Synanthropic Flies—A Review Including How They Obtain Nutrients, along with Pathogens, Store Them in the Crop and Mechanisms of Transmission
Abstract
:Simple Summary
Abstract
1. Introduction
2. What Is a Synanthropic Fly?
3. Spillovers and Flies
4. Were Synanthropic Flies Originally Associated with NHP and Other Animals?
5. Meal Analysis of Filth, Necrophilic and Synanthropic Flies
6. Are Female Flies Better Transmitters of Pathogens/Parasites Than Males?
7. The Role of Fly Regurgitation and Grooming in Pathogen Handling
8. The Role of the Fly Crop, the Role It Plays Serving as a Storage Organ for Pathogens and Parasites That Are Both Obtained from an External Source, and Later Transferred to NHP and Other Animal Hosts
9. Importance of the Fly Crop and Significance of Regurgitation/Defecation in Pathogen Transmission
10. The Ability of the Fly to Infect a New Host
11. The Dipteran Crop as the Site for Horizontal Transmission of Resistant Genes, Possibly Mutations, and Reassortment of Genetic Material in Pathogen Spillovers
12. Do Non-Human Primates, or Other Animals, Feed on Insects and/or the Infectious Droplets of the 3 Families of Flies?
13. Do Flies Feed on the Feces and Other Fluids, such as Urine, of Gorillas, Chimpanzees, Monkeys?
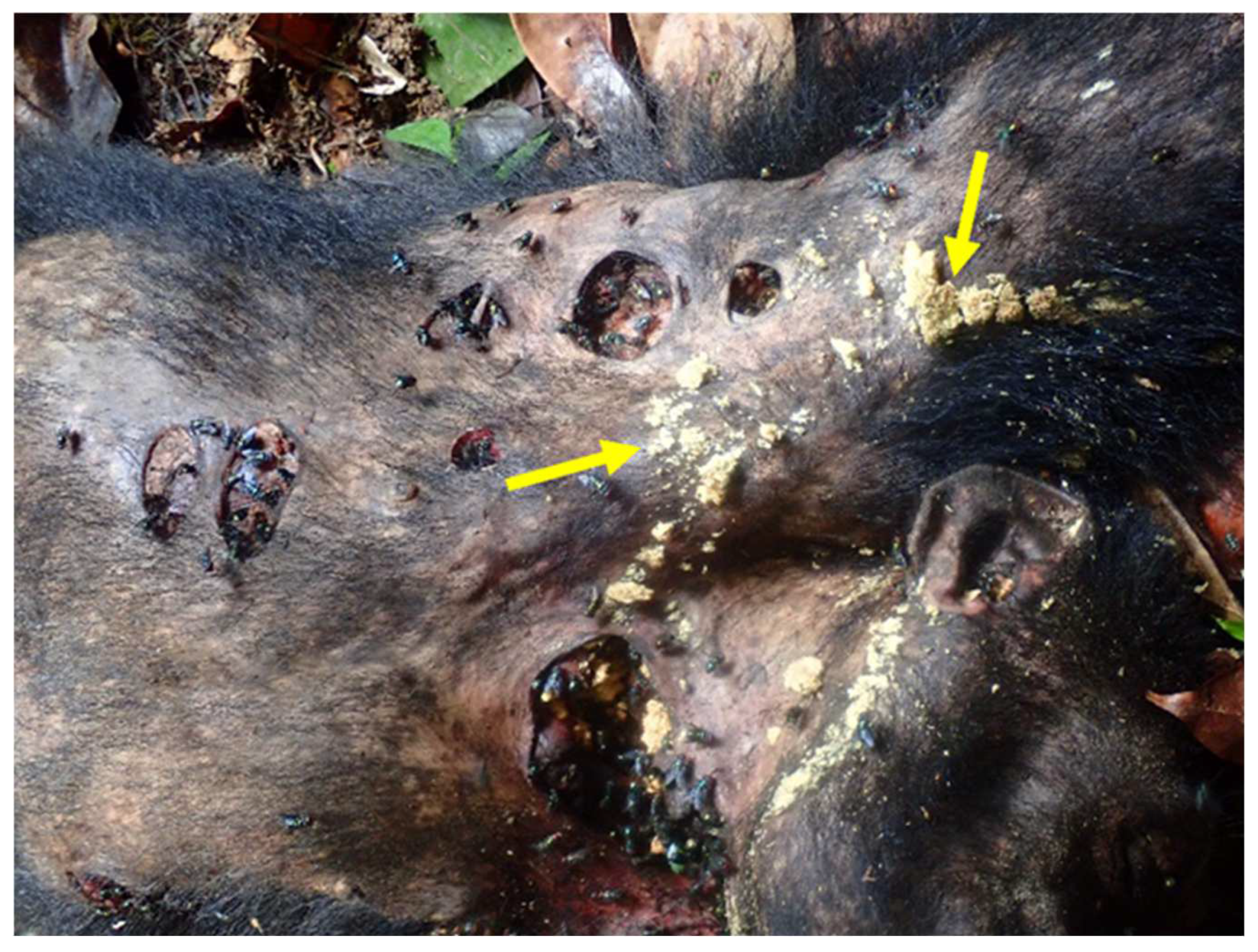
14. Do Flies Feed on Dead Non-Human Primates and Other Zoonotic Animals?
15. Do Bats Feed on these 3 Dipteran Families or the Fruit These Flies Regurgitated or Defecated Upon? How Long Can Pathogens from Fly Regurgitate or Defecation Remain Infective?
16. Examples from the Literature Supporting Flies as Extremely Likely Flying Capsules of Pathogens
17. The Immune System of Flies and Other Vertebrates
18. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohn, S.K., Jr. Epidemiology of the Black Death and successive waves of plague. Med. Hist. Suppl. 2008, 52, 74–100. [Google Scholar] [CrossRef]
- Balaraman, V.; Drolet, B.S.; Mitzel, D.N.; Wilson, W.C.; Owens, J.; Gaudreault, N.N.; Meekins, D.A.; Bold, D.; Trujillo, J.D.; Noronha, L.E.; et al. Mechanical transmission of SARS-CoV-2 by house flies. Parasites Vectors 2021, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Phil. Trans. R. Soc. Lond. 2017, 372, 20150490. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- Kritsky, G.; Mader, D.; Smith, J.J. Surreal entomology—The insect imagery of Salvador Dalí. Am. Entomol. 2013, 59, 29–37. [Google Scholar] [CrossRef]
- Devaux, C.A.; Mediannikov, O.; Medkour, H.; Raoult, D. Infectious disease risk across the growing human-nonhuman primate interface: A review of the evidence. Front. Public Health 2019, 7, 305. [Google Scholar] [CrossRef]
- Quammen, D. Spillover: Animal Infections and the Next Human Pandemic, 1st ed.; W. W. Norton and Co., Inc.: New York, NY, USA, 2012. [Google Scholar]
- Hugh-Jones, M.E.; de Vos, V. Anthrax and wildlife. Rev. Sci. Tech. 2002, 21, 359–383. [Google Scholar] [CrossRef]
- Gortazar, C.; Diez-Delgado, I.; Barasona, J.A.; Vicente, J.; De La Fuente, J.; Boadella, M. The wild side of disease control at the wildlife-livestock-human interface: A review. Front. Vet. Sci. 2015, 1, 27. [Google Scholar] [CrossRef]
- Lehman, M.W.; Craig, A.S.; Al, M.W.L.E.; Kapina-Kany’Anga, M.; Malenga, P.; Munsaka, F.; Muwowo, S.; Shadomy, S.; Marx, M.A. Role of Food Insecurity in Outbreak of Anthrax Infections among Humans and Hippopotamuses Living in a Game Reserve Area, Rural Zambia. Emerg. Infect. Dis. 2017, 23, 1471–1477. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [Green Version]
- Aghokeng, A.F.; Ayouba, A.; Mpoudi-Ngole, E.; Loul, S.; Liegeois, F.; Delaporte, E.; Peeters, M. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provides insights into risks for potential new cross-species transmissions. Infect. Genet. Evol. 2010, 10, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mildenstein, T.; Tanshi, I.; Racey, P.A. Chapter 12: Exploitation of bats for bushmeat and medicine. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 325–375. [Google Scholar] [CrossRef]
- Bonwitt, J.; Kandeh, M.; Rashid Ansumana, R.; Sahr, F.; Hannah Brown, H.; Kelly, A.H. Unintended consequences of the ‘bushmeat ban’ in West Africa during the 2013-2016 Ebola virus disease epidemic. Soc. Sci. Med. 2018, 200, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Bennett, A.J.; Bushmaker, T.; Cameron, K.; Ondzie, A.; Niama, F.R.; Parra, H.-J.; Mombouli, J.-V.; Olson, S.H.; Munster, V.J.; Goldberg, T.L. Corrigendum to “Diverse RNA viruses of arthropod origin in the blood of fruit bats suggest a link between bat and arthropod viromes”. Virology 2019, 528, 207. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.T.; Ahn, M.; Goh, G.; Anderson, D.E.; Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature 2021, 589, 363–370. [Google Scholar] [CrossRef]
- Gessain, A. Mechanisms of viral emergence and interspecies transmission: The example of simian foamy viruses in Central Africa. Bull. Acad. Natl. Med. 2013, 197, 1655–1667, discussion 1667-8. [Google Scholar]
- Stoffolano, J.G., Jr.; Bartley, M.M.; Yin, C.-M. Male and female Phormia regina (Diptera: Calliphoridae) trapped at two different baits in the field. Ann. Entomol. Soc. Am. 1990, 83, 603–606. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr.; Li, M.F.; Sutton, J.A., Jr.; Yin, C.M. Faeces feeding by adult Phormia regina (Diptera: Calliphoridae): Impact on reproduction. Med. Vet. Entomol. 1995, 9, 388–392. [Google Scholar] [CrossRef]
- Echeverria, P.; Harrison, B.; Tirapat, C.; McFarland, A. Flies as a source of enteric pathogens in a rural village in Thailand. Appl. Environ. Microb. 1983, 46, 32–36. [Google Scholar] [CrossRef]
- Oldstone, M.B.A. Viruses, Plagues and History: Past, Present and Future; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Chowdhury, S.; Aleem, M.A.; Khan, M.S.I.; Hossain, M.E.; Ghosh, S.; Rahman, M.Z. Major zoonotic diseases of public health importance in Bangladesh. Vet. Med. Sci. 2021, 7, 1199–1210. [Google Scholar] [CrossRef]
- Anthony, S.J.; Epstein, J.H.; Murray, K.A.; Navarrete-Macias, I.; Zambrana-Torrelio, C.M.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Ali Khan, S.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13. [Google Scholar] [CrossRef] [PubMed]
- Förster, M.; Klimpel, S.; Mehlhorn, H.; Sievert, K.; Messler, S.; Pfeffer, K. Pilot study on synanthropic flies (e.g., Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 2007, 101, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Stoffolano, J.G., Jr.; Haselton, A.T. The adult dipteran crop: A unique and overlooked organ. Annu. Rev. Entomol. 2013, 58, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Gogarten, J.F.; Düx, A.; Mubemba, B.; Pléh, K.; Hoffmann, C.; Mielke, A.; Müller-Tiburtius, J.; Sachse, A.; Wittig, R.M.; Calvignac-Spencer, S.; et al. Tropical rainforest flies carrying pathogens form stable associations with social nonhuman primates. Mol. Ecol. 2019, 28, 4242–4258. [Google Scholar] [CrossRef] [PubMed]
- Cerretti, P.; Stireman, J.O., III; Pape, T.; O’Hara, J.E.; Marinho, M.A.T.; Rognes, K.; Grimaldi, D.A. First fossil of an oestroid fly (Diptera: Calyptratae: Oestroidea) and the dating of oestroid divergences. PLoS ONE. 2017, 12, e0182101. [Google Scholar] [CrossRef]
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House Fly–Microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Chaudhury, M.F.B.; Singh, B.; Cammack, J.A.; Meisel, R.P. Review of bacterial interactions with blow flies (Diptera: Calliphoridae) of medical, veterinary, and forensic importance. Ann. Entomol. Soc. Am. 2017, 110, 19–36. [Google Scholar] [CrossRef]
- Greenberg, B. Flies and Disease, Vol. 1: Ecology, Classification and Biotic Associations; Flies and Disease. Volume II. Biology and Disease Transmission; Princeton University Press: Princeton, NJ, USA, 1971. [Google Scholar]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef]
- Dethier, V.G. The Hungry Fly; Harvard University Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Stevens, N.J.; Seiffert, E.R.; O’Connor, P.M.; Roberts, E.M.; Schmitz, M.D.; Krause, C.; Gorscak, E.; Ngasala, S.; Hieronymus, T.; Temu, J. Palaeontological evidence for an Oligocene divergence between Old World monkeys and apes. Nature 2013, 497, 611–614. [Google Scholar] [CrossRef]
- Evans, T.S.; Gilardi, K.V.K.; Barry, P.A.; Ssebide, B.J.; Kinani, J.F.; Nizeyimana, F.; Noheri, J.B.; Byarugaba, D.K.; Mudakikwa, A.; Cranfield, M.R.; et al. Detection of viruses using discarded plants from wild mountain gorillas and golden monkeys. Am. J. Primatol. 2016, 78, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.J. “Winged sponges”: Houseflies as carriers of typhoid fever in 19th- and early 20th-century military camps. Perspect Biol. Med. 2006, 49, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.; McKenzie, M.; Melkonjan, N.; Zaky, M.; Stoffolano, J.; Webley, W. Infection, persistence, and significance of Chlamydia trachomatis in housefly, Musca domestica L. Vector Borne Zoonotic Dis. 2021, 21, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, A.; Scasciamacchia, S.; Garofolo, G.; Giangaspero, A.; Tarsitano, E.; Adone, R. Evaluation of the house fly Musca domestica as a mechanical vector for an anthrax. PLoS ONE 2010, 5, e12219. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Pijoan, C. Studies on the carriage and transmission of porcine reproductive and respiratory syndrome virus by individual houseflies (Musca domestica). Vet. Rec. 2004, 154, 80–85. [Google Scholar] [CrossRef]
- Hoppe, E.; Pauly, M.; Gillespie, T.R.; Akoua-Koffi, C.; Hohmann, G.; Fruth, B.; Karhemere, S.; Madinda, N.F.; Mugisha, L.; Muyembe, J.-J.; et al. Multiple Cross-Species Transmission Events of Human Adenoviruses (HAdV) during Hominine Evolution. Mol. Biol. Evol. 2015, 32, 2072–2084. [Google Scholar] [CrossRef]
- Sistiaga, A.; Mallol, C.; Galván, B.; Summons, R.E. The Neanderthal meal: A new perspective using faecal biomarkers. PLoS ONE. 2014, 9, e101045. [Google Scholar] [CrossRef]
- Sistiaga, A.; Wrangham, R.; Rothman, J.M.; Summons, R.E. New Insights into the evolution of the human diet from faecal biomarker analysis in wild chimpanzee and gorilla faeces. PLoS ONE. 2015, 10, e0128931. [Google Scholar]
- Downes, W.L.; Dahlem, G.A. Keys to the evolution of Diptera: Role of Homoptera. Environ. Entomol. 1987, 16, 847–854. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Ann. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr. Regulation of a carbohydrate meal in the adult Diptera, Lepidoptera, and Hymenoptera. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Boer, G., Eds.; Chapman & Hall: New York, NY, USA, 1995; p. 210. [Google Scholar]
- Maldonado, A.M.; Centeno, N. Quantifying the potential pathogens transmission of the blowflies (Diptera: Calliphoridae). Mem. Do Inst. Oswaldo Cruz 2003, 98, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Olson, S.H.; Cameron, K.; Reed, P.; Ondzie, A.; Joly, D. Maximizing nonhuman primate fecal sampling in the republic of congo. J. Wildl. Dis. 2012, 48, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; To, K.K.-W.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Stoffolano, J.G., Jr. Fly foregut and transmission of microbes. Adv. Insect Physiol. 2019, 57, 29–95. [Google Scholar]
- Khoso, F.N.; Pueh, M.; Tan, I.; Talib, S.M.B.; Lau, W.H. Molecular identification and composition of cyclorrhaphan flies associated with cafeterias. Pakistan. J. Zool. 2015, 47, 1743–1752. [Google Scholar]
- Owings, C.G.; Banerjee, A.; Asher, T.M.; Gilhooly, W.P., III; Tuceryan, A.; Hufne, M.; Skaggs, C.L.; Adebowale, I.M.; Manicke, N.E.; Picard, C.J. Female blow flies as vertebrate resource indicators. Sci. Rep. 2019, 9, 10594. [Google Scholar] [CrossRef]
- Foil, L.D.; Gorham, J.R. Mechanical transmission of disease agents by arthropods. In Medical Entomology; Chapter 12; Eldridge, B.F., Edman, J.D., Eds.; Kluwer Academic Publishers: London, UK, 2000; p. 461. [Google Scholar]
- Graczyk, T.K.; Fayer, R.; Cranfield, M.R.; Mhangami-Ruwende, B.; Knight, R.; Trout, J.M.; Bixler, H. Filth flies are transport hosts of Cryptosporidium parvum. Emerg. Infect. Dis. 1999, 5, 726. [Google Scholar] [CrossRef]
- Carn, V.M. The role of dipterous insects in the mechanical transmission of animal viruses. Br. Vet. J. 1996, 152, 377–393. [Google Scholar] [CrossRef]
- Nuroteva, P. Synanthropy of blowflies (Dipt., Calliphoridae) in Finland. Ann. Entomol. Fennici. 1963, 29, 1–49. [Google Scholar]
- Mihályi, F. The danger-index of the synanthropic flies. Acta Zool. Hung. 1967, 13, 373–377. [Google Scholar]
- Blacio, K.; Liria, J.; Soto-Vivas, A. Diversity and synanthropy of flies (Diptera: Calyptratae) from Ecuador, with new records for the country. J. Threat. Taxa 2020, 12, 15784–15793. [Google Scholar] [CrossRef]
- Rich, S.M.; Leendertz, F.H.; Xu, G.; LeBreton, M.; Djoko, C.F.; Aminake, M.N.; Takang, E.E.; Diffo, J.L.D.; Pike, B.L.; Rosenthal, B.M.; et al. The origin of malignant malaria. Proc. Natl. Acad. Sci. USA 2009, 106, 14902–14907. [Google Scholar] [CrossRef] [PubMed]
- Duval, L.; Fourment, M.; Nerrienet, E.; Rousset, D.; Sadeuh, S.A.; Goodman, S.M.; Andriaholinirina, N.V.; Randrianarivelojosia, M.; Paul, R.E.; Robert, V.R.; et al. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc. Natl. Acad. Sci. USA 2010, 107, 10561–10566. [Google Scholar] [CrossRef] [PubMed]
- Kappel, H.B.; Oliveira, A.G.; da Silva, P.R.; Pelli, A. Non-biting flying insects as carriers of pathogenic bacteria in a Brazilian hospital. Rev. Da Soc. Bras. De Med. Trop. 2013, 46, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Spillar, D. Nutrition and diet of muscoid flies. Bull. World Health Organ. 1964, 31, 551. [Google Scholar]
- Schnuch, M.; Seebauer, H. Sugar cell responses to lactose and sucrose in labellar and tarsal taste hairs of Musca domestica. J. Comp. Physiol. A. 1998, 182, 767–775. [Google Scholar] [CrossRef]
- Wiesmann, R. Zum Nahrungsproblem der freilebenden Stubeniegen Musca domestica L. Z. Angew. Zool. 1960, 2, 159–181. [Google Scholar]
- Oftedal, O.T. Origin and Evolution of the Major Constituents of Milk. In Advanced Dairy Chemistry Proteins, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2013; Volume 1A, pp. 1–42. [Google Scholar]
- Gogarten, J.F. Widespread fly-primate associations: Larger monkey groups harbor higher fly densities. EcoHealth 2022, IDECH-21-0115.R1, Manuscript submitted as short communication. [Google Scholar]
- Brusatte, S.L.; O’Connor, J.K.; Jarvis, E.D. The origin and diversification of birds. Curr. Biol. 2015, 25, R888–R898. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess, E.R.; Gerry, A.C.; Kaufman, E.P.; Thomson, J.; Pickens, V.; Machtinger, E.T. House Fly (Musca domestica [Diptera: Muscidae])—Biology, pest status, current Management prospects, and research needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Hoffmann, C.; Stockhausen, M.; Merkel, K.; Calvignac-Spencer, S.; Leendertz, F.H. Assessing the feasibility of fly based surveillance of wildlife infectious diseases. Sci. Rep. 2016, 6, 37952. [Google Scholar] [CrossRef] [PubMed]
- Satchell, G.H.; Harrison, R.A., II. Experimental observations on the possibility of transmission of yaws by wound-feeding Diptera, in Western Samoa. Trans. R. Soc. Trop. Med. Hyg. 1953, 47, 148–153. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr.; Duan, H.; Yin, C.M. Crop and midgut filling and emptying in a female Phormia regina (Diptera: Calliphoridae) fed a liver diet. J. Med. Entomol. 1995, 32, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Stamm, L.V. Flies and yaws: Molecular studies provide new Insight. EBioMedicine 2016, 11, 9–10. [Google Scholar] [CrossRef]
- Gupta, A.K.; Nayduch, D.; Verma, P.; Shah, B.; Ghate, H.G.; Patole, M.S.; Shouche, Y.S. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.). FEMS Microbiol. Ecol. 2012, 79, 581–593. [Google Scholar] [CrossRef]
- Calibeo-Hayes, D.; Denning, S.S.; Stringham, S.M.; Guy, J.S.; Smith, L.G.; Watson, D.W. Mechanical transmission of turkey coronavirus by domestic houseflies (Musca domestica L.). Avian Dis. 2003, 47, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Sasaki, T.; Saito, N.; Tamura, K.; Suzuki, K.; Watanabe, H.; Agui, N. Houseflies: Not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H. Am. J. Trop. Med. Hyg. 1999, 61, 625–629. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Wang, L.-F.; Stoffolano, J.G., Jr.; McLandsborough, L. Development of the fly ‘crop vessel’ bioassay for fly/microbial studies. Afr. J. Microbiol. 2017, 11, 1027–1034. [Google Scholar] [CrossRef]
- Chaiwong, T.; Srivoramas, T.; Sukontason, K.; Sanford, M.R.; Moophayak, K.; Sukontason, K.L. Survey of the Synanthropic Flies Associated with Human Habitations in Ubon Ratchathani Province of Northeast Thailand. J. Parasitol. Res. 2012, 2012, 613132. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kobayashi, M.; Agui, N. Epidemiological Potential of Excretion and Regurgitation by Musca domestica (Diptera: Muscidae) in the Dissemination of Escherichia coli O157: H7 to Food. J. Med. Entomol. 2000, 37, 945–949. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiony, G.M.; Stoffolano, J.G., Jr. Comparison of sucrose intake and production of elimination spots among adult Musca domestica, Musca autumnalis, Phormia regina and Protophormia terraenovae. Asian Pac. J. Trop. Biomed. 2016, 6, 640–645. [Google Scholar] [CrossRef]
- Braack, L.E.; De Vos, V. Feeding habits and flight range of blow-flies (Chrysomyia pp.) in relation to anthrax transmission in the Kruger National Park, South Africa. Onderstepoort J. Vet. Res. 1990, 57, 141–142. [Google Scholar]
- Jacques, B.J.; Bourret, T.J.; Shaffer, J.J. Role of fly cleaning behavior on carriage of Escherichia coli and Pseudomonas aeruginosa. J. Med. Entomol. 2017, 54, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Macovei, L.; Zurek, L. Ecology of antibiotic resistance genes: Characterization of enterococci from house flies collected in food settings. Appl. Environ. Microbiol. 2006, 72, 4028–4035. [Google Scholar] [CrossRef]
- Petridis, M.; Bagdasarian, M.; Waldor, M.; Walker, M.K.E. Horizontal transfer of Shiga toxin and antibiotic resistance genes among Escherichia coli strains in house fly (Diptera: Muscidae) gut. J. Med. Entomol. 2006, 43, 288–295. [Google Scholar] [CrossRef]
- Onwugamba, F.C.; Fitzgerald, J.R.; Rochonc, K.; Guardabassid, L.; Alabie, A.; Kühneg, S.; Grobusche, M.P.; Schaumburg, F. The role of ‘filth flies’ in the spread of antimicrobial resistance. Travel Med. Infect. Dis. 2018, 22, 8–17. [Google Scholar] [CrossRef]
- Sadaqat, Z.; Kaushik, S.; Kain, P. Gut Feeding the Brain: Drosophila Gut An Animal Model for Medicine to Understand Mechanisms Mediating Food Preferences; IntechOpen: London, UK, 2022; p. 1. [Google Scholar] [CrossRef]
- Stoffolano, J.G., Jr.; Acaron, A.; Conway, M. “Bubbling” or droplet regurgitation in both sexes of adult Phormia regina (Diptera: Calliphoridae) fed various concentrations of sugar and protein solutions. Ann. Entomol. Soc. Am. 2008, 101, 964–970. [Google Scholar] [CrossRef]
- El-Bassiony, G.M.; Luizzi, V.; Nguyen, D.; Stoffolano, J.G., Jr.; Purdy, A.E. Vibrio cholerae laboratory infection of the adult house fly Musca domestica. Med. Vet. Entomol. 2016, 30, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Raele, D.A.; Stoffolano, J.G., Jr.; Vasco, I.; Pennuzzi, G.; La Porta, M.C.N.; Cafiero, M.A. Study on the role of the common house fly, Musca domestica, in the spread of ORF virus (Poxviridae) DNA under Laboratory Conditions. Microorganisms 2021, 9, 2185. [Google Scholar] [CrossRef] [PubMed]
- Knauf, S.; Raphael, J.; Mitjà, O.; Lejora, I.; Chuma, I.S.; Batamuzi, E.K.; Keyyu, J.D.; Fyumagwa, R.; Lüert, S.; Godornes, C.; et al. Isolation of Treponema DNA from necrophagous flies in a natural ecosystem. EBioMedicine 2016, 11, 85–90. [Google Scholar] [CrossRef]
- Graham-Smith, G.S. Non-bloodsucking flies. In Flies in Relation to Disease; Cambridge University Press: Cambridge, UK, 1914; pp. 180–186. [Google Scholar]
- Basson, L.; Hassim, A.; Dekker, A.; Gilbert, A.; Beyer, W.; Rossouw, J.; Van Heerden, H. Blowflies as vectors of Bacillus anthracis in the Kruger National Park. Koedoe 2018, 60, a1468. [Google Scholar] [CrossRef]
- Patrona, L.V.; Pléh, K.; Samuni, L.; Ulrich, M.; Röthemeier, C.; Sachse, A.; Muschter, S.; Nitsche, A.; Couacy-Hymann, R.; Boesch, C.; et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat. Microbiol. 2020, 5, 955–965. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, R.; Sleigh, A.; McMichael, T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian–Australasian Region. EcoHealth 2012, 9, 24–35. [Google Scholar] [CrossRef]
- Rivers, D.B.; Hammerschmidt, C.; Carrigan, A.; Melvin, K. Retention of human body fluids in adults of Calliphora vicina (Diptera: Calliphoridae). J. Med. Entomol. 2021, 58, 1663–1672. [Google Scholar] [CrossRef]
- Akter, S.; Sabuj, A.A.M.; Haque, Z.F.; Rahman, M.T.; Kafi, M.A.; Saha, S. Detection of antibiotic-resistant bacteria and their resistance genes from houseflies. Vet. World 2020, 13, 266–274. [Google Scholar] [CrossRef]
- Marshall, B.; Petrowski, D.; Levy, S.B. Inter- and intraspecies spread of Escherichia coli in a farm environment in the absence of antibiotic usage (ecology/colonization/antibiotic resistance). Proc. Natl. Acad. Sci. USA 1990, 87, 6609–6613. [Google Scholar] [CrossRef]
- Akhtar, M.; Hirt, H.; Zurek, L. Horizontal transfer of the tetracycline resistance gene tetM mediated by pCF10 among Enterococcus faecalis in the house fly (Musca domestica L.) alimentary canal. Microb. Ecol. 2009, 58, 509–518. [Google Scholar] [CrossRef]
- Hayes, J.D.; Wolf, C.R. Molecular mechanisms of drug resistance. Biochem. J. 1990, 272, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Review Article. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Bourgarel, M.; Cappelle, J.; Liégeois, F.; De Nys, H.M.; Roger, F. Ebola virus maintenance: If not (only) bats, what else? Viruses 2018, 10, 549. [Google Scholar] [CrossRef]
- Janiak, M.C.; Chaney, M.E.; Tosi, A.J. Evolution of acidic mammalian chitinase genes (CHIA) is related to body mass and insectivory in primates. Mol. Biol. Evol. 2018, 35, 607–622. [Google Scholar] [CrossRef]
- Lesnik, J.J. Edible Insects and Human Evolution; University Press of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Bermejo, M.; Rodríguez-Teijeiro, J.D.; Iller, G.; Barroso, A.; Vilà, C.; Walsh, P.D. Ebola outbreak killed 5000 gorillas. Science 2006, 314, 5805. [Google Scholar] [CrossRef]
- Hamad, I.; Delaporte, E.; Raoult, D.; Bittar, F. Detection of termites and other insects consumed by African great apes using molecular fecal analysis. Sci. Rep. 2014, 4, 4478. [Google Scholar] [CrossRef]
- Qvarnström, M.; Fikáček, M.; Wernström, J.V.; Huld, S.; Beutel, R.G.; Arriaga-Varela, E.; Ahlberg, P.E.; Niedźwiedzki, G. Exceptionally preserved beetles in a Triassic coprolite of putative dinosauriform origin. Curr. Biol. 2021, 31, 3374–3381.e5. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. 2015, 24, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, J.Z.; Tollit, D.J.; Thompson, P.M.; Amos, W. Molecular scatology: The use of molecular genetic analysis to assign species, sex and individual identity to seal faeces. Mol. Ecol. 1997, 6, 225–234. [Google Scholar] [CrossRef]
- Lubar, R.S. Dali: The Salvador Dali Museum Collection Hardcover; Bulfinch Press: Boston, MA, USA, 2000. [Google Scholar]
- Anderson, J.R. Chimpanzees and death. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 5, 373. [Google Scholar] [CrossRef]
- Porter, A.; Eckardt, W.; Vecellio, V.; Guschanski, K.; Niehoff, P.P.; Ngobobo-As-Ibungu, U.; Pekeyake, R.N.; Stoinski, T.; Caillaud, D. Behavioral responses around conspecific corpses in adult eastern gorillas (Gorilla beringei spp.). Peer J. 2019, 7, e6655. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Eby, P.; Hudson, P.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.; Martin, G.; et al. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef] [PubMed]
- Rydell, J.; Bogdanowicz, W.; Boonman, A.; Pettersson, S.; Suchecka, E.; Pomorski, J.J. Bats may eat diurnal flies that rest on wind turbines. Mamm. Biol. 2016, 81, 331–339. [Google Scholar] [CrossRef]
- Bean, B.; Moore, B.M.; Sterner, B.; Peterson, L.R.; Gerding, D.N.; Balfour, H.H. Survival of Influenza Viruses on Environmental Surfaces. J. Infect. Dis. 1982, 146, 47–51. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef]
- Wasala, L.; Talley, J.L.; DeSilva, U.; Fletcher, J.; Wayadande, A. Transfer of Escherichia coli O157:H7 to spinach by house flies, Musca domestica (Diptera: Muscidae). Phytopathology 2013, 103, 373–380. [Google Scholar] [CrossRef]
- Jones, C.J.; Isard, S.A.; Cortinas, M.R. Dispersal of Synanthropic Diptera: Lessons from the Past and Technology for the Future. Ann. Entomol. Soc. Am. 1999, 92, 829–839. [Google Scholar] [CrossRef]
- Blackburn, J.K.; Curtis, A.; Hadfield, T.L.; O’Shea, B.; Mitchell, M.A.; Hugh-Jones, M.E. Confirmation of Bacillus anthracis from Flesh-eating Flies Collected during a West Texas Anthrax Season. J. Wildl. Dis. 2010, 46, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Ferrandon, D.; Jung, A.; Criqui, M.; Lemaitre, B.; Uttenweiler-Joseph, S.; Michaut, L.; Reichhart, J.; Hoffmann, J.A. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998, 17, 1217–1227. [Google Scholar] [CrossRef]
- Calvignac-Spencer, S.; Merkel, K.; Kutzner, N.; Kühl, H.; Boesch, C.; Kappeler, P.M.; Metzger, S.; Schubert, G.; Leendertz, F.H. Carrion fly-derived DNA as a tool for comprehensive and cost-effective assessment of mammalian biodiversity. Mol. Ecol. 2013, 22, 915–924. [Google Scholar] [CrossRef]
- Calvignac-Spencer, S.; Leendertz, F.H.; Gilbert, M.T.; Schubert, G. An invertebrate stomach’s view on vertebrate ecology: Certain invertebrates could be used as “vertebrate samplers” and deliver DNA-based information on many aspects of vertebrate ecology. Bioessays 2013, 35, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Friedman, T.L. The World Is Flat: A Brief History of the Twenty-First Century; Farrar, Straus and Giroux: New York, NY, USA, 2005. [Google Scholar]
- Rose, N.H.; Sylla, M.; Badolo, A.; White, B.J.; Crawford, J.E.; McBride, C.S. Climate and urbanization drive mosquito preference for humans. Curr. Biol. 2020, 30, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and disease emergence: Dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoffolano, J.G., Jr. Synanthropic Flies—A Review Including How They Obtain Nutrients, along with Pathogens, Store Them in the Crop and Mechanisms of Transmission. Insects 2022, 13, 776. https://doi.org/10.3390/insects13090776
Stoffolano JG Jr. Synanthropic Flies—A Review Including How They Obtain Nutrients, along with Pathogens, Store Them in the Crop and Mechanisms of Transmission. Insects. 2022; 13(9):776. https://doi.org/10.3390/insects13090776
Chicago/Turabian StyleStoffolano, John G., Jr. 2022. "Synanthropic Flies—A Review Including How They Obtain Nutrients, along with Pathogens, Store Them in the Crop and Mechanisms of Transmission" Insects 13, no. 9: 776. https://doi.org/10.3390/insects13090776
APA StyleStoffolano, J. G., Jr. (2022). Synanthropic Flies—A Review Including How They Obtain Nutrients, along with Pathogens, Store Them in the Crop and Mechanisms of Transmission. Insects, 13(9), 776. https://doi.org/10.3390/insects13090776





