Simple Summary
Aeolothrips intermedius is a native predator of Thrips tabaci, one of the most harmful thrips for many open field crops. Our study evaluated the predatory efficiency of A. intermedius against T. tabaci adults. The results showed that A. intermedius can be considered a promising resource for the biological control of onion thrips.
Abstract
The onion thrips, Thrips tabaci, is a main insect pest for many field crops worldwide, with a particular preference for the species of the genus Allium. Aeolothrips intermedius is a banded thrips, whose larvae are considered the primary native predator of T. tabaci. Due of their predatory behaviour, A. intermedius larvae are considered a good candidate for biological control against thrips pests. However, limited information is available on the specific predation rate of A. intermedius against T. tabaci. The aim of our study was to evaluate the predatory efficiency of A. intermedius larvae against T. tabaci adults. Predation assays, performed under laboratory conditions, indicated that A. intermedius larvae begin to prey after an average of about 23 min, and the time taken by an A. intermedius larva to capture and subdue the prey until its death is about 26 min. Furthermore, the maximum number of prey that the A. intermedius larvae are able to kill in 12 h is up to eight adults of T. tabaci/A. intermedius larva.
1. Introduction
The onion thrips Thrips tabaci Lindeman, 1889 (Thysanoptera: Thripidae) (Figure 1), is one of the most harmful polyphagous pests for many field crops [1]. In particular, T. tabaci causes huge damage to Alliaceae [2] and Brassicaceae crops [3]. In onion crops, T. tabaci, if uncontrolled, can reduce the yield by as much as 75% [4]. In addition, T. tabaci is also a vector of the Iris Yellow Spot Virus (IYSV), a significant disease affecting onion, leek, iris, and wild Allium species [5].

Figure 1.
Adult of the onion thrips Thrips tabaci (Photo by R. Antonelli).
The use of synthetic insecticides is the most adopted method for the control of this pest. However, because of its high reproductive rate, short generation time, and wide host range [6], T. tabaci is difficult to control and the treatments have to be repeated frequently, which results in the presence of insecticide residues in food products, resistance development, and high costs [7,8,9,10,11]. Due to the increasing concern about the side effects of the overuse of synthetic insecticides, new eco-friendly approaches for managing thrips pests, such as biological controls, are demanded by growers and consumers. In this scenario, biological control systems may help to reduce both pest populations and virus incidence not only in conventional and IPM farming, but also in organic farming. Among the biological control systems, some natural products [12,13,14] seem useful for harmful thrips control, even if they are not decisive [15].
Among the T. tabaci biological control agents, some predators, such as predatory mites (Neoseiulus spp.) and Hemiptera (Orius spp.), have been reported [16]. However, the efficacy of these predators is not considered decisive [6]. Among the T. tabaci predators, Aeolothrips intermedius Bagnall, 1934 (Thysanoptera: Aeolothripidae), is a cosmopolitan species [17,18], also known as “banded thrips” (Figure 2), even if all the congeneric species present the same characteristic of black and white banded wings. Both adults and larvae are floricolous (mainly found in Leguminosae, Rosaceae, and Poaceae; Table 1) and while adults feed mainly on flowers and pollen by their stem-sucking-rasping mouthparts, A. intermedius larvae prey on more than 44 species of Thysanoptera [19].

Figure 2.
Female of the predatory thrips Aeolothrips intermedius (Photo by R. Antonelli).
In Europe, A. intermedius larvae are reported as predators of Heliothrips haemorrhoidalis Bouché, Odontothrips confusus Priesner, and some species belonging to the genus Haplothrips [20]. A. intermedius is considered the primary native predator of Thrips tabaci [20,21,22,23,24] and a good candidate for the biological control of onion thrips in field conditions [25]. However, despite that A. intermedius has been reported as a very effective predator that may play an important role in the biological control of Thysanoptera [26], there is little information on its specific predation rate against T. tabaci. Thus, the aim of our study was to assess, using laboratory tests, the predatory efficiency of A. intermedius larvae against the crop insect pest T. tabaci. In particular, the predation tests carried out in this study were aimed to evaluate (a) the predation times of A. intermedius larvae on T. tabaci adults; (b) the predation rate of A. intermedius larvae on T. tabaci adults.

Table 1.
Plant species for which the predatory thrips Aeolothrips intermedius have been recorded.
Table 1.
Plant species for which the predatory thrips Aeolothrips intermedius have been recorded.
| Plant Species | Family | References |
|---|---|---|
| Albizzia juliprissin | Leguminosae | Marullo, 1991 [27] |
| Allium cepa | Alliaceae | Trdan, 2005 [20] |
| Avena sativa | Poaceae | Trdan, 2005 [20] |
| Brassica sp. | Brassicaceae | Marullo, 1993 [22] |
| Coronilla varia | Leguminosae | Marullo, 1993 [22] |
| Dianthus sp. | Cariophillaceae | Trdan, 2005 [20] |
| Diplotaxis sp. | Brassicaceae | Marullo, 1993 [22] |
| Echium vulgare | Boraginaceae | Zur Strassen, 1987 [28] |
| Galeca officinalis | Leguminosae | Zur Strassen, 1987 [28] |
| Galeopsis ladanum | Lamiaceae | Zur Strassen, 1991 [29] |
| Gallium sp. | Rubiaceae | Zur Strassen, 1991 [29] |
| Genista sagittalis | Leguminosae | Zur Strassen, 1991 [29] |
| Helianthus annuus | Asteraceae | Trdan, 2005 [20] |
| Helianthemum nummularium | Cistaceae | Zur Strassen, 1991 [29] |
| Hordeum vulgare | Poaceae | Trdan, 2005 [20] |
| Jasione montana | Campanulaceae | Zur Strassen, 1991 [29] |
| Lactuca sativa | Cichoriaceae | Trdan, 2005 [20] |
| Malus communis | Rosaceae | Marullo, 1993 [22] |
| Medicago sativa | Leguminosae | Mound, 1968 [30] |
| Melilotus sp. | Leguminosae | Marullo, 1993 [22] |
| Nicotiana tabacum | Solanaceae | Trdan, 2005 [20] |
| Pisum sativum | Leguminosae | Trdan, 2005 [20] |
| Pteridium aquilinum | Hypolepidaceae | Marullo, 1991 [27] |
| Quercus pubescens | Fagaceae | Marullo, 1991 [27] |
| Sinapis sp. | Brassicaceae | Zur Strassen, 1991 [29] |
| Trifolium pratense | Leguminosae | Trdan, 2005 [20] |
| Trifolium repens | Leguminosae | Trdan, 2005 [20] |
| Triticum aestivum | Poaceae | Trdan, 2005 [20] |
| Zea mays | Poaceae | Trdan, 2005 [20] |
2. Materials and Methods
2.1. Insect Specimens
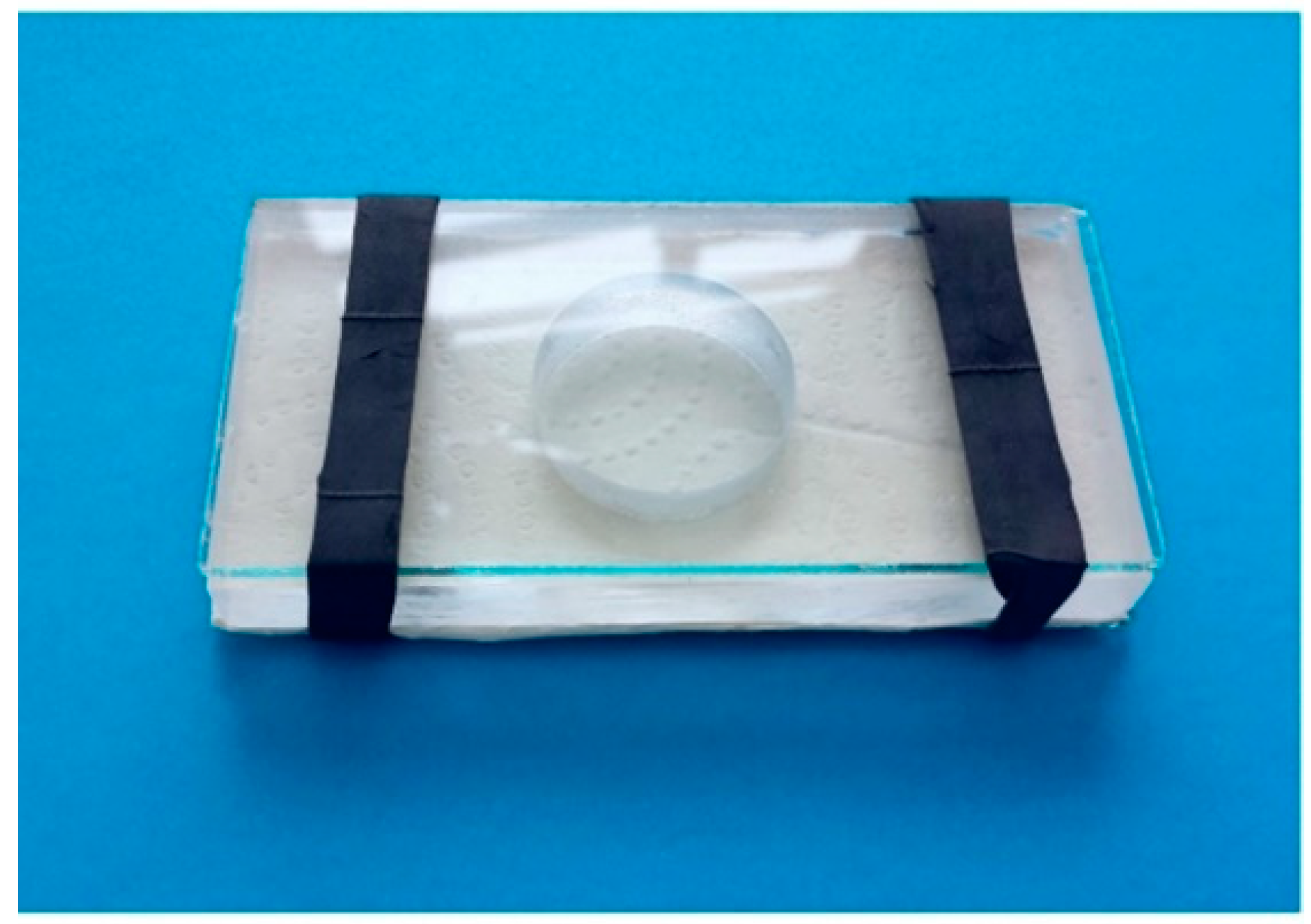
A. intermedius and T. tabaci were reared in four alfalfa (Medicago sativa L.) square parcels (1 m × 1 m) and covered with a screen (0.6–0.8 mm mesh size) at the Centro Enrico Avanzi of the University of Pisa (CiRAA) (Pisa, Italy) during the period May–September 2021. The two species were collected daily from alfalfa flowers, present in the parcels from July until the end of September, to be tested. The alfalfa flowers collected were shacked on white paper to allow for an easy visual inspection of the larval and adult A. intermedius and T. tabaci populations. One A. intermedius larva (predator) and one T. tabaci adult (prey) were collected from the surface with a fine-tipped wet brush and were transferred into a Huffaker cage [31] (Figure 3) with a green linden (Tilia platyphyllos) leaf as vegetal support to allow for observations during the predation tests [24].
Figure 3.
Huffaker cage used in the experiments (Photo by R. Antonelli).
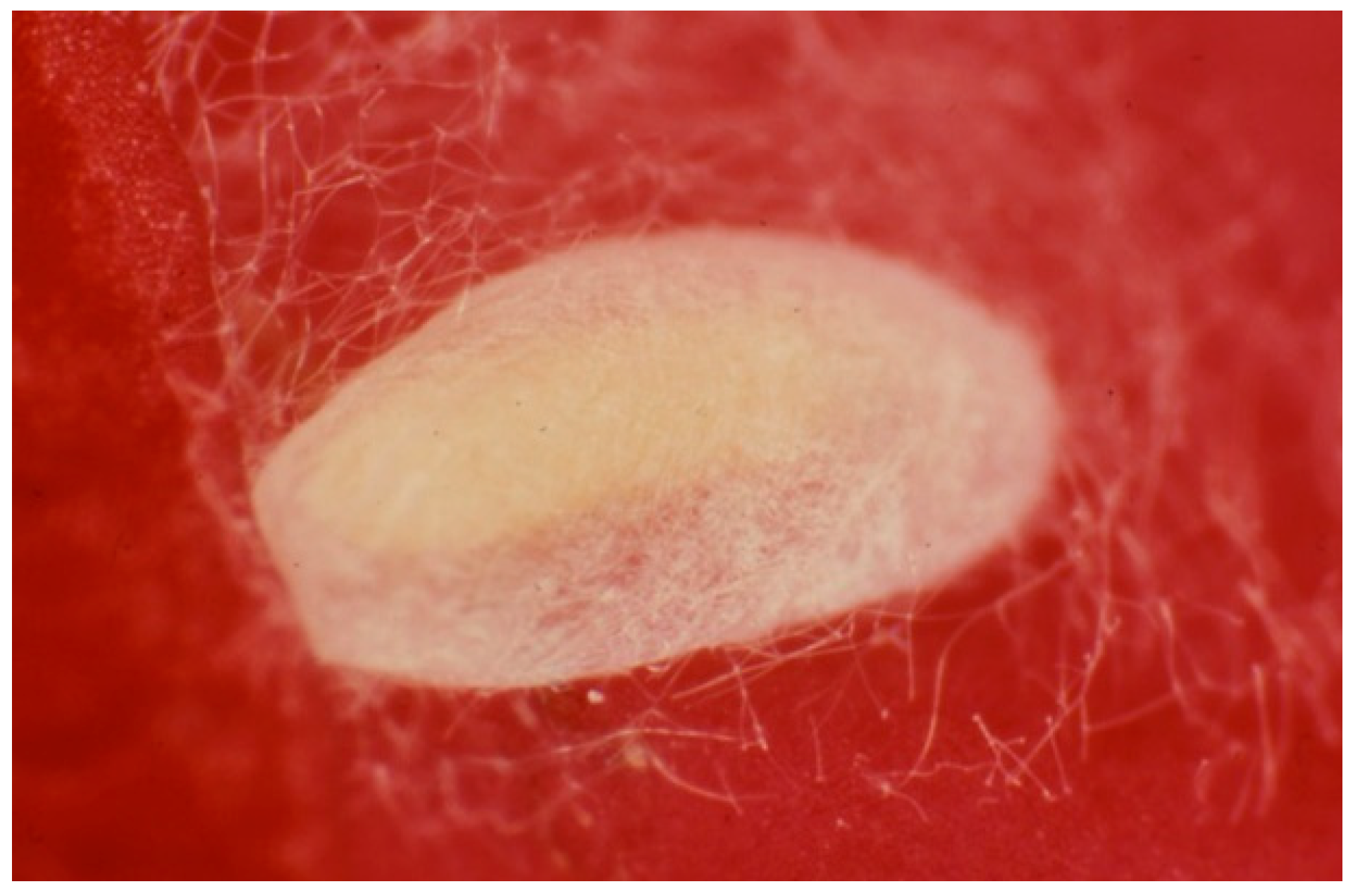
The Huffaker cages were maintained under laboratory conditions at 25 ± 2 °C, 65 ± 5% RH, and 16:8 = L:D photoperiod. Since contamination of the species in the rearing parcels could occur, to ensure the correct identification of both the prey and the predator, some randomly chosen specimens were collected and mounted onto slides by the current methodology [32], which were then observed under the microscope for a specific identification confirmation. In particular, the predator larvae were fed until they produced cocoons and the adults emerged (Figure 4).
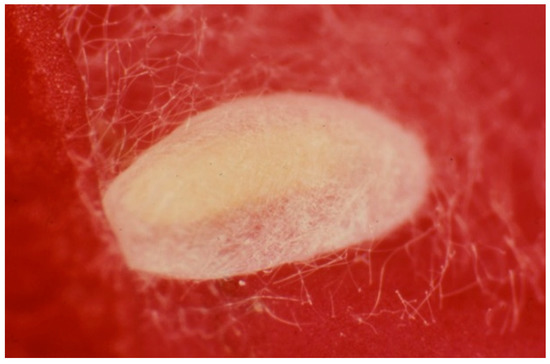
Figure 4.
Cocoon of the predatory thrips Aeolothrips intermedius (Photo by R. Antonelli).
2.2. Predation Time
To evaluate the time spent by the predator to attack and kill the prey, the A. intermedius larvae, collected the day before the test, were starved for 24 h. On the day of the test, an A. intermedius larva (L-II) and a T. tabaci adult were put in a Huffaker cage and the time when the predation started, the time spent to capture and subdue the prey, and the final time when the predator stops sucking the prey were recorded (Figure 5). In the rare case that a predation did not occur, the test was discarded. The test was replicated until 30 valid predations occurred.

Figure 5.
Predation of an Aeolothrips intermedius larva on an onion thrips Thrips tabaci adult. (A) start of the predation (the predator captures and subdues the prey); (B) end of the predation (the predator stops feeding on the prey).
2.3. Predation Rate
To determine the predation rate of the A. intermedius larvae on the T. tabaci adults, the number of prey killed by each predator (prey/predator) over 12 h was assessed. The predators were collected the day before the test and were starved for 24 h. For each replica, one A. intermedius larva and three T. tabaci adults were put in a Huffaker cage. The predation was recorded every three hours from 9.00 AM until 9.00 PM (controls were performed at 12 AM, 3 PM, 6 PM, and 9 PM). If at the check time the predator had killed all prey, three more prey were added. The number of prey inserted in the Huffaker cage at each check, the number of prey killed, and the percentage of prey killed over 12 h were recorded. The test was replicated until 30 valid predations occurred. Three T. tabaci were put in a Huffaker cage as a control for each test and the eventual natural mortality was checked every three hours from 9.00 AM until 9.00 PM.
3. Results
The predation time bioassay results indicated that the A. intermedius larvae begin to prey on the T. tabaci adults after about 23 ±14 min, and the time spent by the A. intermedius larvae in capturing and subduing the prey until it leaves the victim dead is about 26 ± 12 min. The total time from the first visual contact of the predator with the prey until the end of predation (when the predator leaves the prey) is about 49 ± 18 min.
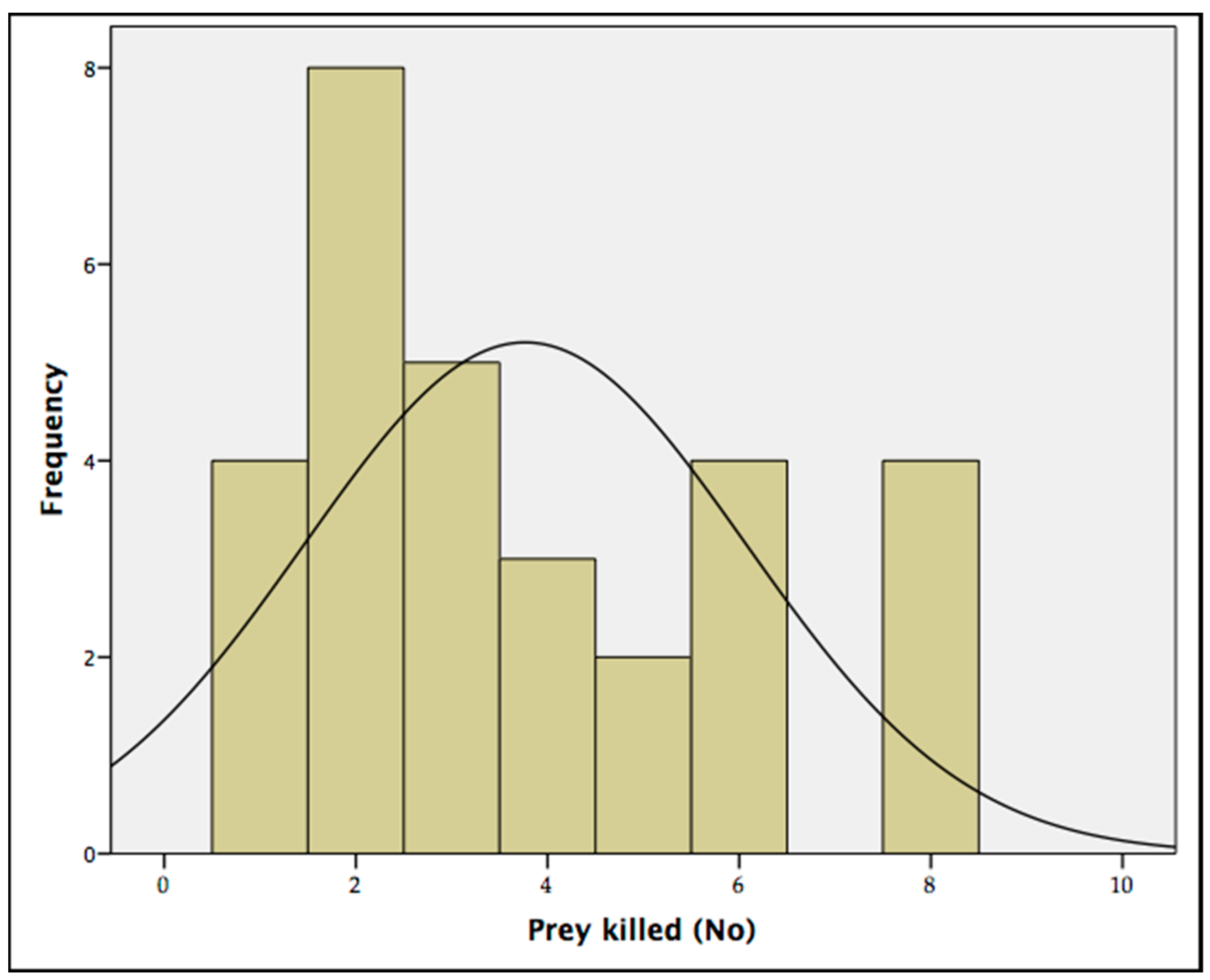
The predation rate tests showed that the maximum number of T. tabaci adults that the A. intermedius larvae were able to kill over a period of 12 h was eight prey/predator (88.89%). The average quantity of prey killed by each predator over 12 h was 3.77 ± 0.15 (Table 2), with a mode of two prey/predator (Figure 6). No natural mortality was recorded in the control tests.

Table 2.
Predation rate of Aeolothrips intermedius larvae on the onion pest Thrips tabaci adults.
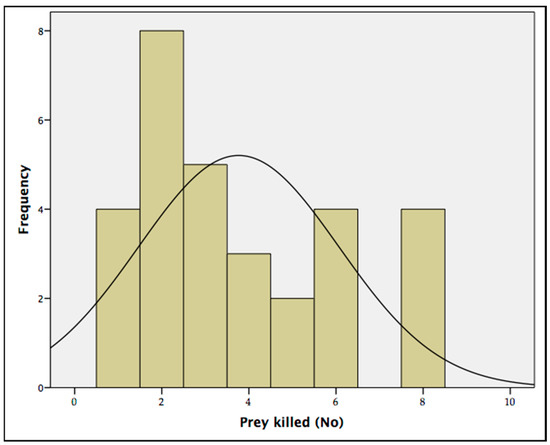
Figure 6.
Mode of the predation rate of the predator larvae Aeolothrips intermedius on onion pest Thrips tabaci adults. The histogram represents the frequencies of Thrips tabaci preyed over 12 h. The curve represents a standard normal distribution.
4. Discussion
Thrips tabaci is a problematic pest, whose control is hampered by a high reproductive rate of the species, a short generation time, and a wide range of hosts [6]. Biological controls by predatory Thysanoptera [25,33,34] showed promising results. In particular, A. intermedius has been tested to determine the correct timing for chemical treatments, the factors affecting the dynamics of its interaction with the T. tabaci population, its susceptibility to commonly used insecticides, and its interaction with other predators [25,33,34]. However, key information on the biological and ecological aspects of the predation of A. intermedius on onion thrips is still lacking [20,33,34], and to our knowledge, this is the first time that the predatory efficacy of A. intermedius against T. tabaci has been assessed.
Our results are in line with the predatory efficacy reported by Fathi et al. [33] for Orius niger (Hemiptera: Anthocoridae) and A. intermedius against T. tabaci. The authors observed high predation rates with an additive effect when the two predators were used together, while the predation was lower when each predator was used separately as follows: 77% for the A. intermedius predation and 91% for the O. niger predation [33]. Such percentages are very close to the 88.9% (eight prey killed over the nine administrated) obtained as the maximum predation rate in our tests. Similarly, Deligeorgidis [35] observed that, at a ten prey density, one O. niger female was able to prey on 73.63% of the T. tabaci and 59.66% of the F. occidentalis over 24 h. However, a lower predation efficacy was observed for Coccinella septempunctata (Coleoptera Coccinellidae) with about 51.37% of the preyed T. tabaci adults [36].
The predation rates observed in this experiment for the second instar A. intermedius larvae were close to those of the same instar of other Aeolothripidae predators currently commercialized. In fact, Mahendran and Radhakrishnan [37] observed that the commercialized Aeolothripidae predator Franklinothrips vespiformis larvae are able to prey on 12 Scirtothrips bispinosus (a Thripidae harmful for tea plantations) adults within 24 h. In our experiment, the prey (T. tabaci adults) were administrated sequentially (three prey every three hours) in order to keep the prey/predator rate quite constant during the experiment. However, Khamis and Jabbar [38] and El-Sheikh et al. [39] observed that the number of prey (T. tabaci adults) killed by Orius albipennis was significantly correlated with the prey density varying from 10 to 25 prey per day for prey densities from 10 to 60 per predator. This indicates that under field working conditions with higher prey densities, A. intermedius could increase its predatory efficacy and be a very effective predator for the control of T. tabaci, with predation rates in line with other predators already commercialized (F. vespiformis, Orius sp., and predatory mites).
As expected, due to the difference in the size of the mites compared to the predatory Thysanoptera, the predation rates obtained in this experiment are higher than those reported by other authors for mites. This could be due to due to the difference in size between the mites and the Thysanoptera. Actually, Messelink et al. [40] observed that the predator mite Amblyseius swirskii (Athias-Henriot) is able to prey on about six Frankliniella occidentalis adults in 48 h, while Berndt et al. [41] reported predation rates of 3.5 (±0.5) and 1.64 (±0.3) F. occidentalis per day by Hypoaspis aculeifer females and Stratiolaelaps miles (Berlese) females, respectively. Lower predation rates than the one we observed for A. intermedius were obtained by Walzer et al. [42] also for the mite Neoseiulus californicus, which preyed on an average of 3.19 T. tabaci larvae per day.
A main drawback to the widespread use of A. intermedius could be the difficulty of rearing. In fact, even if Bournier et al. [21] published a complete breading protocol for A. intermedius, other authors reported the difficulties in rearing the species under laboratory conditions [43]. In this work, we obtained A. intermedius larvae from spontaneous breeding in open fields. Under such conditions, it was quite easy to obtain samples for the biological assays. In this regard, in previous observations in the open field, Conti et al. [44] reported three peaks in the presence of an A. intermedius adult as follows: the first in early June, the second in mid-July, and the third in early August. This is in accordance with the results of Orosz et al. [25] and Trdan et al. [45], who recorded the first and second adult peaks of A. intermedius in the same period (except for August, probably due to the different climatic conditions between central Italy and Hungary). Overall, these results support the possibility of obtaining large numbers of A. intermedius larvae for use in the biological control of Thrips tabaci without a complicated breeding procedure.
5. Conclusions
In conclusion, the present study allowed us to describe the T. tabaci–A. intermedius prey/predator interaction. The results demonstrated that A. intermedius could be considered a promising resource for the biological control of onion thrips in fields. Overall, our data show that A. intermedius can contribute to reducing the population of T. tabaci. In addition, since A. intermedius has been observed in over 40 vegetal species as a predator of 18 thrips species, especially those belonging to the suborder of Terebrantia, such as the onion thrips [19], it may represent a very versatile biocontrol agent that is able to adapt to different plant hosts and a wide range of harmful thrips.
Author Contributions
Conceptualization, B.C.; data curation, S.B.; formal analysis, S.B.; investigation, L.A., A.G. and P.G.; writing—original draft, L.A.; writing—review and editing, L.A., S.B., A.G., P.G. and B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Murai, T. Effect of temperature on development and reproduction of the onion thrips, Thrips tabaci Lindeman (Thysanoptera: Thripidae), on pollen and honey solution. Appl. Entomol. Zool. 2000, 35, 499–504. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Miller, M.E.; Edelson, J.V.; Cartwright, B. Injury to Onions by Thrips tabaci (Thysanoptera: Thripidae) and Its Role in the Development of Purple Blotch. Environ. Entomol. 1993, 22, 1266–1277. [Google Scholar] [CrossRef]
- Shelton, A.M.; Wilsey, W.T.; Schmaedick, M.A. Management of Onion Thrips (Thysanoptera: Thripidae) on Cabbage by Using Plant Resistance and Insecticides. J. Econ. Entomol. 1998, 91, 329–333. [Google Scholar] [CrossRef]
- Hely, P.C. Control of Thrips tabaci on onions. Agric. Gaz. N. S. W. 1946, 57, 467–471. [Google Scholar]
- Gent, D.H.; du Toit, L.J.; Fichtner, S.F.; Mohan, S.K.; Pappu, H.R.; Schwartz, H.F. Iris yellow spot virus: An emerging threat to onion bulb and seed production. Plant Dis. 2006, 90, 1468–1480. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of in-creasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- MacIntyre Allen, J.K.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Resistance of Thrips tabaci to pyrethroid and organo-phosphorous insecticides in Ontario, Canada. Pest. Manag. Sci. 2005, 61, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Zhao, J.Z.; Nault, B.A.; Plate, J.; Musser, F.R.; Larentzaki, E. Patterns of insecticide resistance in onion thrips (Thysanoptera: Thripidae) in onion fields in New York. J. Econ. Entomol. 2006, 99, 1798–1804. [Google Scholar] [CrossRef]
- Bielza, P. Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest. Manag. Sci. 2008, 64, 1131–1138. [Google Scholar] [CrossRef]
- Herron, G.A.; James, T.M.; Rophail, J.; Mo, J. Australian populations of onion thrips, Thrips tabaci Lindeman (Thysanoptera: Thripidae), are resistant to some insecticides used for their control. Aust. J. Entomol. 2008, 47, 361–364. [Google Scholar] [CrossRef]
- Gao, Y.; Lei, Z.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest. Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Conti, B. Eco-friendly control strategies against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae): Repellency and toxic activity of plant essential oils and extracts. Pharmacologyonline 2014, 1, 44–50. [Google Scholar]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasit. Vectors 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential Oil Compounds for Thrips Control—A Review. Nat. Prod. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Loomans, A.J.M.; Murai, T. Culturing thrips and parasitoids. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International Harpenden: Herts, UK, 1997; pp. 477–503. [Google Scholar]
- Conti, B. Thysanoptera collected by suction trap in the San Rossore-Migliarino-Massaciuccoli nature reserve (Pisa, Italy). Boll. Soc. Entomol. Ital. 2002, 134, 3–19. [Google Scholar]
- Conti, B. Thysanoptera collected in the San Rossore-Migliarino-Massaciuccoli nature reserve (Pisa, Italy). II Contribution. Boll. Soc. Entomol. Ital. 2009, 141, 9–16. [Google Scholar]
- Riudavets, J. Predators of Frankliniella occidentalis (Perg.) and Thrips tabaci Lind.: A review. WUR 1995, 95, 43–87. [Google Scholar]
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest. Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Bournier, A.; Lacasa, A.; Pivot, Y. Biologie d’un thrips prédateur Aeolothrips intermedius [Thys.: Aeolothripidae]. Entomophaga 1978, 23, 403–410. [Google Scholar] [CrossRef]
- Marullo, R. I Tisanotteri dell’Italia Meridionale: II Contributo. Le specie italiane del genere Aeolothrips Haliday. Boll. Lab. di Entomol. Agrar. Portici 1993, 50, 121–140. [Google Scholar]
- Conti, B.; Vesmanis, A. The entomofauna of Corsica, coastal Tuscany and the islands of the Tuscan Archipelago: Thysanoptera (Insecta). Frustula Entomol. 2001, XXIV, 125–142. [Google Scholar]
- Conti, B. Notes on the presence of Aeolothrips intermedius in northwestern Tuscany and on its development under laboratory conditions. Bull. Insectol. 2009, 62, 107–112. [Google Scholar]
- Orosz, S.; Bujdos, L.; Varga, L.; Fekete, T. Investigations of Thrips tabaci and Aeolothrips intermedius population dynamics in tobacco plantations. Acta Agrar. Debr. 2018, 74, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.; Sparks Jr, A.; Srinivasan, R.; Kennedy, G.; Fonsah, G.; Scott, J.; Olson, S. Thrips: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato; Academic Press: Cambridge, MA, USA, 2018; pp. 49–71. [Google Scholar]
- Marullo, R. Thysanoptera of southern Italy. First contribution. Species collected from cultivated, spontaneous and forest plants and their regional distribution. Redia 1991, 74, 185–209. [Google Scholar]
- Zur Strassen, R. On some Thysanoptera of an agricultural area located on woody slopes in northern Umbria, Italy. Redia 1987, 70, 203–228. [Google Scholar]
- Zur Strassen, R. Öekologische und phäenologistiche Daten von Fransenflüeglern aus dem Gebiet des Rotenfels/Nahe bei Bad Krenuznach (Isecta: Thysanoptera). Mitt. Internat. Entomol. Ver. 1991, 16, 119–139. [Google Scholar]
- Mound, L.A. A review of R.S. Bagnall’s Thysanoptera Collection. In Bulletin of the British Museum (Natural History). Entomology. Supplement; Biodiversity Heritage Library: London, UK, 1968; Volume 11, pp. 1–181. [Google Scholar]
- Huffaker, C.B. An improved cage for work with small insects. J. Econ. Entomol. 1948, 41, 648–649. [Google Scholar] [CrossRef]
- Mound, L.A.; Marullo, R. The Thrips of Central and South America: An Introduction. Mem. Entomol. Int. 1996, 6, 1–488. [Google Scholar]
- Fathi, S.A.A.; Asghari, A.; Sedghi, M. Interaction of Aeolothrips intermedius and Orius niger in controlling Thrips tabaci on potato. Int. J. Agric. Biol. 2008, 10, 521–525. [Google Scholar]
- Mautino, G.C.; Bosco, L.; Tavella, L. Impact of control strategies on Thrips tabaci and its predator Aeolothrips intermedius on onion crops. Phytoparasitica 2014, 42, 41–52. [Google Scholar] [CrossRef][Green Version]
- Deligeorgidis, P.N. Predatory effect of Orius niger (Wolff) (Hem. Anthocoridae) on Frankliniella occidentalis (Pergande) and Thrips tabaci Lindeman (Thysan. Thripidae). J. Appl. Entomol. 2002, 126, 82–85. [Google Scholar] [CrossRef]
- Deligeorgidis, P.N.; Ipsilandis, C.G.; Vaiopoulou, M.; Kaltsoudas, G.; Sidiropoulos, G. Predatory effect of Coccinella septempunctata on Thrips tabaci and Trialeurodes vaporariorum. J. Appl. Entomol. 2005, 129, 246–249. [Google Scholar] [CrossRef]
- Mahendran, P.; Radhakrishnan, B. Franklinothrips vespiformis Crawford (Thysanoptera: Aeolothripidae), a potential predator of the tea thrips, Scirtothrips bispinosus Bagnall in south Indian tea plantations. Entomon 2019, 44, 49–56. [Google Scholar] [CrossRef]
- Khamis, A.R.; Jabbar, A.S. Evaluation of The Predatory Efficiency of Orius Albidipennis Reuter for Two Prey Species Myzus Persicae (Sulzer) and Thrips tabaci Lind. on The Carrot Plant in Laboratory. Al-Qadisiyah J. Agric. Sci. 2021, 11, 14–22. [Google Scholar] [CrossRef]
- El-Sheikh, W.E.; Fadl, H.A.A.; El Kenway, A.H. Prey-Predator Interaction between Orius albidipennis (Hemiptera: Anthocoridae) and Thrips tabaci (Thysanoptera: Thripidae). J. Appl. Plant Prot. 2021, 10, 1–8. [Google Scholar]
- Messelink, G.J.; van Maanen, R.; van Steenpaal, S.E.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef]
- Berndt, O.; Meyhöfer, R.; Poehling, H.M. The edaphic phase in the ontogenesis of Frankliniella occidentalis and comparison of Hypoaspis miles and Hypoaspis aculeifer as predators of soil-dwelling thrips stages. Biol. Control 2004, 30, 17–24. [Google Scholar] [CrossRef]
- Walzer, A.; Paulus, W.; Schausberger, P. Ontogenetic shifts in intraguild predation on thrips by phytoseiid mites: The relevance of body size and diet specialization. Bull. Entomol. Res. 2004, 94, 577–584. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. Newsletter on biological control in greenhouses. WUR 1999, 19, 32. [Google Scholar]
- Conti, B.; Berti, F.; Mercati, D.; Giusti, F.; Dallai, R. The ultrastructure of Malpighian tubules and the chemical composition of the cocoon of Aeolothrips intermedius Bagnall (Thysanoptera). J. Morphol. 2010, 271, 244–254. [Google Scholar] [PubMed]
- Trdan, S.; Rifelj, M.; Valic, N. Population dynamics of banded thrips (Aeolothrips intermedius Bagnall, Thysanoptera, Aeolothripidae) and its potential prey Thysanoptera species on white clover. Commun. Agric. Appl. Biol. Sci. 2005, 70, 753–758. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).