Electrophysiological and Morphological Characterization of Contact Chemosensilla in Adults and Larvae of the Butterfly, Atrophaneura alcinous
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Stimulants
2.3. Preparation for Electron Microscopy
2.4. Electrophysiological Experiments
3. Results
3.1. Characterization of Sensilla Types in the Forelegs of Male and Female Adults
3.2. Electrophysiological Response to Stimuli in Adults
3.3. Different Cells Respond to Stimulants of Host or Nonhost Plants
3.4. Contact Chemosensilla Are Present on Larval Mouthparts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Hallem, E.A.; Dahanukar, A.; Carlson, J.R. Insect odor and taste receptors. Annu. Rev. Entomol. 2006, 51, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Rausher, M.D. Search image for leaf shape in a butterfly. Science 1978, 200, 1071–1073. [Google Scholar] [CrossRef]
- Nishida, R.; Fukami, H. Oviposition stimulants of an aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alicinous. J. Chem. Ecol. 1989, 15, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, T.; Nishida, R.; Fukami, H. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae). Appl. Entmol. Zool. 1991, 26, 29–40. [Google Scholar] [CrossRef]
- Sánchez-Gracia, A.; Vieira, F.G.; Rozas, J. Molecular evolution of the major chemosensory gene families in insects. Heredity 2009, 103, 208–216. [Google Scholar] [CrossRef]
- Feeny, P.; Rosenberry, L.; Carter, M. Chemical aspects of oviposition behavior in butterflies. In Herbivorous Insects: Host-Seeking Behavior and Mechanisms; Ahmad, S., Ed.; Academic Press: New York, NY, USA, 1983; pp. 27–76. [Google Scholar]
- Honda, K. Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J. Chem. Ecol. 1990, 15, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishida, R.; Kuwahara, Y. Oviposition stimulant for a Rutaceae-feeding swallowtail butterfly, Papilio bianor (Lepidoptera: Papilionidae): Hydroxycinnamic acid derivative from Orixa japonica. Appl. Entomol. Zool. 2000, 35, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Honda, K.; Ômura, H.; Hayashi, N. Oviposition stimulants for the tropical swallowtail butterfly, Papilio polytes, feeding on a rutaceous plant, Toddalia asiatica. J. Chem. Ecol. 2003, 29, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Oviposition stimulants of some papilionid butterflies contained in their host plants. Botyu-Kagaku 1977, 42, 33–140. [Google Scholar]
- Ichinose, T.; Honda, K. Ovipositional behavior of Papilio protenor demetrius Cramer and the factors involved in its host plants. Appl. Entomol. Zool. 1978, 13, 103–114. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Ueno, K.; Yamanaka, A.; Isono, K.; Endo, K.; Nishida, R.; Yoshihara, K.; Tokunaga, F. A putative binding protein for lipophilic substances related to butterfly oviposition. FEBS Lett. 2000, 478, 299–303. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Wazawa, T.; Ishii, Y.; Yanagida, T.; Nishida, R.; Zheng, X.-G.; Ishiguro, M.; Yoshihara, K.; Hisatomi, O.; Tokunaga, F. Characterization of chemoreceptive protein binding to an oviposition stimulant using a fluorescent micro-binding assay in a butterfly. FEBS Lett. 2009, 583, 345–349. [Google Scholar] [CrossRef]
- Tsuchihara, K.; Hisatomi, O.; Tokunaga, F.; Asaoka, K. An oviposition stimulant binding protein in a butterfly: Immunohistochemical localization and electrophysiological responses to plant compounds. Commun. Integr. Biol. 2009, 2, 356–358. [Google Scholar] [CrossRef]
- Tsuchihara, K. Mother knows the best taste: Molecular and physiological characterization of oviposition stimulant binding protein in Atrophaneura alcinous. In Advances in Medicine and Biology; Nova Science Publishers, Inc.: New York, NY, USA, 2012; Volume 16, pp. 167–193. [Google Scholar]
- Ryuda, M.; Calas-List, D.; Yamada, A.; Marion-Poll, F.; Yoshikawa, H.; Tanimura, T.; Ozaki, K. Gustatory sensing mechanism coding for multiple oviposition stimulants in the swallowtail butterfly, Papilio xuthus. J. Neurosci. 2013, 33, 914–924. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Loon, J.A.J. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. Hung. 2002, 48, 215–263. [Google Scholar]
- Crava1, C.M.; Bobkov, Y.V.; Sollai, G.; Anfora, G.; Crnjar, R.; Cattaneo, A.M. Chemosensory receptors in the larval maxilla of Papilio hospiton. Front. Ecol. Evol. 2022, 9, 795994. [Google Scholar] [CrossRef]
- Hodgson, E.S.; Lettvin, J.Y.; Roeder, K.D. Physiology of a primary chemoreceptor unit. Science 1995, 122, 417–418. [Google Scholar] [CrossRef]
- Asaoka, K.; Shibuya, T. Morphological and electrophysiological characteristics of the epipharyngeal sensilla of the silkworm, Bombyx mori. Entomol. Exp. Appl. 1995, 77, 167–176. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Davis, A.; Ramaswamy, S. Contribution of different taste cells and signaling pathways to the discrimination of “bitter” taste stimuli by an insect. J. Neurosci. 2002, 22, 7281–7287. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, L.M.; Loon, J.A.J.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Glendinning, J.I.; Tarre, M.; Asaoka, K. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar (Manduca sexta). Behav. Neurosci. 1999, 113, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I.; Davis, A.; Rai, M. Temporal coding mediates discrimination of “bitter” taste stimuli by an insect. J. Neurosci. 2006, 26, 8900–8908. [Google Scholar] [CrossRef] [Green Version]
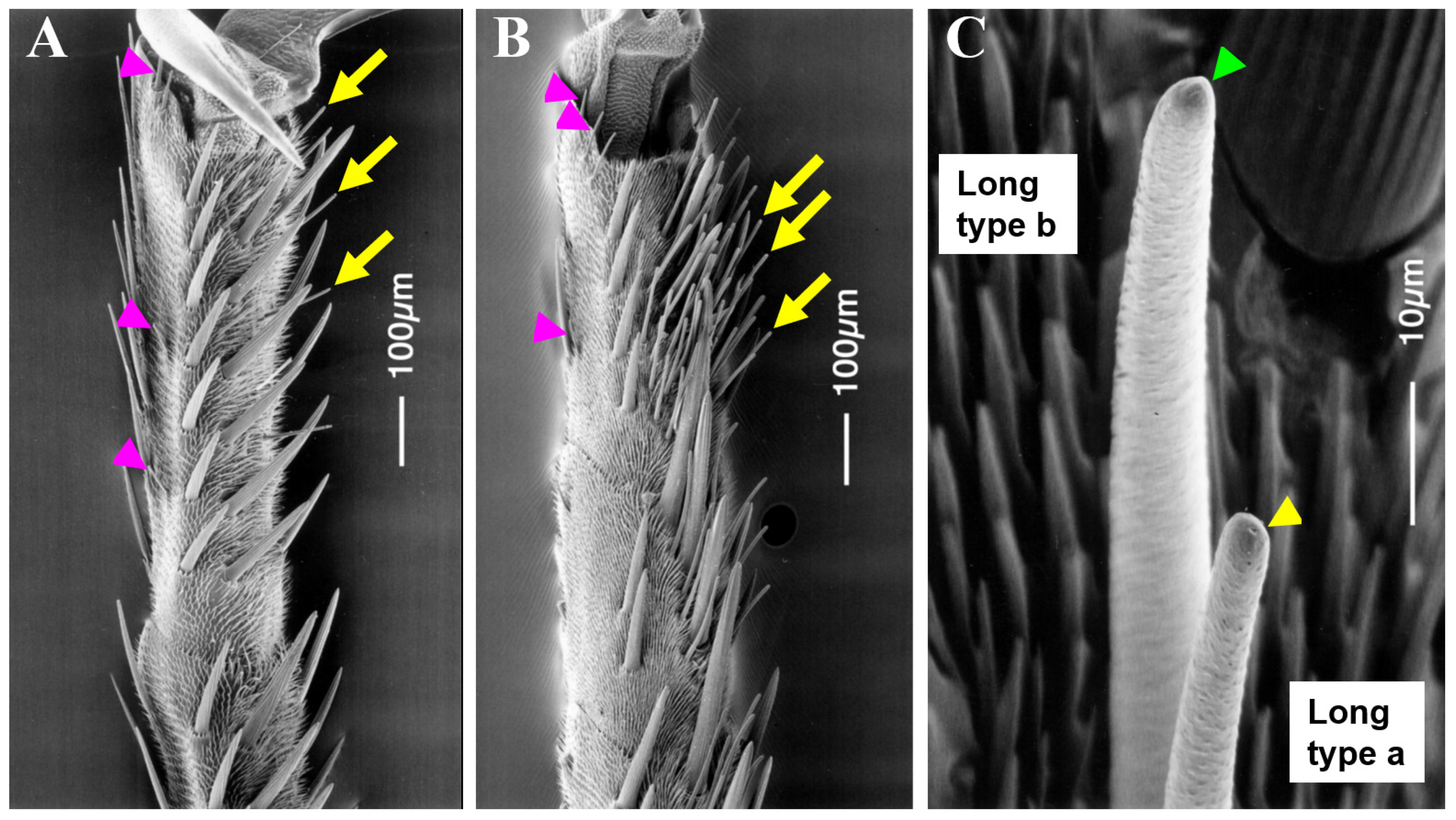
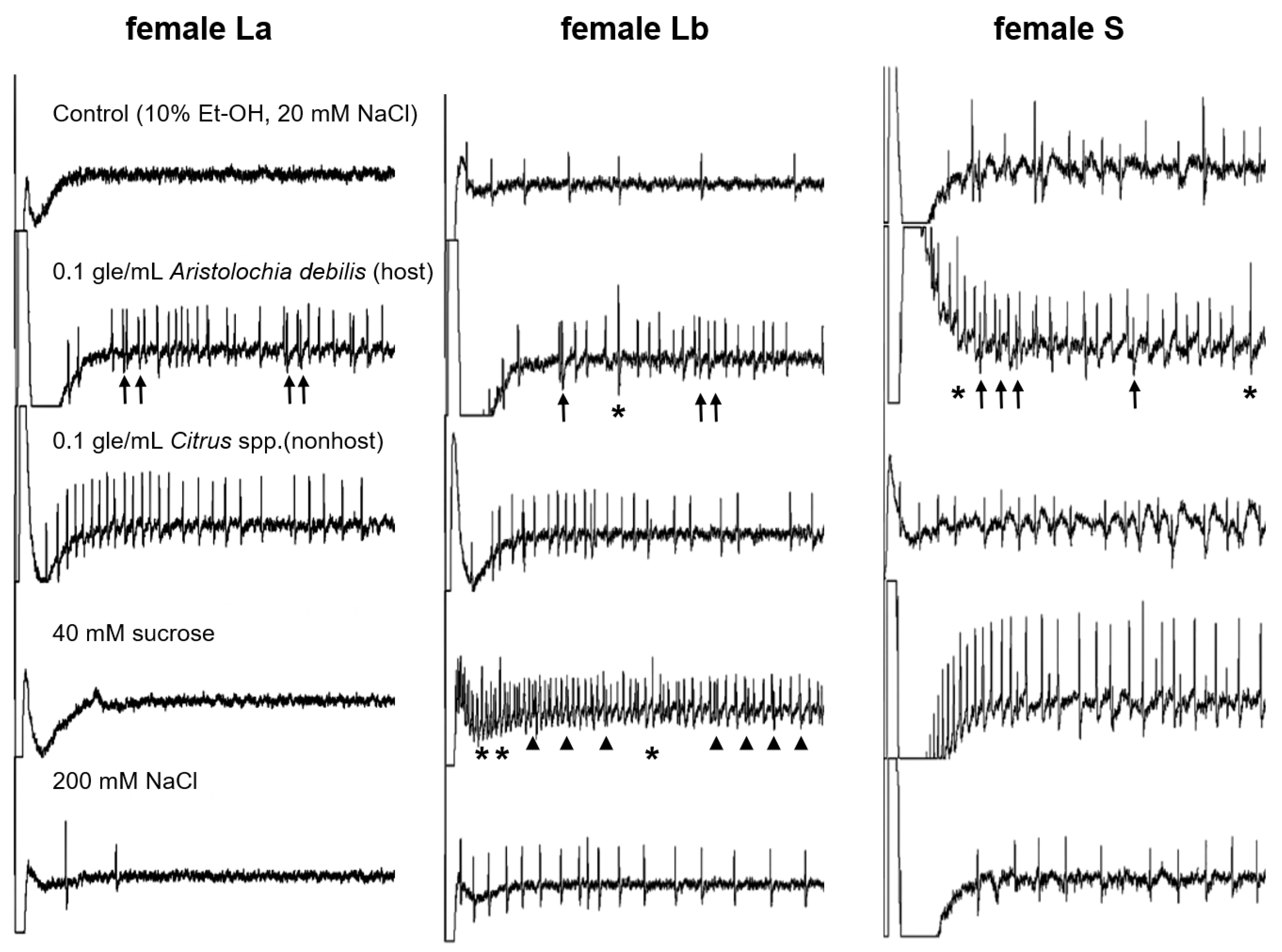
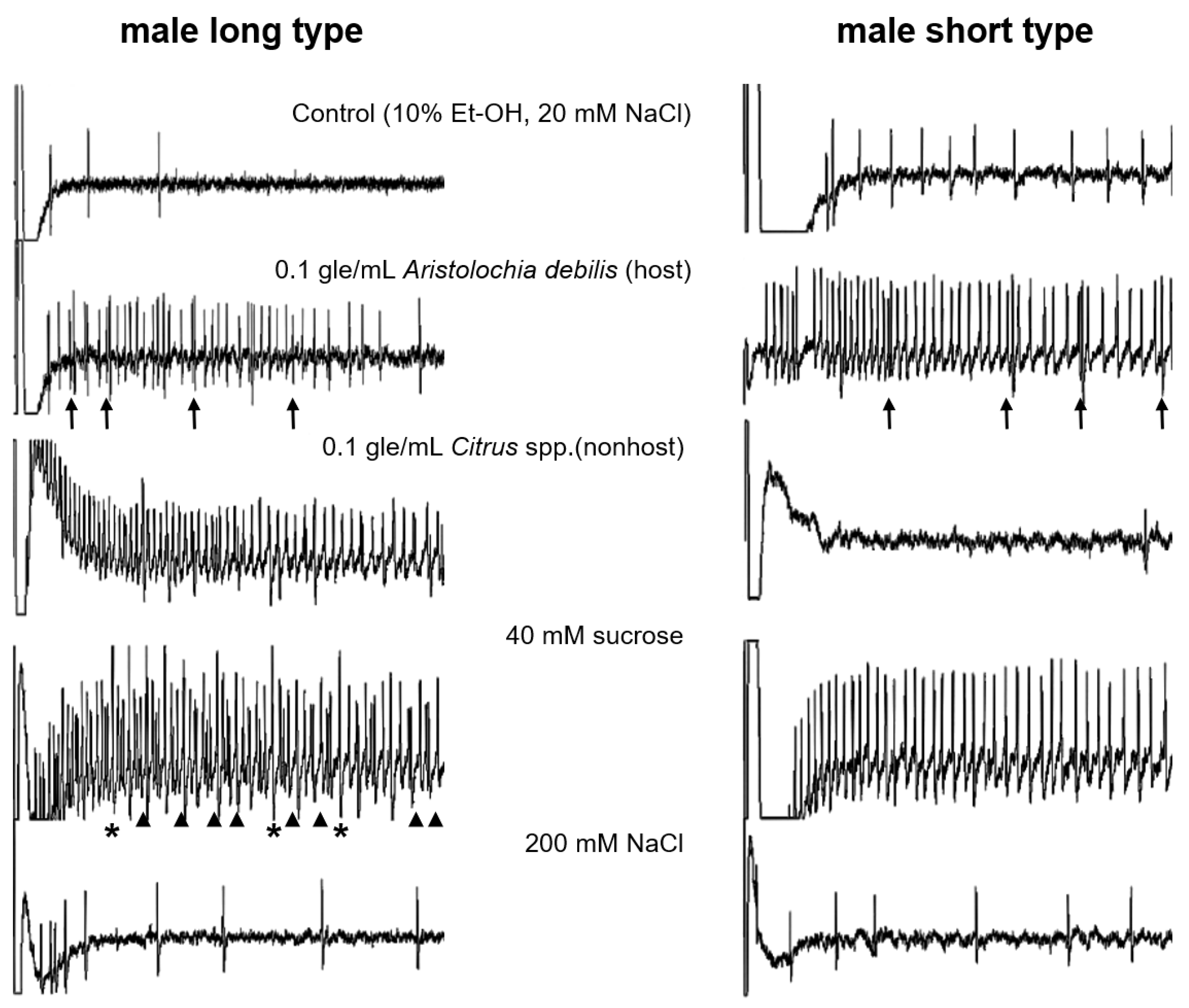
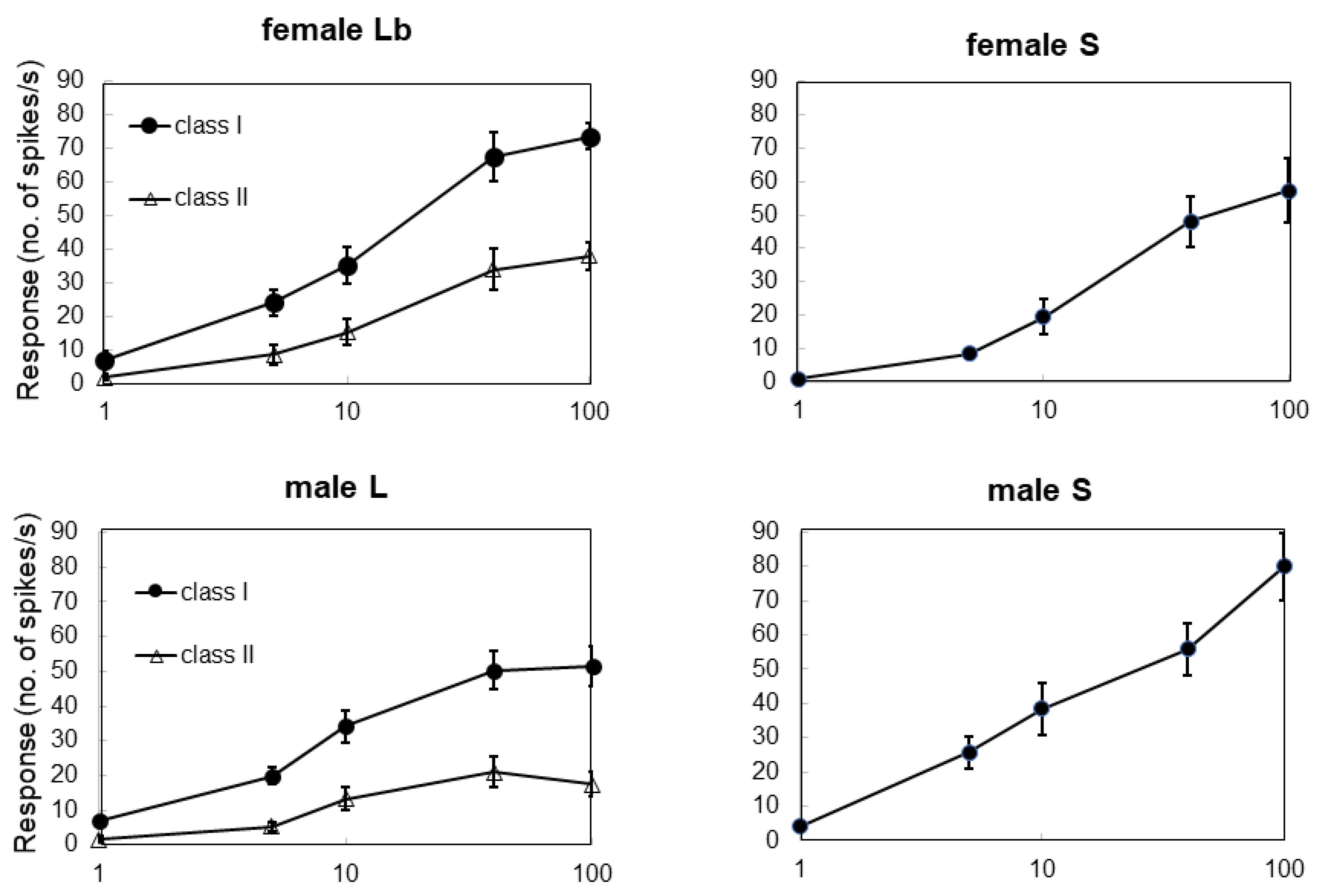
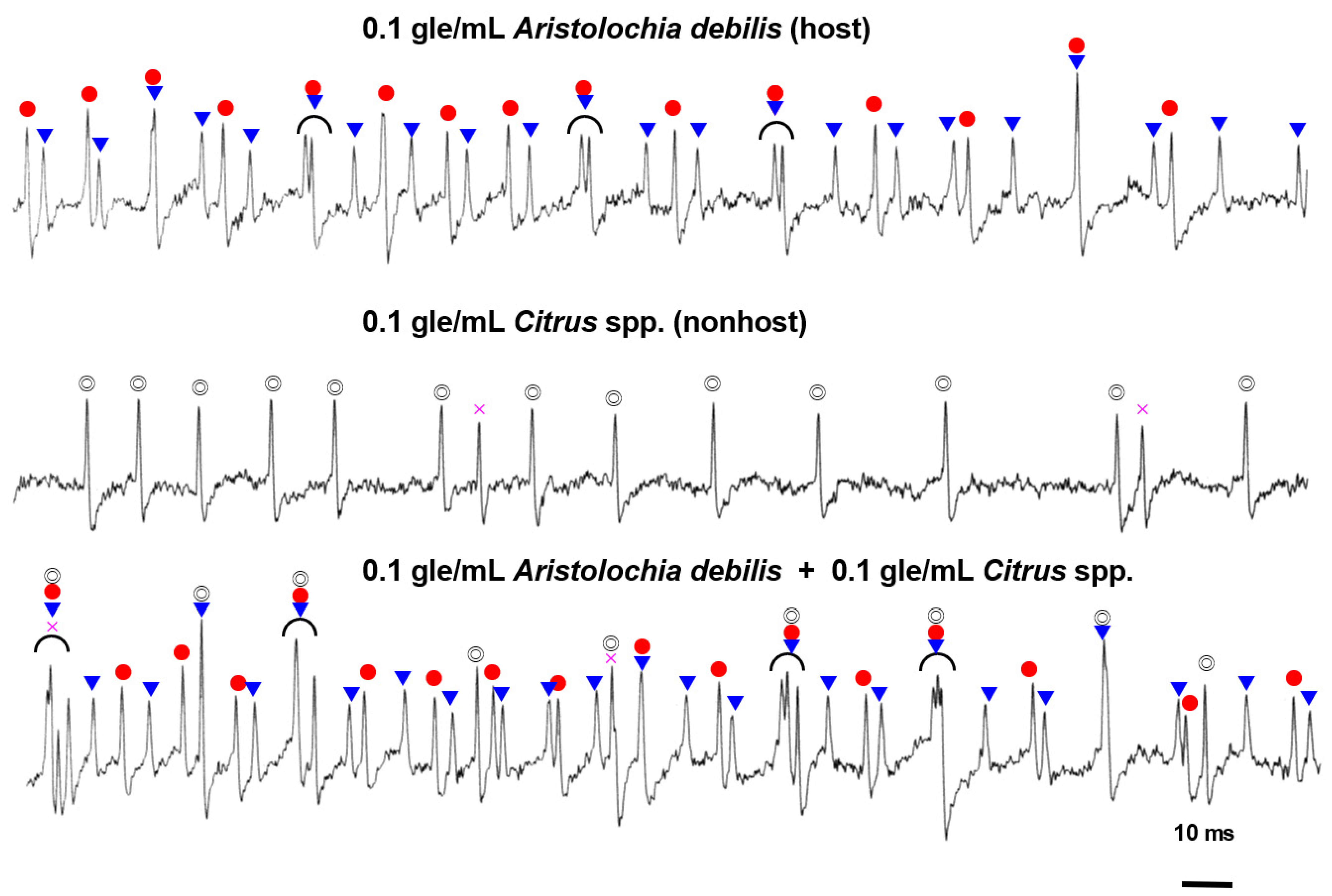
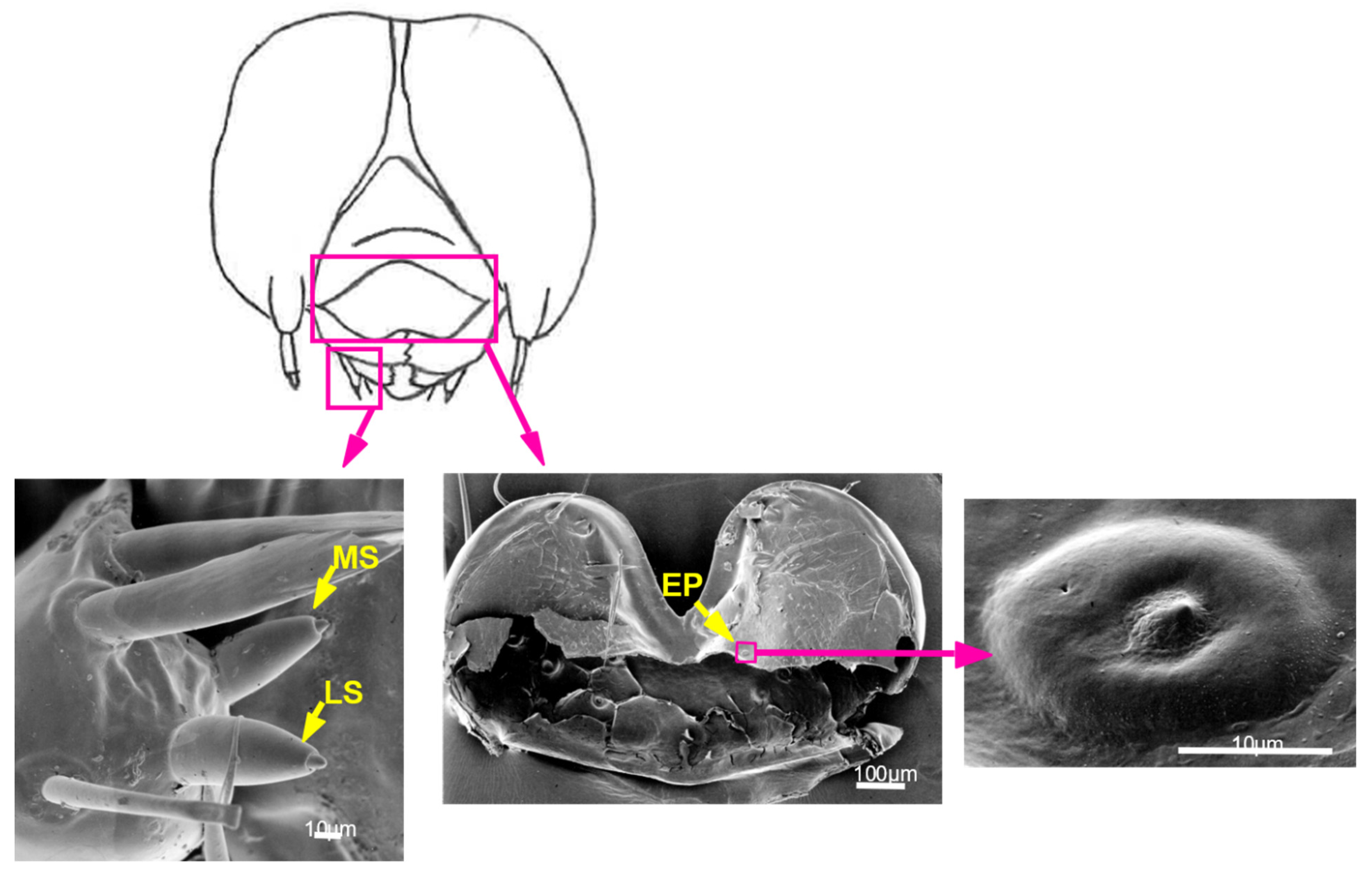

| Type of Sensilla | 0.1 gle/mL Aristolochia debilis | 0.1 gle/mL Citrus spp. | 100 mM Sucrose | 200 mM NaCl |
|---|---|---|---|---|
| Female La (70) | 2 | 1 | 0 | 1 |
| Female Lb (3–5) | 2 | 1 | 2 | 1 |
| Female S (6–8) | 2 | 1 | 1 | 1 |
| Male L (6–8) | 2 | 1 | 2 | 1 |
| Male S (6–8) | 2 | 1 | 1 | 1 |
| Type of Sensilla | 0.1 gle/mL Aristolochia debilis | 0.1 gle/mL Citrus spp. | 100 mM Sucrose | 15 mM Sequoyitol | 1 mM Aristolochic Acid |
|---|---|---|---|---|---|
| LS (1) | 2 | 1 | 0 | 1 | 1 |
| MS (1) | 2 | 1 | 1 | 0 | 1 |
| EP (1) | 2 | 1 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuchihara, K.; Takanashi, T.; Asaoka, K. Electrophysiological and Morphological Characterization of Contact Chemosensilla in Adults and Larvae of the Butterfly, Atrophaneura alcinous. Insects 2022, 13, 802. https://doi.org/10.3390/insects13090802
Tsuchihara K, Takanashi T, Asaoka K. Electrophysiological and Morphological Characterization of Contact Chemosensilla in Adults and Larvae of the Butterfly, Atrophaneura alcinous. Insects. 2022; 13(9):802. https://doi.org/10.3390/insects13090802
Chicago/Turabian StyleTsuchihara, Kazuko, Takuma Takanashi, and Kiyoshi Asaoka. 2022. "Electrophysiological and Morphological Characterization of Contact Chemosensilla in Adults and Larvae of the Butterfly, Atrophaneura alcinous" Insects 13, no. 9: 802. https://doi.org/10.3390/insects13090802
APA StyleTsuchihara, K., Takanashi, T., & Asaoka, K. (2022). Electrophysiological and Morphological Characterization of Contact Chemosensilla in Adults and Larvae of the Butterfly, Atrophaneura alcinous. Insects, 13(9), 802. https://doi.org/10.3390/insects13090802





