Phylogenetic-Related Divergence in Perceiving Suitable Host Plants among Five Spider Mites Species (Acari: Tetranychidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plants
2.2. Spider Mites
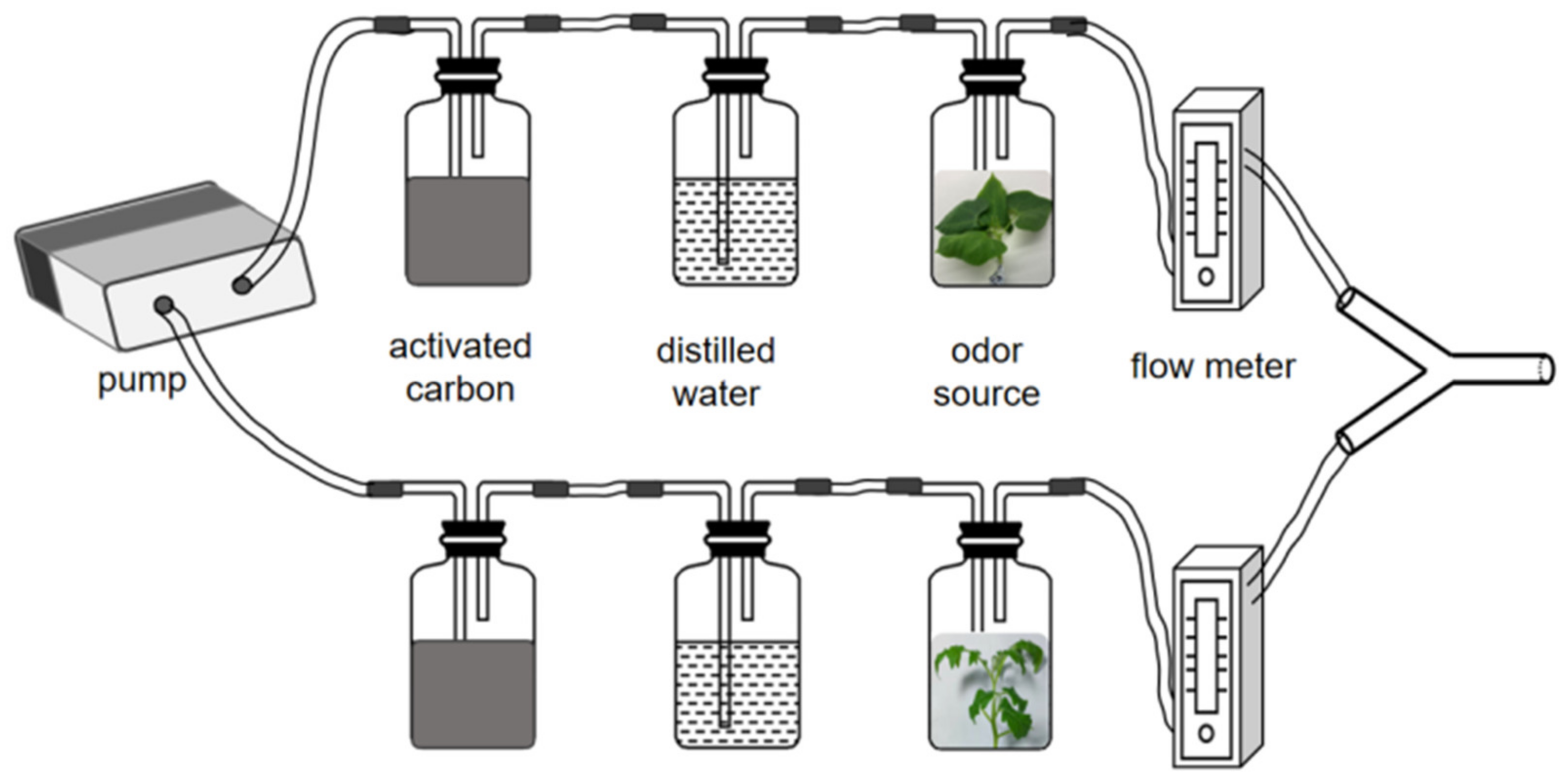
2.3. Y-Tube Olfactometer Experiments
2.4. Two-Choice Disc Bioassay
2.5. Data Analysis
3. Results
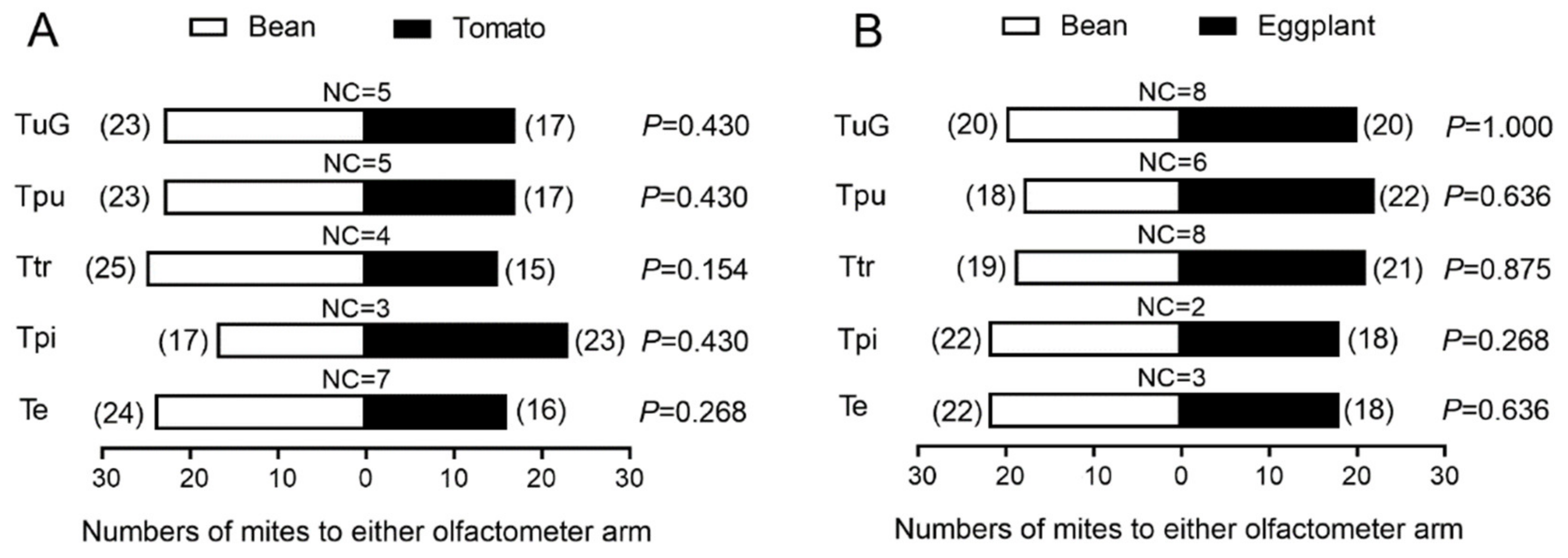
3.1. Performance in Y-Type Olfactometer Bioassay
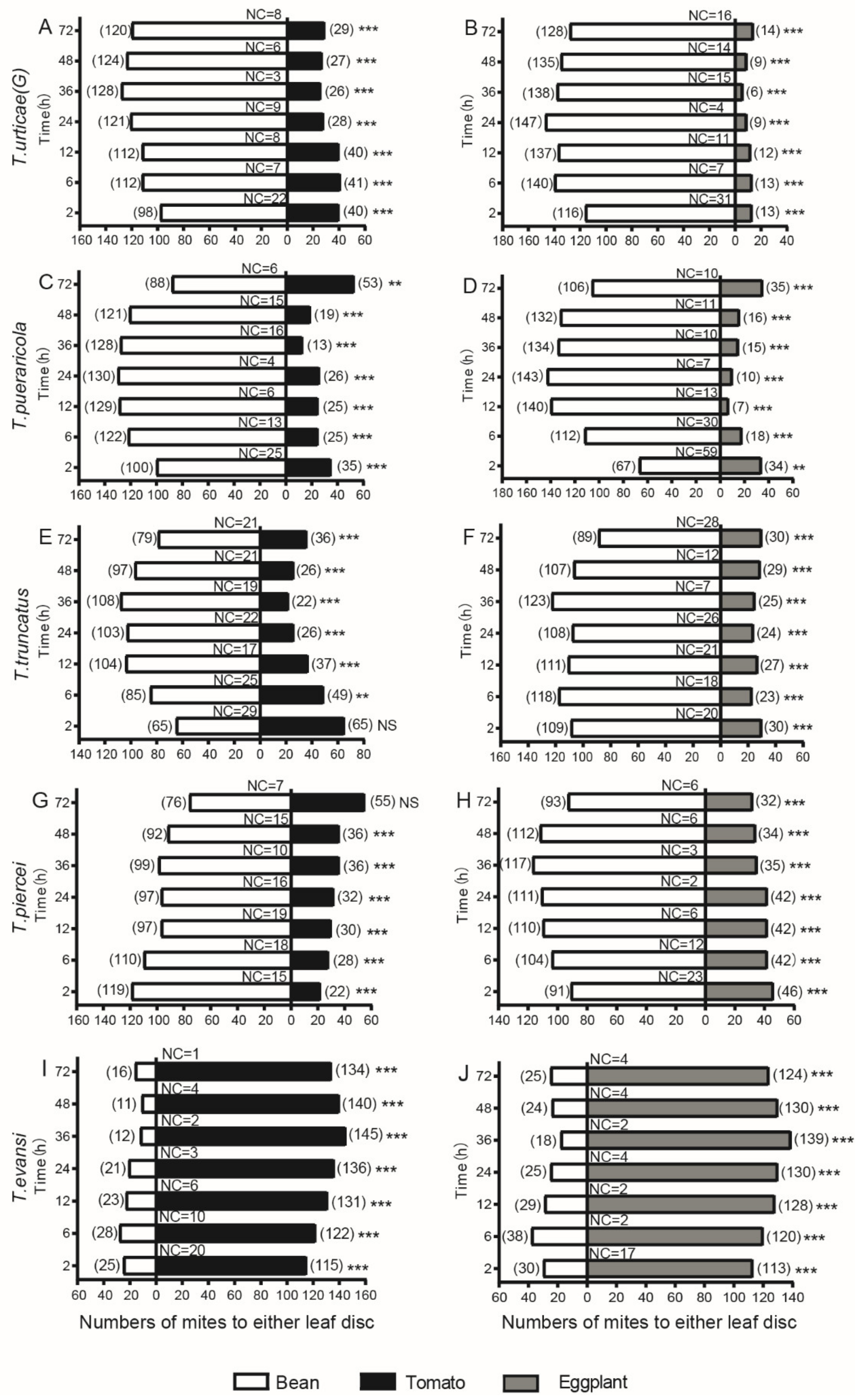
3.2. Performance in Two-Choice Disc Bioassays
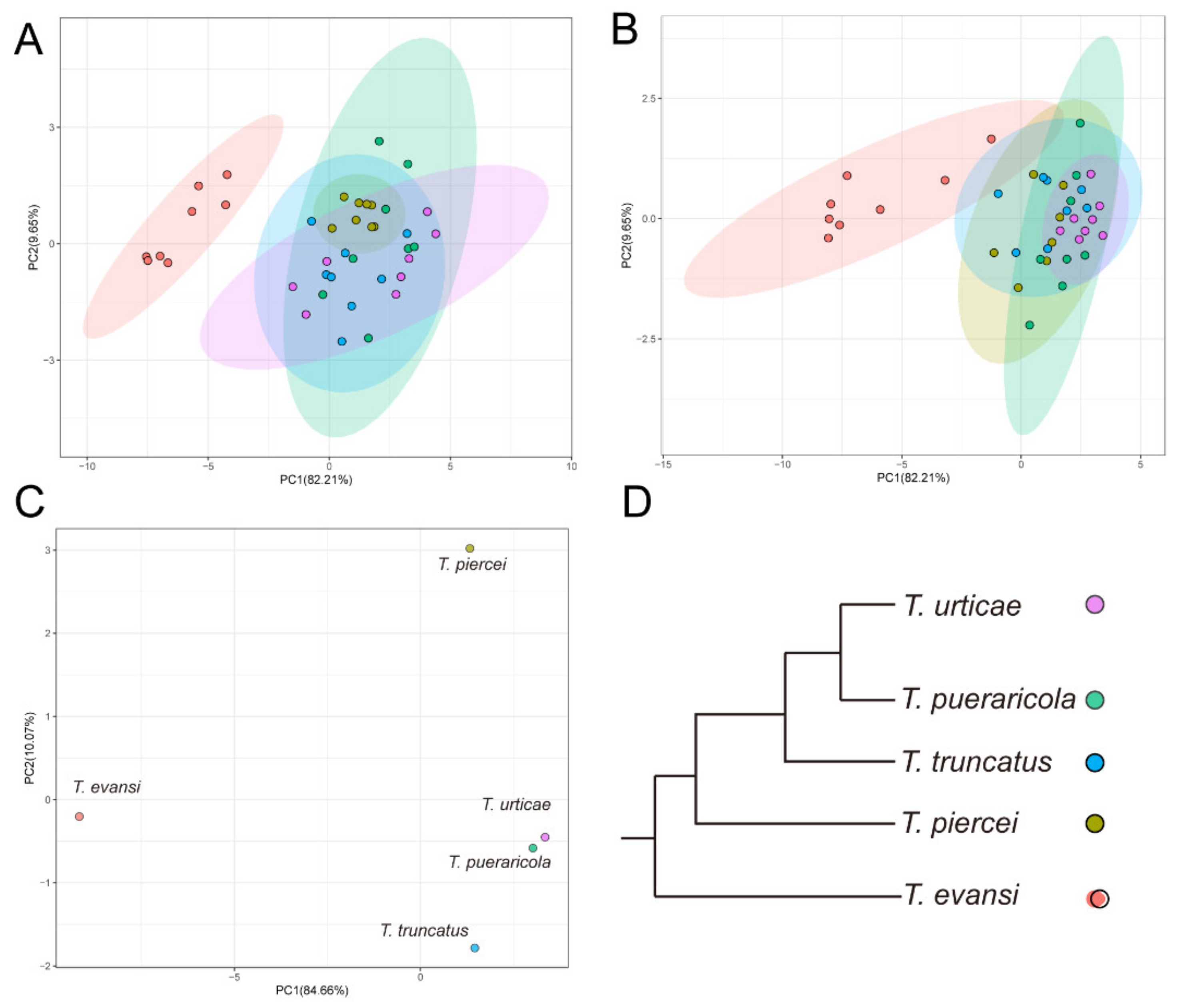
3.3. Overall Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migeon, A.; Dorkeld, F. A Comprehensive Database for the Tetranychidae; Montpellier. 2015. Available online: http://www1.montpellier.inra.fr/CBGP/spmweb (accessed on 28 July 2022).
- Jin, P.Y.; Tian, L.; Chen, L.; Hong, X.Y. Spider mites of agricultural importance in China, with focus on species composition during the last decade (2008–2017). Syst. Appl. Acarol. 2018, 23, 2087–2098. [Google Scholar] [CrossRef]
- Jin, P.-Y.; Sun, J.-T.; Hoffmann, A.; Guo, Y.-F.; Zhou, J.-C.; Zhu, Y.-X.; Chen, L.; Hong, X.-Y. Phylogenetic signals in pest abundance and distribution range of spider mites. BMC Evol. Biol. 2019, 19, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.C. Spider-mite problems and control in Taiwan. Exp. Appl. Acarol. 2000, 24, 453–462. [Google Scholar] [CrossRef]
- Takafuji, A.; Ozawa, A.; Nemoto, H.; Gotoh, T. Spider mites of Japan: Their biology and control. Exp. Appl. Acarol. 2000, 24, 319–335. [Google Scholar] [CrossRef]
- Islam, M.T.; Jahan, M.; Gotoh, T.; Ullah, M.S. Host-dependent life history and life table parameters of Tetranychus truncatus (Acari: Tetranychidae). Syst. Appl. Acarol. 2017, 22, 2068–2082. [Google Scholar] [CrossRef]
- Seeman, O.D.; Beard, J.J. Identification of exotic pest and Australian native and naturalised species of Tetranychus (Acari: Tetranychidae). Zootaxa 2011, 2961, 1–72. [Google Scholar] [CrossRef] [Green Version]
- Gotoh, T.; Bruin, J.; Sabelis, M.W.; Menken, S.B.J. Host race formation in Tetranychus urticae: Genetic differentiation, host-plant preference, and mate choice in a tomato and a cucumber strain. Entomol. Exp. Appl. 1993, 68, 171–178. [Google Scholar] [CrossRef]
- Sun, J.T.; Lian, C.; Navajas, M.; Hong, X.Y. Microsatellites reveal a strong subdivision of genetic structure in Chinese populations of the mite Tetranychus urticae Koch (Acari: Tetranychidae). BMC Genet. 2012, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhang, F.; Peng, Z.; Liu, K.; Jin, Q. The effects of temperature on the development and reproduction of Tectranychus Tetranychus piercei McGregor (Acari: Tetranychidae) in banana. Syst. Appl. Acarol. 2002, 7, 69–76. [Google Scholar] [CrossRef]
- Tian, L.; Jin, P.Y.; Sun, C.P.; Hong, X.Y. First distribution record of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) in mainland China. Syst. Appl. Acarol. 2019, 24, 965–970. [Google Scholar] [CrossRef]
- Navajas, M.; de Moraes, G.J.; Auger, P.; Migeon, A. Review of the invasion of Tetranychus evansi: Biology, colonization pathways, potential expansion and prospects for biological control. Exp. Appl. Acarol. 2013, 59, 43–65. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Auger, P.; Martinez, M.; Migeon, A.; Castanera, P.; Diaz, I.; Navajas, M.; Ortego, F. Host plant use by two distinct lineages of the tomato red spider mite, Tetranychus evansi, differing in their distribution range. J. Pest Sci. 2018, 91, 169–179. [Google Scholar] [CrossRef]
- Ge, C.; Ding, X.-L.; Zhang, J.-P.; Hong, X.-Y. Tetranychus urticae (green form) on Gossypium hirsutum in China: Two records confirmed by aedeagus morphology and RFLP analysis. Syst. Appl. Acarol. 2013, 18, 239–244. [Google Scholar]
- Boubou, A.; Migeon, A.; Roderick, G.K.; Navajas, M. Recent emergence and worldwide spread of the red tomato spider mite, Tetranychus evansi: Genetic variation and multiple cryptic invasions. Biol. Invasions 2011, 13, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Morishita, M.; Hinomoto, N.; Gotoh, T. Phylogenetic analysis of the spider mite sub-family Tetranychinae (Acari: Tetranychidae) based on the mitochondrial COI gene and the 18s and the 5′ end of the 28s rrna genes indicates that several genera are polyphyletic. PLoS ONE 2014, 9, e108672. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Kozaki, T.; Ishii, K.; Gotoh, T. Phylogeny of the spider mite sub-family Tetranychinae (Acari: Tetranychidae) inferred from RNA-Seq data. PLoS ONE 2018, 13, e0203136. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-T.; Lin, J.-H.; Zhang, Q.; Zhao, D.-S.; Chen, L.; Gao, W.-N.; Xue, X.-F.; Hong, X.-Y. The mitochondrial genome of the red tomato spider mite, Tetranychus evansi Baker & Pritchard (Acari: Tetranychidae) and its implications for phylogenetic analysis. Syst. Appl. Acarol. 2019, 24, 1724–1735. [Google Scholar] [CrossRef]
- Ozaki, M. Feeding behavior regulation in the fly: Effect of a noxious substance through the taste and olfactory neurons. Chem. Senses 2005, 30 (Suppl. S1), i289–i290. [Google Scholar] [CrossRef]
- Fujii, S.; Yavuz, A.; Slone, J.; Jagge, C.; Song, X.; Amrein, H. Drosophila sugar receptors in sweet taste perception, olfaction, and internal nutrient sensing. Curr. Biol. 2015, 25, 621–627. [Google Scholar] [CrossRef] [Green Version]
- DeGennaro, M.; McBride, C.S.; Seeholzer, L.; Nakagawa, T.; Dennis, E.J.; Goldman, C.; Jasinskiene, N.; James, A.A.; Vosshall, L.B. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 2013, 498, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.J.; Zhang, S.S.; Niu, B.L.; Ji, D.F.; Liu, X.J.; Li, M.W.; Bai, H.; Palli, S.R.; Wang, C.Z.; Tan, A.J. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef] [Green Version]
- Ngoc, P.C.; Greenhalgh, R.; Dermauw, W.; Rombauts, S.; Bajda, S.; Zhurov, V.; Grbic, M.; Van de Peer, Y.; Van Leeuwen, T.; Rouze, P.; et al. Complex evolutionary dynamics of massively expanded chemosensory receptor families in an extreme generalist chelicerate herbivore. Genome Biol. Evol. 2016, 8, 3323–3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostanian, N.J.; Morrison, F.O. Morphology and ultrastructure of sense organs in the twospotted spider mite (Acarina: Tetranychidae). Ann. Entomol. Soc. Am. 1973, 66, 379–383. [Google Scholar] [CrossRef]
- Sanchez-Gracia, A.; Vieira, F.G.; Rozas, J. Molecular evolution of the major chemosensory gene families in insects. Heredity 2009, 103, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Smitley, D.R.; Kennedy, G.G. Aerial dispersal of the two-spotted spider mite (Tetranychus urticae) from field corn. Exp. Appl. Acarol. 1988, 5, 33–46. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Granett, J.; Normington, S.M. Influence of dispersal from almonds on the population dynamics and acaricide resistance frequencies of spider mites infesting neighboring cotton. Exp. Appl. Acarol. 1991, 10, 187–212. [Google Scholar] [CrossRef]
- Dres, M.; Mallet, J. Host races in plant-feeding insects and their importance in sympatric speciation. Philos. Trans. R. Soc. B 2002, 357, 471–492. [Google Scholar] [CrossRef] [Green Version]
- Forbes, A.A.; Devine, S.N.; Hippee, A.C.; Tvedte, E.S.; Ward, A.K.G.; Widmayer, H.A.; Wilson, C.J. Revisiting the particular role of host shifts in initiating insect speciation. Evolution 2017, 71, 1126–1137. [Google Scholar] [CrossRef]
- Peccoud, J.; Ollivier, A.; Plantegenest, M.; Simon, J.C. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. USA 2009, 106, 7495–7500. [Google Scholar] [CrossRef] [Green Version]
- Villacis-Perez, E.; Snoeck, S.; Kurlovs, A.H.; Clark, R.M.; Breeuwer, J.A.J.; Van Leeuwen, T. Adaptive divergence and post-zygotic barriers to gene flow between sympatric populations of a herbivorous mite. Commun. Biol. 2021, 4, 853. [Google Scholar] [CrossRef]
| Species | Sampling Date | Location | Host Plant | Coordinates |
|---|---|---|---|---|
| T. urticae (green form) | 2015.08 | Rizhao, Shandong | Arachis hypogaea | 119.46° E, 35.42° N |
| T. pueraricola | 2016.08 | Huizhou, Guangdong | Solanum melongena | 114.74° E, 22.98° N |
| T. truncatus | 2016.08 | Huizhou, Guangdong | Vigna sinensis | 114.42° E, 23.12° N |
| T. piercei | 2020.06 | Sanya, Hainan | Trachycarpus fortunei | 118.80° E, 32.06° N |
| T. evansi | 2017.07 | Yaan, Sichuan | Lycopersicum esculentum | 103.00° E, 29.98° N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.-Q.; Yu, X.-Y.; Xue, X.-F.; Hong, X.-Y.; Zhang, J.-P.; Sun, J.-T. Phylogenetic-Related Divergence in Perceiving Suitable Host Plants among Five Spider Mites Species (Acari: Tetranychidae). Insects 2022, 13, 705. https://doi.org/10.3390/insects13080705
Hu Q-Q, Yu X-Y, Xue X-F, Hong X-Y, Zhang J-P, Sun J-T. Phylogenetic-Related Divergence in Perceiving Suitable Host Plants among Five Spider Mites Species (Acari: Tetranychidae). Insects. 2022; 13(8):705. https://doi.org/10.3390/insects13080705
Chicago/Turabian StyleHu, Qi-Qi, Xin-Yue Yu, Xiao-Feng Xue, Xiao-Yue Hong, Jian-Ping Zhang, and Jing-Tao Sun. 2022. "Phylogenetic-Related Divergence in Perceiving Suitable Host Plants among Five Spider Mites Species (Acari: Tetranychidae)" Insects 13, no. 8: 705. https://doi.org/10.3390/insects13080705
APA StyleHu, Q.-Q., Yu, X.-Y., Xue, X.-F., Hong, X.-Y., Zhang, J.-P., & Sun, J.-T. (2022). Phylogenetic-Related Divergence in Perceiving Suitable Host Plants among Five Spider Mites Species (Acari: Tetranychidae). Insects, 13(8), 705. https://doi.org/10.3390/insects13080705







