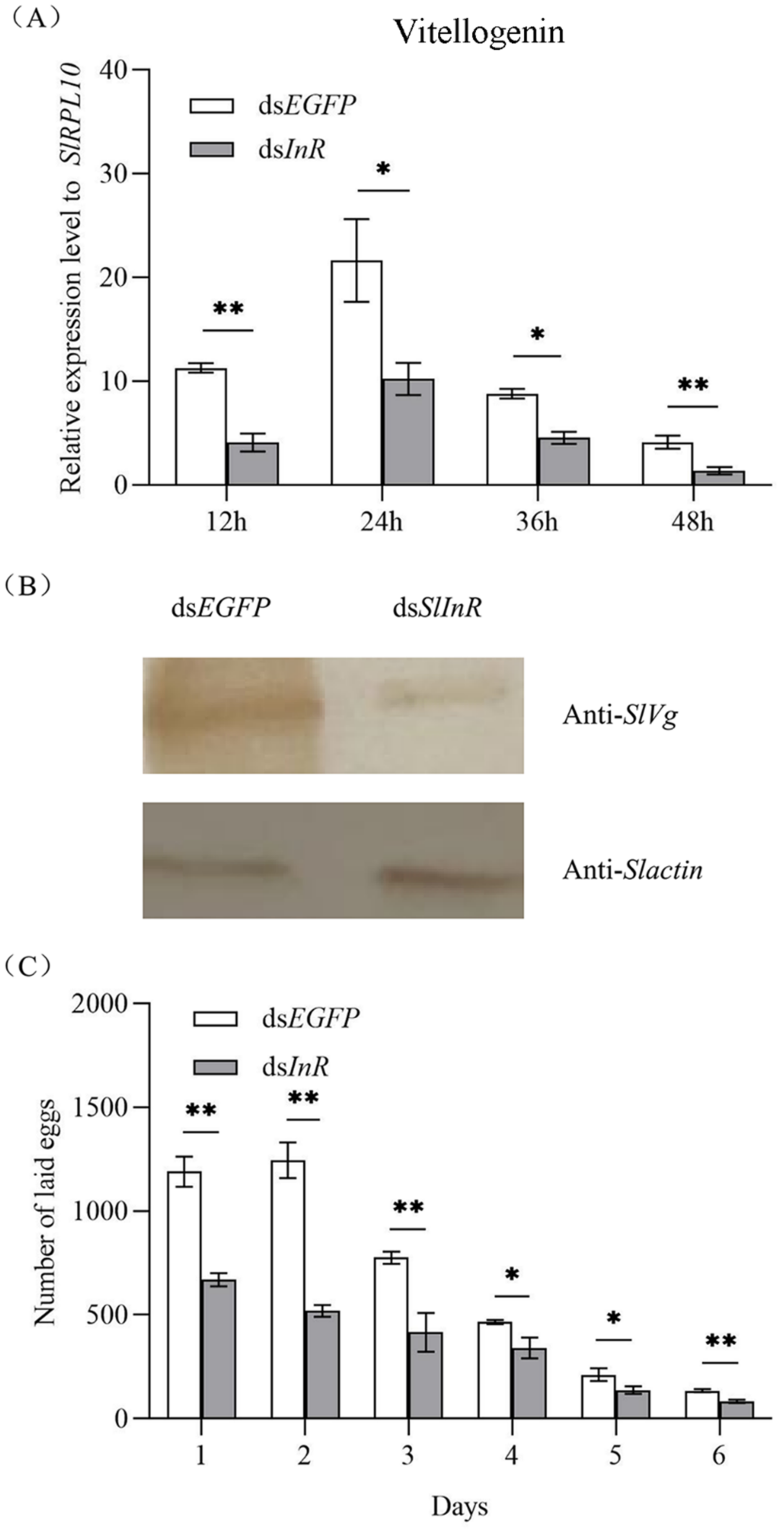
3.5. Effect of SlInR Silence on SlVg Expression and Reproduction
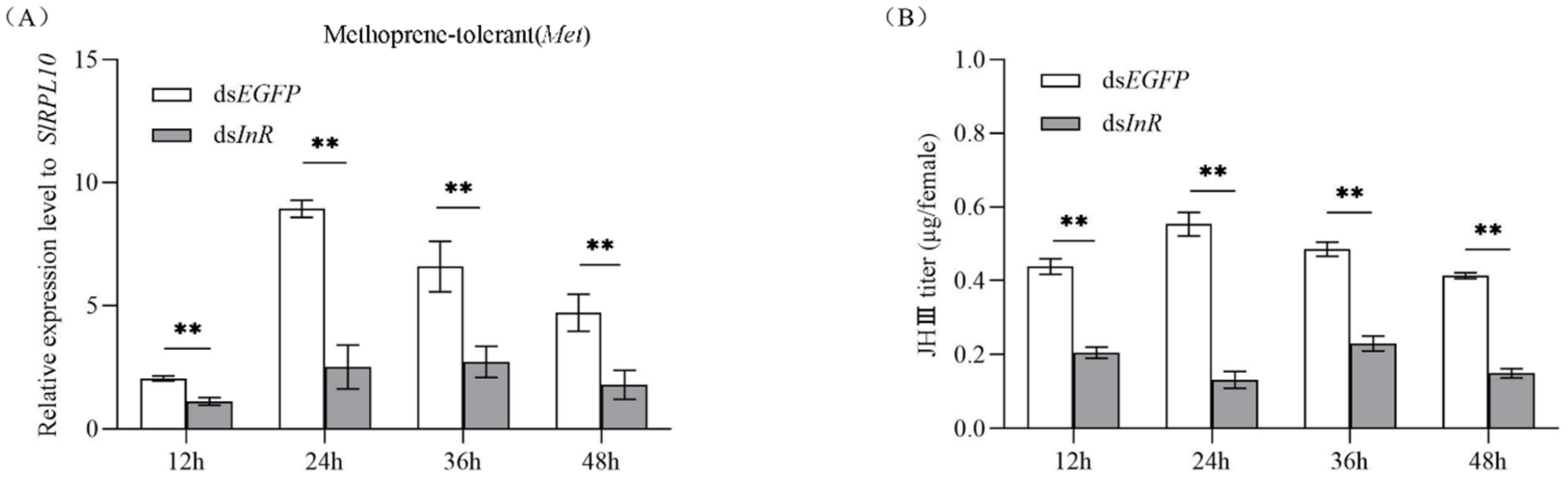
3.6. Effect of SlInR Interference on SlMet Expression and JH-III titer
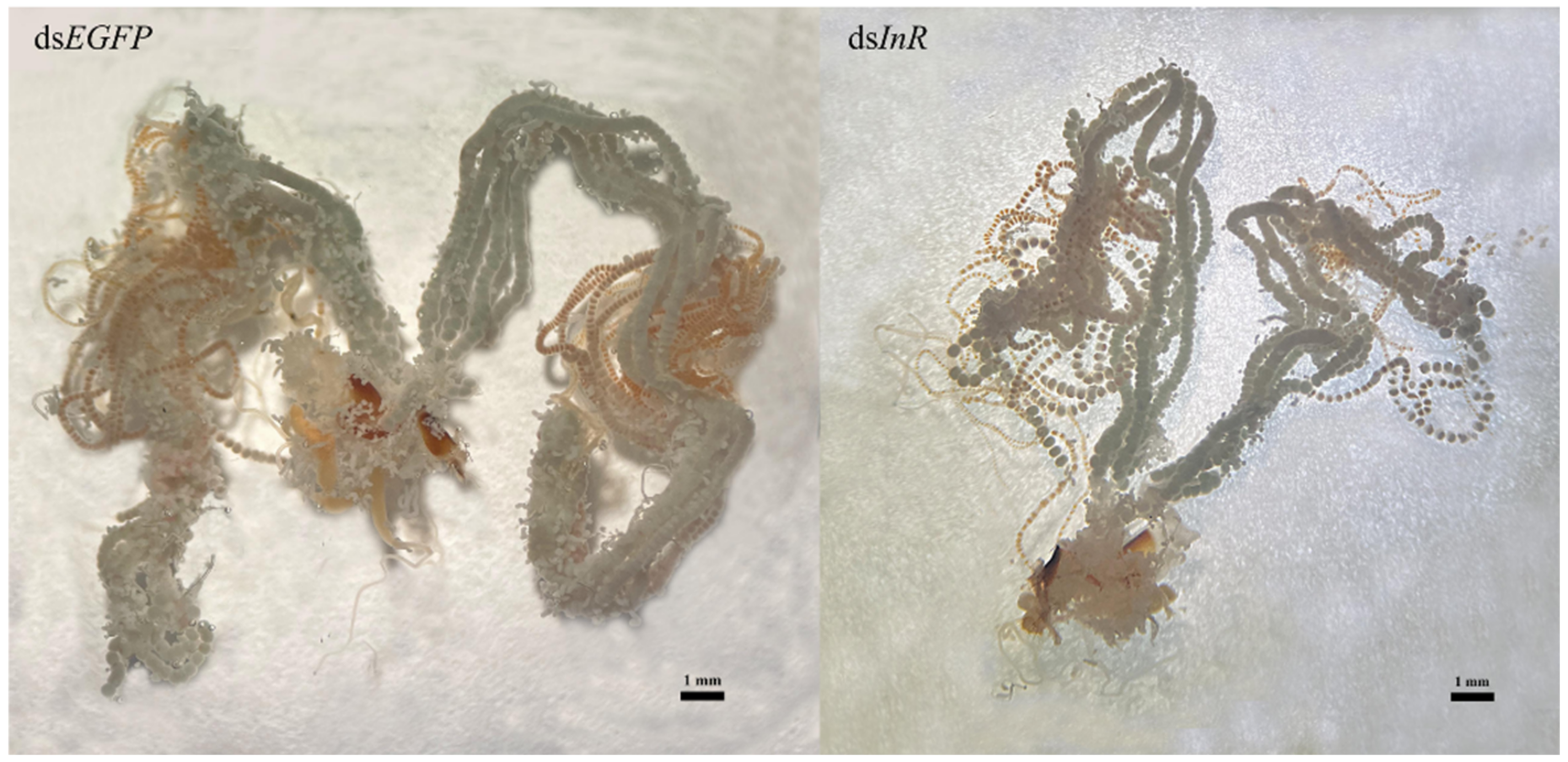
3.7. Effect of SlInR Knockdown on Ovarian Development
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Niu, J.Z.; Ding, B.Y.; Zhang, Q.; Ye, C.; Zhang, W.; Smagghe, G.; Wang, J.J. Vitellogenin and its receptor play essential roles in the development and reproduction of the brown citrus aphid, Aphis (Toxoptera) citricidus. Insect Mol. Biol. 2018, 27, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.H.; Kunkel, J.G. Vitellogenin and Vitellin in Insects. Annu. Rev. Entomol. 1979, 24, 475–505. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, N.; Jiang, H.; Dong, F.; Yang, X.; Xu, X.; Qian, K.; Meng, X.; Wang, J. Involvement of Two Paralogous Methoprene-Tolerant Genes in the Regulation of Vitellogenin and Vitellogenin Receptor Expression in the Rice Stem Borer, Chilo suppressalis. Front. Genet. 2020, 11, 609. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Zhang, M.; Chen, W.; Zhang, G. Food source affects the expression of vitellogenin and fecundity of a biological control agent, Neoseiulus cucumeris. Exp. Appl. Acarol. 2014, 63, 333–347. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Granger, N.A.; Roe, R.M. The juvenile hormones: Historical facts and speculations on future research directions. Insect Biochem. Mol. Biol. 2000, 30, 617–644. [Google Scholar] [CrossRef]
- Jindra, M.; Bellés, X.; Shinoda, T. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 2015, 11, 39–46. [Google Scholar] [CrossRef]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA 2011, 108, 638–643. [Google Scholar] [CrossRef]
- Naruse, S.; Washidu, Y.; Miura, K.; Shinoda, T.; Minakuchi, C. Methoprene-tolerant is essential for embryonic development of the red flour beetle Tribolium castaneum. J. Insect Physiol. 2020, 121, 104017. [Google Scholar] [CrossRef]
- Hiruma, K.; Kaneko, Y. Hormonal regulation of insect metamorphosis with special reference to juvenile hormone biosynthesis. Curr. Top. Dev. Biol. 2013, 103, 73–100. [Google Scholar]
- Xu, J.; Sheng, Z.; Palli, S.R. Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003535. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Leinwand, S.G.; Scott, K. Juvenile hormone drives the maturation of spontaneous mushroom body neural activity and learned behavior. Neuron 2021, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Malka, O.; Grozinger, C.; Hefetz, A. Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Fronstin, R.B.; Hatle, J.D. A cumulative feeding threshold required for vitellogenesis can be obviated with juvenile hormone treatment in lubber grasshoppers. J. Exp. Biol. 2008, 211 Pt 1, 79–85. [Google Scholar] [CrossRef][Green Version]
- Hatle, J.D.; Juliano, S.A.; Borst, D.W. Hemolymph ecdysteroids do not affect vitellogenesis in the lubber grasshopper. Arch. Insect Biochem. Physiol. 2003, 52, 45–57. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sun, Z.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef]
- Gujar, H.; Palli, S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 35546. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Cardoso-Jaime, V.; Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Juvenile hormone controls ovarian development in female Anopheles albimanus mosquitoes. Sci. Rep. 2019, 9, 2127. [Google Scholar] [CrossRef]
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, G.C.; Zeng, F.F.; Liu, C.Y.; Mao, J.J. Insulin-like peptides regulate vitellogenesis and oviposition in the green lacewing, Chrysopa septempunctata. Bull. Entomol. Res. 2017, 107, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Colombani, J.; Andersen, D.S.; Boulan, L.; Boone, E.; Romero, N.; Virolle, V.; Texada, M.; Léopold, P. Drosophila Lgr3 Couples Organ Growth with Maturation and Ensures Developmental Stability. Curr. Biol. 2015, 25, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef]
- Badisco, L.; Marchal, E.; Van Wielendaele, P.; Verlinden, H.; Vleugels, R.; Vanden Broeck, J. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria. Peptides 2011, 32, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, T.; Feng, Y.; Liu, X.; Zhang, L.; Chen, H.; Zeng, F.; Wang, M.; Liu, C.; Li, Y.; et al. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020, 123, 104049. [Google Scholar] [CrossRef] [PubMed]
- Werz, C.; Köhler, K.; Hafen, E.; Stocker, H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet 2009, 5, e1000596. [Google Scholar] [CrossRef]
- Kim, S.E.; Cho, J.Y.; Kim, K.S.; Lee, S.J.; Lee, K.H.; Choi, K.Y. Drosophila PI3 kinase and Akt involved in insulin-stimulated proliferation and ERK pathway activation in Schneider cells. Cell. Signal. 2004, 16, 1309–1317. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Roy, S.G.; Raikhel, A.S. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J. Biol. Chem. 2005, 280, 20565–20572. [Google Scholar] [CrossRef]
- Wang, X.X.; Geng, S.L.; Zhang, X.S.; Xu, W.H. P-S6K is associated with insect diapause via the ROS/AKT/S6K/CREB/HIF-1 pathway in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2020, 120, 103262. [Google Scholar] [CrossRef]
- Nässel, D.R.; Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef]
- Chen, Q.; Li, G.; Pang, Y. A simple artificial diet for mass rearing of some noctuid species. Entomol. Knowl. 2000, 37, 325–327. [Google Scholar]
- Dong, X.; Zhong, G.; Hu, M.; Yi, X.; Zhao, H.; Wang, H. Molecular cloning and functional identification of an insect odorant receptor gene in Spodoptera litura (F.) for the botanical insecticide rhodojaponin III. J. Insect Physiol. 2013, 59, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, J.; Xu, R.; Li, X.; Wang, Y.; Pan, X.; Zhang, C.; Li, Y.; Wang, F.; Li, C. Molecular identification and lipid mobilization role of adipokinetic hormone receptor in Spodoptera litura (F.). Bull. Entomol. Res. 2022, 18, 1–8. [Google Scholar] [CrossRef]
- Plantevin, G.; De Reggi, M.; Nardon, C. Changes in ecdysteroid and juvenile hormone titers in the hemolymph of Galleria mellonella larvae and pupae. Gen. Comp. Endocrinol. 1984, 56, 218–230. [Google Scholar] [CrossRef]
- Uno, T.; Furutani, M.; Sakamoto, K.; Uno, Y.; Kanamaru, K.; Mizoguchi, A.; Hiragaki, S.; Takeda, M. Localization and functional analysis of the insect-specific RabX4 in the brain of Bombyx mori. Arch. Insect Biochem. Physiol. 2017, 96, 1–13. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Liu, C.; Kuei, C.; Sutton, S.; Chen, J.; Bonaventure, P.; Wu, J.; Nepomuceno, D.; Kamme, F.; Tran, D.T.; Zhu, J.; et al. INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J. Biol. Chem. 2005, 280, 292–300. [Google Scholar] [CrossRef]
- Yenush, L.; Fernandez, R.; Myers, M.G.; Grammer, T.C., Jr.; Sun, X.J.; Blenis, J.; Pierce, J.H.; Schlessinger, J.; White, M.F. The Drosophila insulin receptor activates multiple signaling pathways but requires insulin receptor substrate proteins for DNA synthesis. Mol. Cell. Biol. 1996, 16, 2509–2517. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Riehle, M.A.; Fan, Y.; Cao, C.; Brown, M.R. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: Expression, cellular localization, and phylogeny. Peptides 2006, 27, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.S.; Rybczynski, R.; Wilson, T.G.; Wang, Y.; Wayne, M.L.; Zhou, Y.; Partridge, L.; Harshman, L.G. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: Female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 2005, 51, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.W.; Wu, F.Y.; Ye, Y.; Li, T.; Zhang, Z.Y.; Zhang, H. Small GTPase Rab40C is upregulated by 20-hydroxyecdysone and insulin pathways to regulate ovarian development and fecundity. Insect Sci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Li, C.; Wu, W.; Li, B. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene 2016, 585, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Nuss, A.B.; Brown, M.R.; Murty, U.S.; Gulia-Nuss, M. Insulin receptor knockdown blocks filarial parasite development and alters egg production in the southern house mosquito, Culex quinquefasciatus. PLoS Negl. Trop. Dis. 2018, 12, e0006413. [Google Scholar] [CrossRef]
- Pri-Tal, B.M.; Brown, J.M.; Riehle, M.A. Identification and characterization of the catalytic subunit of phosphatidylinositol 3-kinase in the yellow fever mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2008, 38, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Palli, S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef]
- Leyria, J.; Orchard, I.; Lange, A.B. Transcriptomic analysis of regulatory pathways involved in female reproductive physiology of Rhodnius prolixus under different nutritional states. Sci. Rep. 2020, 10, 11431. [Google Scholar] [CrossRef]
- Zhai, Y.; Sun, Z.; Zhang, J.; Kang, K.; Chen, J.; Zhang, W. Activation of the TOR Signalling Pathway by Glutamine Regulates Insect Fecundity. Sci. Rep. 2015, 5, 10694. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, W.; Kang, K.; Pang, R.; Dong, Y.; Liu, K.; Zhang, W. FoxO directly regulates the expression of TOR/S6K and vitellogenin to modulate the fecundity of the brown planthopper. Sci. China Life Sci. 2021, 64, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, N.T.; Sun, G.; Wang, S.F.; Raikhel, A.S. CREB isoform represses yolk protein gene expression in the mosquito fat body. Mol. Cell. Endocrinol. 2003, 210, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Süren-Castillo, S.; Abrisqueta, M.; Maestro, J.L. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2012, 42, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.K.; Yang, W.J.; Tian, Y.; Wu, Y.B.; Wang, J.J. Insulin signaling pathway in the oriental fruit fly: The role of insulin receptor substrate in ovarian development. Gen. Comp. Endocrinol. 2015, 216, 125–133. [Google Scholar] [CrossRef]
- Goodman, M.H.; Potter, M.F.; Haynes, K.F. Effects of juvenile hormone analog formulations on development and reproduction in the bed bug Cimex lectularius (Hemiptera: Cimicidae). Pest Manag. Sci. 2013, 69, 240–244. [Google Scholar] [CrossRef]
- Sim, C.; Denlinger, D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef]
- Shiao, S.H.; Hansen, I.A.; Zhu, J.; Sieglaff, D.H.; Raikhel, A.S. Juvenile hormone connects larval nutrition with target of rapamycin signaling in the mosquito Aedes aegypti. J. Insect Physiol. 2008, 54, 231–239. [Google Scholar] [CrossRef]
- Abrisqueta, M.; Süren-Castillo, S.; Maestro, J.L. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2014, 49, 14–23. [Google Scholar] [CrossRef]
- Lu, K.; Chen, X.; Liu, W.T.; Zhang, X.Y.; Chen, M.X.; Zhou, Q. Nutritional Signaling Regulates Vitellogenin Synthesis and Egg Development through Juvenile Hormone in Nilaparvata lugens (Stål). Int. J. Mol. Sci. 2016, 17, 269. [Google Scholar] [CrossRef]
- Wheeler, D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996, 41, 407–431. [Google Scholar] [CrossRef]
- Tufail, M.; Elmogy, M.; Ali Fouda, M.M.; Elgendy, A.M.; Bembenek, J.; Trang, L.T.; Shao, Q.M.; Takeda, M. Molecular cloning, characterization, expression pattern and cellular distribution of an ovarian lipophorin receptor in the cockroach, Leucophaea maderae. Insect Mol. Biol. 2009, 18, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chase, J.; Sevala, V.L.; Schal, C. Lipophorin-facilitated hydrocarbon uptake by oocytes in the German cockroach Blattella germanica (L.). J. Exp. Biol. 2002, 205 Pt 6, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Fruttero, L.L.; Frede, S.; Rubiolo, E.R.; Canavoso, L.E. The storage of nutritional resources during vitellogenesis of Panstrongylus megistus (Hemiptera: Reduviidae): The pathways of lipophorin in lipid delivery to developing oocytes. J. Insect Physiol. 2011, 57, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, X.; Li, W.; Cheng, Y.; Li, Y.; Zhou, J.; You, K.; Song, Y.; et al. Adipokinetic Hormone Receptor Mediates Trehalose Homeostasis to Promote Vitellogenin Uptake by Oocytes in Nilaparvata lugens. Front. Physiol. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Ziegler, R.; Van Antwerpen, R. Lipid uptake by insect oocytes. Insect Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef]
- Fox, C.W.; Czesak, M.E. Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 2000, 45, 341–369. [Google Scholar] [CrossRef]

| Gene | Name | Sequence (5′-3′) |
|---|---|---|
| For qRT-PCR | ||
| SlInR (XM_022966387.1) | SlInR- qF | CATTGACGCCGTCTGGTTTG |
| SlInR- qR | CAGCGGAGGTGTTTCCTGAG | |
| SlPI3K (XM_022968595.1) | SlPI3K- qF | ATACATGACGAGTACGCCCGAG |
| SlPI3K- qR | TCAGTACGGAGGCAGACAGTAG | |
| SlAKT (XM_022971351.1) | SlAKT- qF | GGACAAGGACGGACACATCAAG |
| SlAKT- qR | GCTCAGGATCAGCGAGAACAAC | |
| SlFoxO (XM_022969964.1) | SlFoxO- qF | AACGGACTTCGGACAAGACG |
| SlFoxO- qR | TCGCGGACACCCACTATAAG | |
| SlTOR (XM_022966802.1) | SlTOR- qF | GACAGAACAACAGCCAAGGGAG |
| SlTOR- qR | CGGAAGTGGAGTCAGAAACAGG | |
| SlS6K (XM_022972696.1) | SlS6K- qF | AGGACAGACCCAGGATGATGAC |
| SlS6K- qR | ATAGACGACCCCGAGACTCCAC | |
| SlCREB (XM_022973491.1) | SlCREB- qF | CTTCAATGACATCCAGGGCGAC |
| SlCREB- qR | ATTTGATGCTGCTTCCTCCCAC | |
| SlVg (EU095334.1) | SlVg- qF | CCCACCACACTCTTGCTTTCTC |
| SlVg- qR | GTCCTTGTTCACGTTCCCTGTC | |
| SlMet (2595041) | SlMet- qF | ACGCTCTGACAAAGCAAC |
| SlMet- qR | CTGGCATCGAACTAGACAATAC | |
| SlRPL10 (KC866373.1) | SlRPL10- qF | GACTTGGGTAAGAAGAAG |
| SlRPL10- qR | GATGACATGGAATGGATG | |
| For SlInR dsRNA synthesis | ||
| SlInR (XM_022966387.1) | SlInR ds- F1 | TCGGAATGGTATACGAAGGC |
| SlInR ds- R1 | TCTGGTCATACCGAAGTCCC | |
| T7 SlInR ds- F | GGATCCTAATACGACTCACTATAGGG-TCGGAATGGTATACGAAGGC | |
| T7 SlInR ds- R | GGATCCTAATACGACTCACTATAGGG-TCTGGTCATACCGAAGTCCC | |
| EGFP DQ768212 | EGFP ds- F1 | GCTGACCCTGAAGTTCATCTG |
| EGFP ds- R1 | GAACTCCAGCAGGACCATGT | |
| T7 EGFP ds- F | GGATCCTAATACGACTCACTATAGGG-GCTGACCCTGAAGTTCATCTG | |
| T7 EGFP ds- R | GGATCCTAATACGACTCACTATAGGG-GAACTCCAGCAGGACCATGT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Pei, Y.; Zhang, C.; Huang, Y.; Chen, L.; Wei, L.; Li, C.; Dong, X.; Chen, X. Effect of Insulin Receptor on Juvenile Hormone Signal and Fecundity in Spodoptera litura (F.). Insects 2022, 13, 701. https://doi.org/10.3390/insects13080701
Pan X, Pei Y, Zhang C, Huang Y, Chen L, Wei L, Li C, Dong X, Chen X. Effect of Insulin Receptor on Juvenile Hormone Signal and Fecundity in Spodoptera litura (F.). Insects. 2022; 13(8):701. https://doi.org/10.3390/insects13080701
Chicago/Turabian StylePan, Xue, Yanfang Pei, Cuici Zhang, Yaling Huang, Ling Chen, Liqiong Wei, Chuanren Li, Xiaolin Dong, and Xiang Chen. 2022. "Effect of Insulin Receptor on Juvenile Hormone Signal and Fecundity in Spodoptera litura (F.)" Insects 13, no. 8: 701. https://doi.org/10.3390/insects13080701
APA StylePan, X., Pei, Y., Zhang, C., Huang, Y., Chen, L., Wei, L., Li, C., Dong, X., & Chen, X. (2022). Effect of Insulin Receptor on Juvenile Hormone Signal and Fecundity in Spodoptera litura (F.). Insects, 13(8), 701. https://doi.org/10.3390/insects13080701






