Transfer of Copper (Cu) in the Soil–Plant–Mealybug–Ladybird Beetle Food Chain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Insect Cultures
2.3. Experimental Design
2.4. Preparation of Samples for Cu Content Assays
2.5. Statistical Analysis
3. Results
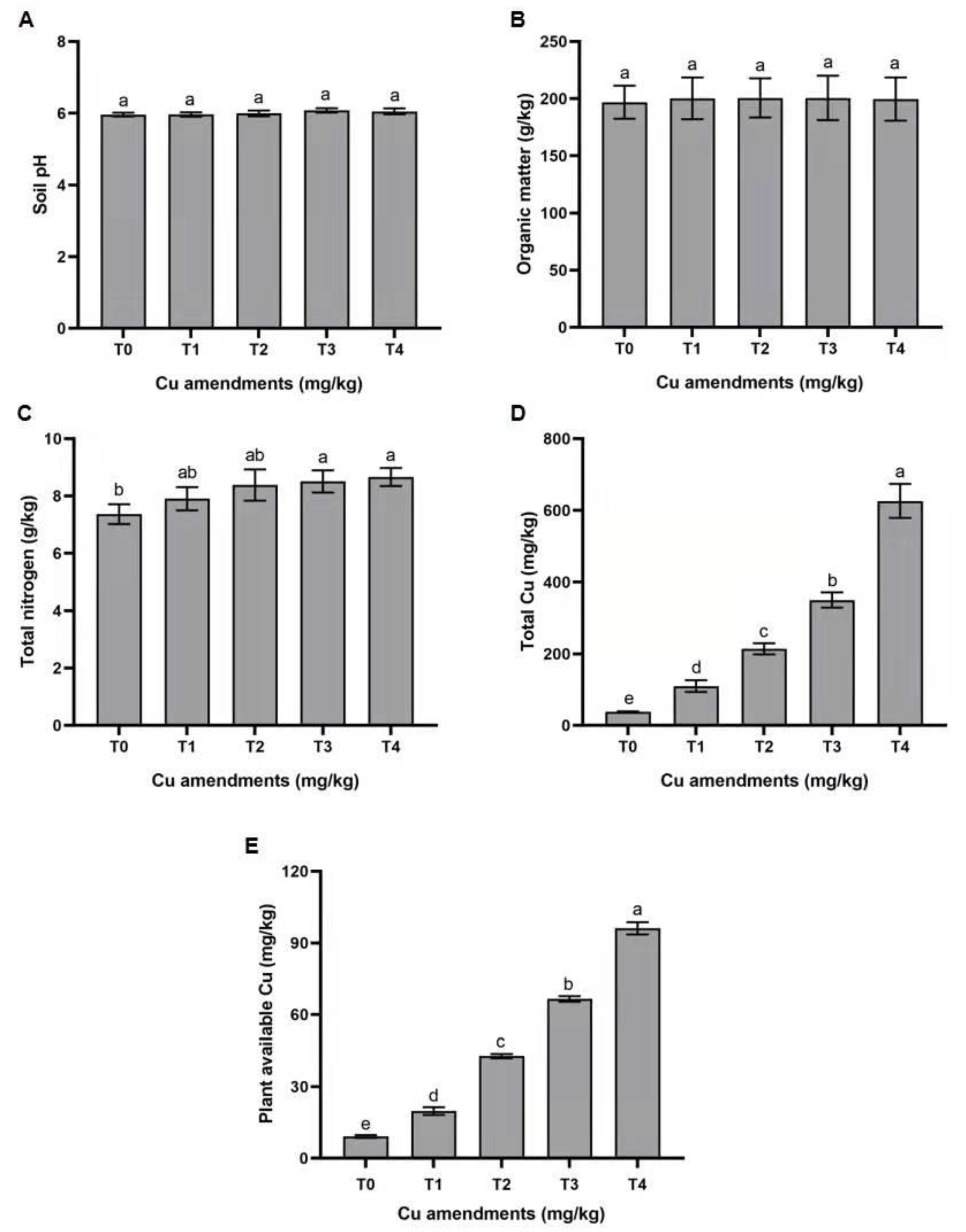
3.1. Effect of Copper Treatments on Soil Properties
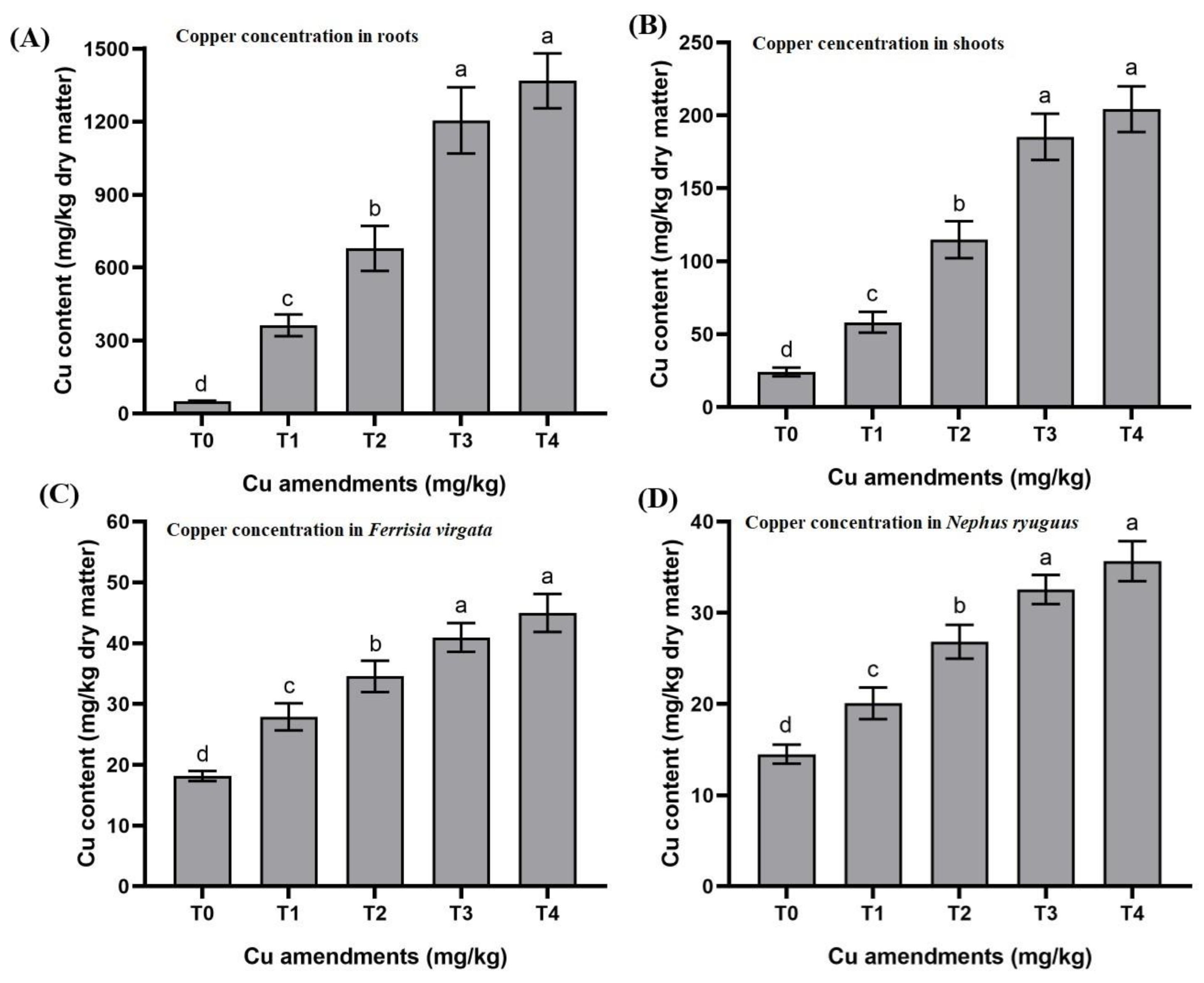
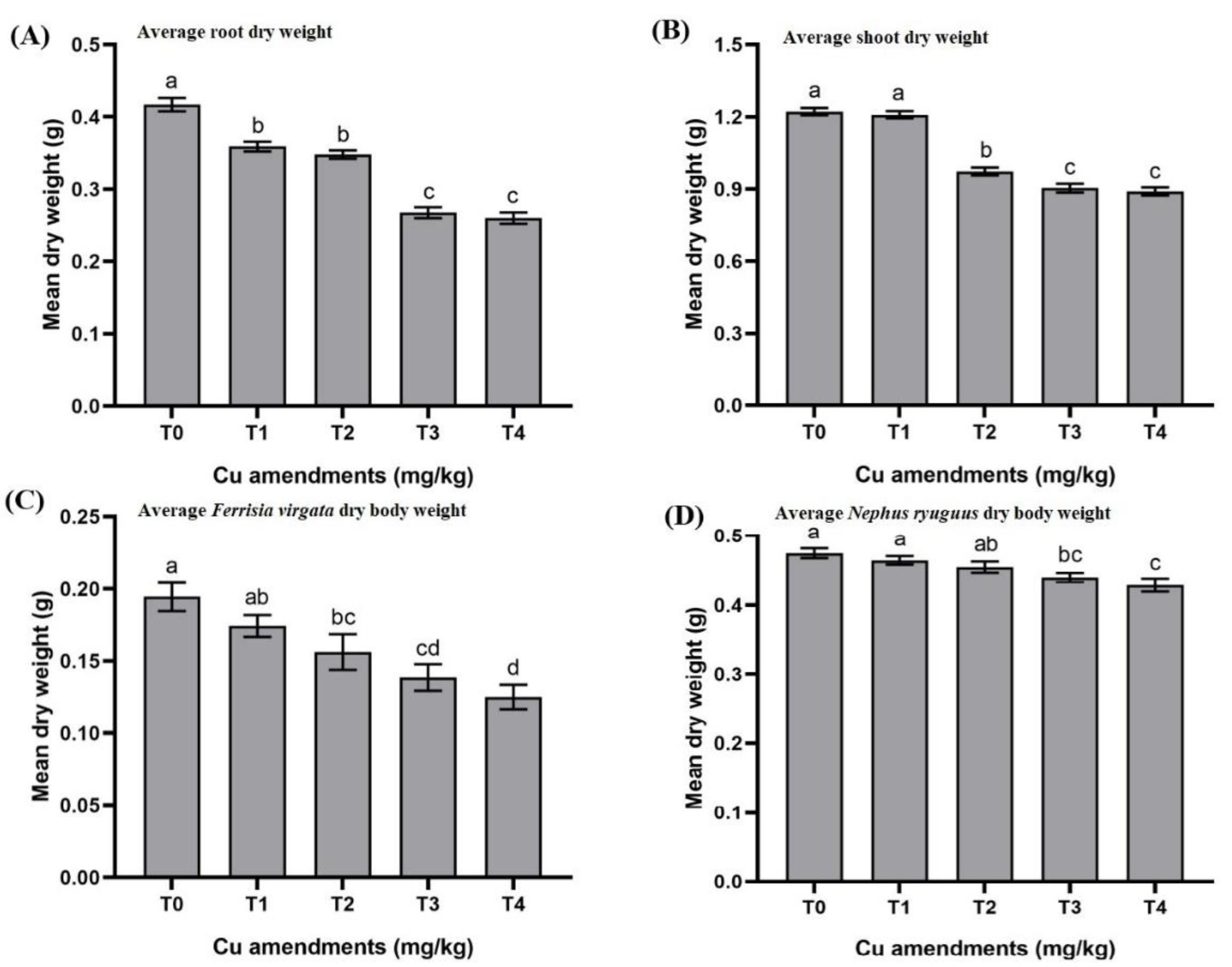
3.2. Effects of Cu Soil Spiking on Soil to Root Cu Transfer and Root Dry Weight
3.3. Effects of Cu Soil Spiking on Root to Shoot Cu Transfer and Shoot Dry Weight
3.4. Effects of Cu Soil Spiking on Shoots to Mealybug Cu Transfer and Mealybug Dry Body Weight
3.5. Effects of Cu Soil Spiking on Mealybug to Cu Transfer and Beetles’ Dry Body Weight
4. Discussion
4.1. Effects of Cu on Soil Properties
4.2. Copper Transfer from Soil to Roots
4.3. Copper Transfer from Roots to Shoots
4.4. Copper Transfer from Shoots to Mealybug and from Mealybug to Adult Beetles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szyczewski, P.; Siepak, J.; Niedzielski, P.; Sobczynski, T. Research on heavy metals in Poland. Polish J. Environ. Stud. 2009, 18, 755–768. [Google Scholar]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal-Contaminated Soils; Khan, M.S., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer: Dordrecht, Germany, 2011; pp. 1–28. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 432. [Google Scholar]
- Zhuang, P.; Zou, H.L.; Shu, W.S. Biotransfer of heavy metals along a soil-plat-chicken food chain: Field study. J. Environ. Sci. 2009, 21, 849–853. [Google Scholar] [CrossRef]
- Mahmood, T.; Islam, K.R. Response of rice seedlings to copper toxicity and acidity. J. Plant Nut. 2006, 29, 943–957. [Google Scholar] [CrossRef]
- Muhammad, A.; Shafaqat, A.; Muhammad, R.; Muhammad, I.; Farhat, A.; Mujahid, F.; Saima, A.B. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Poll. Res. 2015, 22, 8148–8162. [Google Scholar]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Zhang, C.; Qiu, B.L.; Ashraf, U.; Azad, R.; Wu, J.H.; Ali, S. Biotransfer of Cd along a soil-plant-mealybug-ladybird food chain: A comparison with host plants. Chemosphere 2017, 168, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Can, Z.; Wang, X.M.; Ashraf, U.; Qiu, B.L.; Ali, S. Transfer of lead (Pb) in the soil-plant-mealybug-ladybird food chain, a comparison with host plants. Ecotoxicol. Environ. Saf. 2017, 143, 289–295. [Google Scholar]
- Dar, M.I.; Green, I.D.; Khan, F.A. Trace metal contamination: Transfer and fate in food chains of terrestrial invertebrates. Food Webs 2019, 20, e00116. [Google Scholar] [CrossRef]
- Crawford, L.A.; Hodkinson, I.D.; Lepp, N.W. The effects of elevated host-plant cadmium and copper on the performance of aphid Aphis fabae (Homoptera; Aphididae). J. Appl. Ecol. 1995, 32, 528–535. [Google Scholar] [CrossRef]
- Sang, W.; Xu, J.; Bashir, M.H.; Ali, S. Developmental responses of Cryptolaemus montrouuzieri to heavy metals transferred across multi-trophic food chain. Chemosphere 2018, 205, 690–697. [Google Scholar] [CrossRef]
- Garnas, J.R.; Hurley, B.P.; Slippers, B.; Wingfield, M.J. Biological control of forest plantation pests in an interconnected world requires greater international focus. Int. J. Pest Manag. 2012, 58, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Du, C.L.; Wu, J.H.; Bashir, M.H.; Shaukat, M.; Ali, S. Heavy metals transported through a multitrophic food chain influence the energy metabolism and immune responses of Cryptolaemus montrouzieri. Ecotoxicology 2019, 28, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.; Sushil, S.N.; Krishnamoorthy, A. Influence of some selective pesticides on the longevity and progeny production of Leptomastix dactylopii How., a parasitoid of citrus mealybug, Planococcus citri (Risso). Pest Manag. Hort. Ecosyst. 1995, 1, 81–86. [Google Scholar]
- Lapointe, S.L.; Weathersbee, A.A.; Doostdar, H.; Mayer, R.T. Effect of dietry copper on larval Diaprepes abbreviatus (Coleoptera: Curculionidae). Florida Entomol. 2004, 87, 25–29. [Google Scholar] [CrossRef]
- Mani, M.; Shivaraju, C. Mealybugs and Their Management in Agricultural and Horticultural Crops; Springer Nature: Berlin, Germany, 2016; p. 655. [Google Scholar]
- Ren, S.X.; Pang, X.F. The genus Nephus Mulsant (Coleoptera, Coccinellidae) of China. Elytra 1994, 22, 325–333. [Google Scholar]
- Qin, Z.Q.; Wu, J.H.; Qiu, B.L.; Ren, S.X.; Ali, S. Effects of host plant on the development, survivorship and reproduction of Dysmicoccus neobrevipes Beaedley (Hemiptera: Pseudoccocidae). Crop Prot. 2011, 30, 1124–1128. [Google Scholar] [CrossRef]
- Green, I.D.; Walmsley, K. Time-response relationships for the accumulation of Cu, Ni and Zn by seven-spotted ladybirds (Coccinella septempunctata L.) under conditions of single andcombined metal exposure. Chemosphere 2013, 93, 184–189. [Google Scholar] [CrossRef]
- Allison, F.A. Soil Organic Matter and Its Role in Crop Production, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1973; p. 634. [Google Scholar]
- Dar, M.F.; Khan, F.A.; Green, I.D.; Naikoo, M.I. The transfer and fate of Pb from sewage sludge amended soil in a multi-trophic food chain: A comparison with liable elements Cd and Zn. Environ. Sci. Pollut. Res. 2015, 22, 16133–16142. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test forzinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- SAS Institute. SAS Institute. SAS User’s Guide. In Statistics; SAS institute: Cary, NC, USA, 2000. [Google Scholar]
- Ponizovsky, A.A.; Studenikina, T.A.; Mironenko, E.V. Regularities of copper (II) retention by chernozems, dernovo-podsolic and grey forest soils. In Proceedings of the 5th International Conference Biogeochem. Trace Elements, Vienna, Austria, 11–15 July 1999; p. 144. [Google Scholar]
- Bunzl, K.; Trautmannsheimer, M.; Schramel, P.; Raifenhäuse, W. Availability of arsenic, copper, lead, thalium and zinc to various vegetables grown in slag-contamianted soils. J. Environ. Qual. 2001, 30, 934–939. [Google Scholar] [CrossRef]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In Changing Metal Cycles and Human Health; Springer: Berlin, Germany, 1984; pp. 113–141. [Google Scholar]
- Macnicol, R.D.; Beckett, P.H.T. Critical tissue concentrations of potentially toxic elements. Plant Soil. 1985, 85, 107–129. [Google Scholar] [CrossRef]
- Sandmann, G.; Boger, P. Copper-mediated lipid peroxidation process in photosynthetic membranes. Plant Physiol. 1980, 66, 797–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolhouse, H.W.; Walker, S. The physiological basis of copper toxicity and copper tolerance in higher plants. In Copper in Soils and Plants; Academic Press: New York, NY, USA, 1981; p. 235. [Google Scholar]
- Damrongsiri, S.; Chotipong, A. A preliminary investigation for Cu distribution in paddy soil and rice plant in contaminated paddy fields. Paddy Water Environ. 2020, 18, 283–290. [Google Scholar] [CrossRef]
- Yan, Y.P.; He, J.Y.; Zhu, C.; Cheng, C.; Pan, X.B.; Sun, Z.Y. Accumulation of copper on rice growth and accumulation of copper on rice growth and grain yield in different rice cultivars. Chemosphere 2006, 65, 1690–1696. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Shaikh, I.R.; Shaikh, P.R.; Shaikh, R.A.; Shaikh, A.A. Phytotoxic effects of heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L.) seed germination and seedlings growth in black cotton soil of Nanded, India. Res. J. Chem. Sci. 2013, 3, 14–23. [Google Scholar]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, A.R.; Mangi, J.; Abbasi, M.S.; Amin, F. Copper (Cu) tolerance and accumulation potential in four native plant species: A comparative study for effective phytoextraction technique. Geol. Ecol. Landsc. 2021, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.B.; He, X.J.; Chen, L.; Tang, L.; Gao, S.; Chen, F. Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int. J. Agri. Biol. 2010, 12, 119–124. [Google Scholar]
- Mukhopadhyay, M.; Das, A.; Subba, P.; Bantawa, P.; Sarkar, B.; Ghosh, P.D.; Mondal, T.K. Structural, physiological, and biochemical profiling of tea plants under zinc stress. Biol. Plant. 2013, 57, 474–480. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, J.; Jia, L.; Li, Q.; Zhang, T.; Qiao, K.; Ma, S. Zinc induced phytotoxicity mechanism involved in root growth of Triticum aestivum L. Ecotoxicol. Environ. Saf. 2012, 86, 198–203. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Harwood, J.D. Influence of heavy metal contamination on urban natural enemies and biological control. Curr. Opi. Insect. Sci. 2017, 20, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cu Treatments (mg/kg Soil) | Total Soil–Root | Extractable Soil–Root | Root–Shoot | Shoot– Ferrisia Virgata | Ferrisia Virgata– Nephus Ryuguus |
|---|---|---|---|---|---|
| Control | 0.20 | 0.82 | 0.93 | 0.99 | 0.65 |
| 100 | 0.17 | 0.96 | 0.93 | 0.96 | 0.93 |
| 200 | 0.20 | 0.98 | 0.96 | 0.85 | 0.78 |
| 400 | 0.18 | 0.94 | 0.97 | 0.73 | 0.79 |
| 800 | 0.15 | 0.98 | 0.99 | 0.70 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, M.; Cao, H.; Dar, M.I.; Ali, S. Transfer of Copper (Cu) in the Soil–Plant–Mealybug–Ladybird Beetle Food Chain. Insects 2022, 13, 761. https://doi.org/10.3390/insects13090761
Wang X, Zhang M, Cao H, Dar MI, Ali S. Transfer of Copper (Cu) in the Soil–Plant–Mealybug–Ladybird Beetle Food Chain. Insects. 2022; 13(9):761. https://doi.org/10.3390/insects13090761
Chicago/Turabian StyleWang, Xingmin, Mengting Zhang, Huiyi Cao, Mudasir Irfan Dar, and Shaukat Ali. 2022. "Transfer of Copper (Cu) in the Soil–Plant–Mealybug–Ladybird Beetle Food Chain" Insects 13, no. 9: 761. https://doi.org/10.3390/insects13090761
APA StyleWang, X., Zhang, M., Cao, H., Dar, M. I., & Ali, S. (2022). Transfer of Copper (Cu) in the Soil–Plant–Mealybug–Ladybird Beetle Food Chain. Insects, 13(9), 761. https://doi.org/10.3390/insects13090761







