Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution of BODIPY® FL Pirenzepine Binding Sites
2.2. Behavioral Tests
2.2.1. Habituation
2.2.2. Duration of Proboscis Extension
2.3. Data Analysis
3. Results
3.1. Visualization of BODIPY® FL Pirenzepine Binding Sites in Honeybee Brain Slices
3.1.1. Antennal Lobes
3.1.2. Mushroom Bodies
3.1.3. Central Complex
3.1.4. Optic Lobes
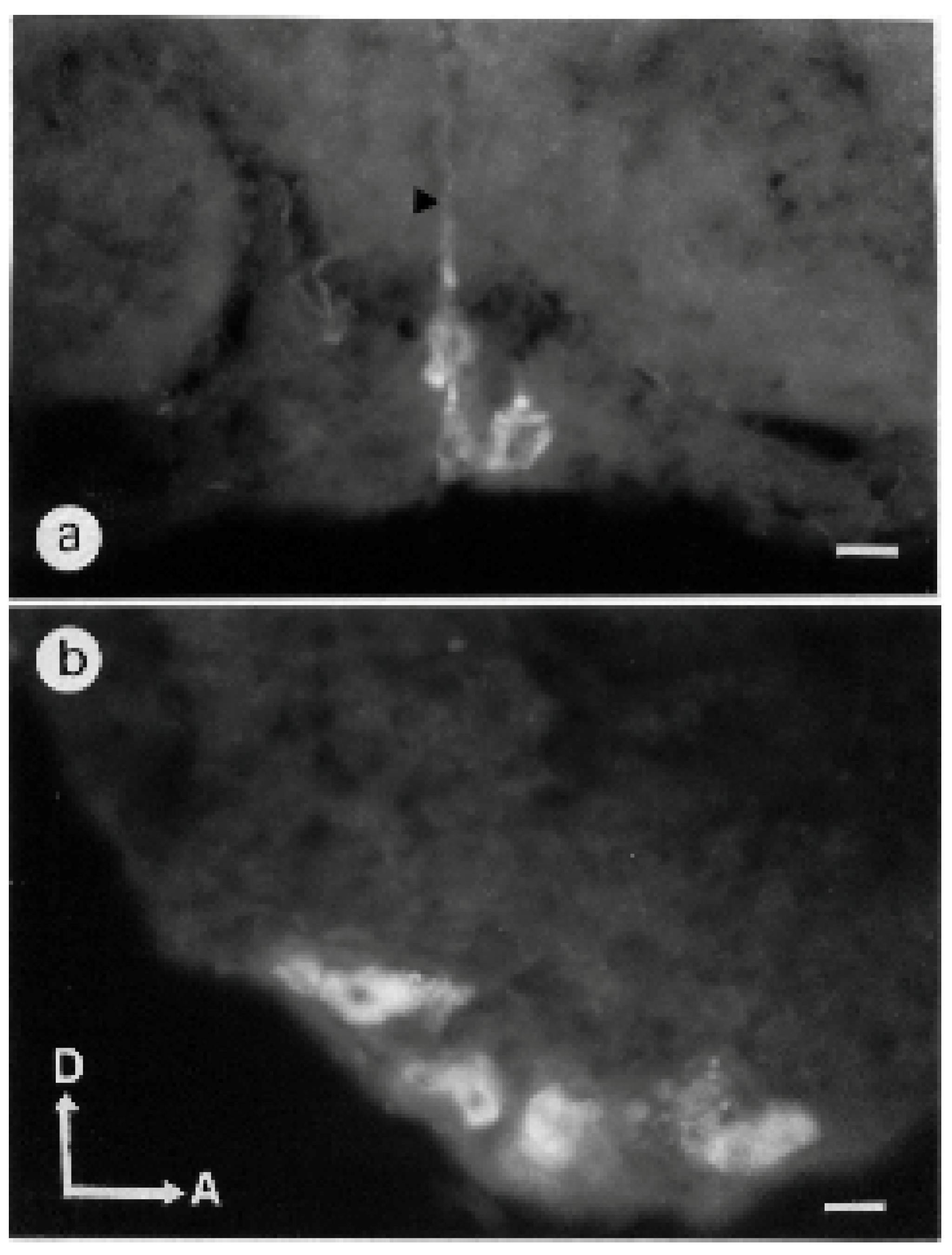
3.1.5. Subesophageal Ganglion
3.2. Behavioral Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taillebois, E.; Thany, S.H. The use of insecticide mixtures containing neonicotinoids as a strategy to limit insect pests: Efficiency and mode of action. Pestic. Biochem. Physiol. 2022, 184, 105126. [Google Scholar] [CrossRef] [PubMed]
- Moreau, E.; Mikulska-Ruminska, K.; Goulu, M.; Perrier, S.; Deshayes, C.; Stankiewicz, M.; Apaire-Marchais, V.; Nowak, W.; Lapied, B. Orthosteric muscarinic receptor activation by the insect repellent IR3535 opens new prospects in insecticide-based vector control. Sci. Rep. 2020, 10, 6842. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Knowles, C.O. Nicotinic and muscarinic cholinergic receptors in honey bee (Apis mellifera) brain. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1990, 97, 275–281. [Google Scholar] [CrossRef]
- Collin, C.; Hauser, F.; Gonzalez de Valdivia, E.; Li, S.; Reisenberger, J.; Carlsen, E.M.; Khan, Z.; Hansen, N.O.; Puhm, F.; Sondergaard, L.; et al. Two types of muscarinic acetylcholine receptors in Drosophila and other arthropods. Cell Mol. Life Sci. 2013, 70, 3231–3242. [Google Scholar] [CrossRef]
- Ren, G.R.; Folke, J.; Hauser, F.; Li, S.; Grimmelikhuijzen, C.J. The A- and B-type muscarinic acetylcholine receptors from Drosophila melanogaster couple to different second messenger pathways. Biochem. Biophys. Res. Commun. 2015, 462, 358–364. [Google Scholar] [CrossRef]
- Xia, R.-Y.; Li, M.-Q.; Wu, Y.-S.; Qi, Y.-X.; Ye, G.-Y.; Huang, J. A new family of insect muscarinic acetylcholine receptors. Insect Mol. Biol. 2016, 25, 362–369. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, X.Y.; Bai, X.; Su, H.A.; Liu, Y.L.; Lu, Y.Y.; Qi, Y.X. Identification of putative muscarinic acetylcholine receptor genes in Bactrocera dorsalis and functional analysis of Bdor-mAChR-B. Insect. Biochem. Mol. Biol. 2021, 139, 103657. [Google Scholar] [CrossRef]
- Lu, S.; Jiang, M.; Tian, X.; Hong, S.; Zhang, J.; Zhang, Y. Characterization of an A-Type Muscarinic Acetylcholine Receptor and Its Possible Non-neuronal Role in the Oriental Armyworm, Mythimna separata Walker (Lepidoptera: Noctuidae). Front. Physiol. 2020, 11, 400. [Google Scholar] [CrossRef]
- Trimmer, B.A. Current excitement from insect muscarinic receptors. Trends Neurosci. 1995, 18, 104–111. [Google Scholar] [CrossRef]
- Trimmer, B.A.; Weeks, J.C. Muscarinic acetylcholine receptors modulate the excitability of an identified insect motoneuron. J. Neurophysiol. 1993, 69, 1821–1836. [Google Scholar] [CrossRef]
- Benson, J.A. Electrophysiological pharmacology of the nicotinic and muscarinic cholinergic responses of isolated neuronal somata from locust thoracic ganglia. J. Exp. Biol. 1992, 170, 203–233. [Google Scholar] [CrossRef]
- Tribut, F.; Duval, A.; Lapied, B. Two distinct receptors are activated by arecoline on cockroach sixth abdominal ganglion DUM neurones. J. Exp. Biol. 1994, 186, 325–331. [Google Scholar] [CrossRef]
- Grolleau, F.; Lapied, B.; Buckingham, S.D.; Mason, W.T.; Sattelle, D.B. Nicotine increases [Ca2+] i and regulates electrical activity in insect neurosecretory cells (DUM neurons) via an acetylcholine receptor with ‘mixed’nicotinic-muscarinic pharmacology. Neurosci. Lett. 1996, 220, 142–146. [Google Scholar] [CrossRef]
- Calas-List, D.; List, O.; Thany, S.H. Nornicotine application on cockroach dorsal unpaired median neurons induces two distinct ionic currents: Implications of different nicotinic acetylcholine receptors. Neurosci. Lett. 2012, 518, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Bielopolski, N.; Amin, H.; Apostolopoulou, A.A.; Rozenfeld, E.; Lerner, H.; Huetteroth, W.; Lin, A.C.; Parnas, M. Inhibitory muscarinic acetylcholine receptors enhance aversive olfactory learning in adult Drosophila. Elife 2019, 8. [Google Scholar] [CrossRef]
- Gauthier, M.; CanoLozano, V.; Zaoujal, A.; Richard, D. Effects of intracranial injections of scopolamine on olfactory conditioning retrieval in the honeybee. Behav. Brain Res. 1994, 63, 5. [Google Scholar] [CrossRef]
- Lozano, V.C.; Armengaud, C.; Gauthier, M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. J. Comp. Physiol. A 2001, 187, 249–254. [Google Scholar] [CrossRef]
- Cano Lozano, V.; Gauthier, M. Effects of the Muscarinic Antagonists Atropine and Pirenzepine on Olfactory Conditioning in the Honeybee. Pharmacol. Biochem. Behav. 1998, 59, 7. [Google Scholar] [CrossRef]
- Abdallah, E.A.M.; Eldefrawi, M.E.; Eldefrawi, A.T. Pharmacologic characterization of muscarinic receptors of insect brains. Arch. Insect Biochem. Physiol. 1991, 17, 107–118. [Google Scholar] [CrossRef]
- Daval, S.B.; Kellenberger, E.; Bonnet, D.; Utard, V.; Galzi, J.-L.; Ilien, B. Exploration of the orthosteric/allosteric interface in human M1 muscarinic receptors by bitopic fluorescent ligands. Mol. Pharmacol. 2013, 84, 71–85. [Google Scholar] [CrossRef]
- Simcock, N.K.; Gray, H.; Bouchebti, S.; Wright, G.A. Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J. Insect. Physiol. 2018, 106, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.; Bicker, G. Habituation of an appetitive reflex in the honeybee. J. Neurophysiol. 1992, 67, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Lambin, M.; Armengaud, C.; Raymond, S.; Gauthier, M. Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 2001, 48, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, S.; Eichmüller, S.; Bicker, G.; Rapus, J.; Eckert, M. Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honeybee. J. Comp. Neurol. 1994, 348, 583–595. [Google Scholar] [CrossRef]
- Qazi, S.; Proulx, D.; Trimmer, B.A. Characterization of muscarinic binding sites in the central nervous system of larval Manduca sexta. Insect Biochem. Mol. Biol. 1996, 26, 721–732. [Google Scholar] [CrossRef]
- Orr, G.L.; Orr, N.; Hollingworth, R.M. Distribution and pharmacological characterization of muscarinic-cholinergic receptors in the cockroach brain. Arch. Insect Biochem. Physiol. 1991, 16, 107–122. [Google Scholar] [CrossRef]
- Shapiro, R.A.; Wakimoto, B.T.; Subers, E.M.; Nathanson, N.M. Characterization and functional expression in mammalian cells of genomic and cDNA clones encoding a Drosophila muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 9039–9043. [Google Scholar] [CrossRef]
- Blake, A.; Anthony, N.; Chen, H.; Harrison, J.; Nathanson, N.; Sattelle, D. Drosophila nervous system muscarinic acetylcholine receptor: Transient functional expression and localization by immunocytochemistry. Mol. Pharmacol. 1993, 44, 716–724. [Google Scholar]
- Harrison, J.B.; Chen, H.H.; Blake, A.D.; Huskisson, N.S.; Barker, P.; Sattelle, D.B. Localization in the nervous system of Drosophila melanogaster of a C-terminus anti-peptide antibody to a cloned Drosophila muscarinic acetylcholine receptor. J. Neuroendocrinol. 1995, 7, 347–352. [Google Scholar] [CrossRef]
- Torkkeli, P.H.; Widmer, A.; Meisner, S. Expression of muscarinic acetylcholine receptors and choline acetyltransferase enzyme in cultured antennal sensory neurons and non-neural cells of the developing moth Manduca sexta. J. Neurobiol. 2005, 62, 316–329. [Google Scholar] [CrossRef]
- Lü, S.M.; Zhao, Z.; Li, K.; Zhang, Y.L.; Xi, G.S. Cloning and expression analysis of a muscarinic cholinergic receptor from the brain of ant, Polyrhachis vicina. Arch. Insect Biochem. Physiol. 2011, 78, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Lapied, B.; Tribut, F.; Hue, B. Effects of McN-A-343 on insect neurosecretory cells: Evidence for muscarinic-like receptor subtypes. Neurosci. Lett. 1992, 139, 165–168. [Google Scholar] [CrossRef]
- Wüstenberg, D.G.; Grünewald, B. Pharmacology of the neuronal nicotinic acetylcholine receptor of cultured Kenyon cells of the honeybee, Apis mellifera. J. Comp. Physiol. A 2004, 190, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Rast, G.; Bräunig, P. Insect mouthpart motor patterns: Central circuits modified for highly derived appendages? Neuroscience 2001, 108, 167–176. [Google Scholar] [CrossRef]
- Okada, J.; Morimoto, Y.; Toh, Y. Antennal motor activity induced by pilocarpine in the American cockroach. J. Comp. Physiol. A 2009, 195, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Kunst, M.; Pförtner, R.; Aschenbrenner, K.; Heinrich, R. Neurochemical Architecture of the Central Complex Related to Its Function in the Control of Grasshopper Acoustic Communication. PLoS ONE 2011, 6, e25613. [Google Scholar] [CrossRef]
- Heinrich, R.; Wenzel, B.; Elsner, N. A role for muscarinic excitation: Control of specific singing behavior by activation of the adenylate cyclase pathway in the brain of grasshoppers. Proc. Natl. Acad. Sci. USA 2001, 98, 9919–9923. [Google Scholar] [CrossRef] [Green Version]
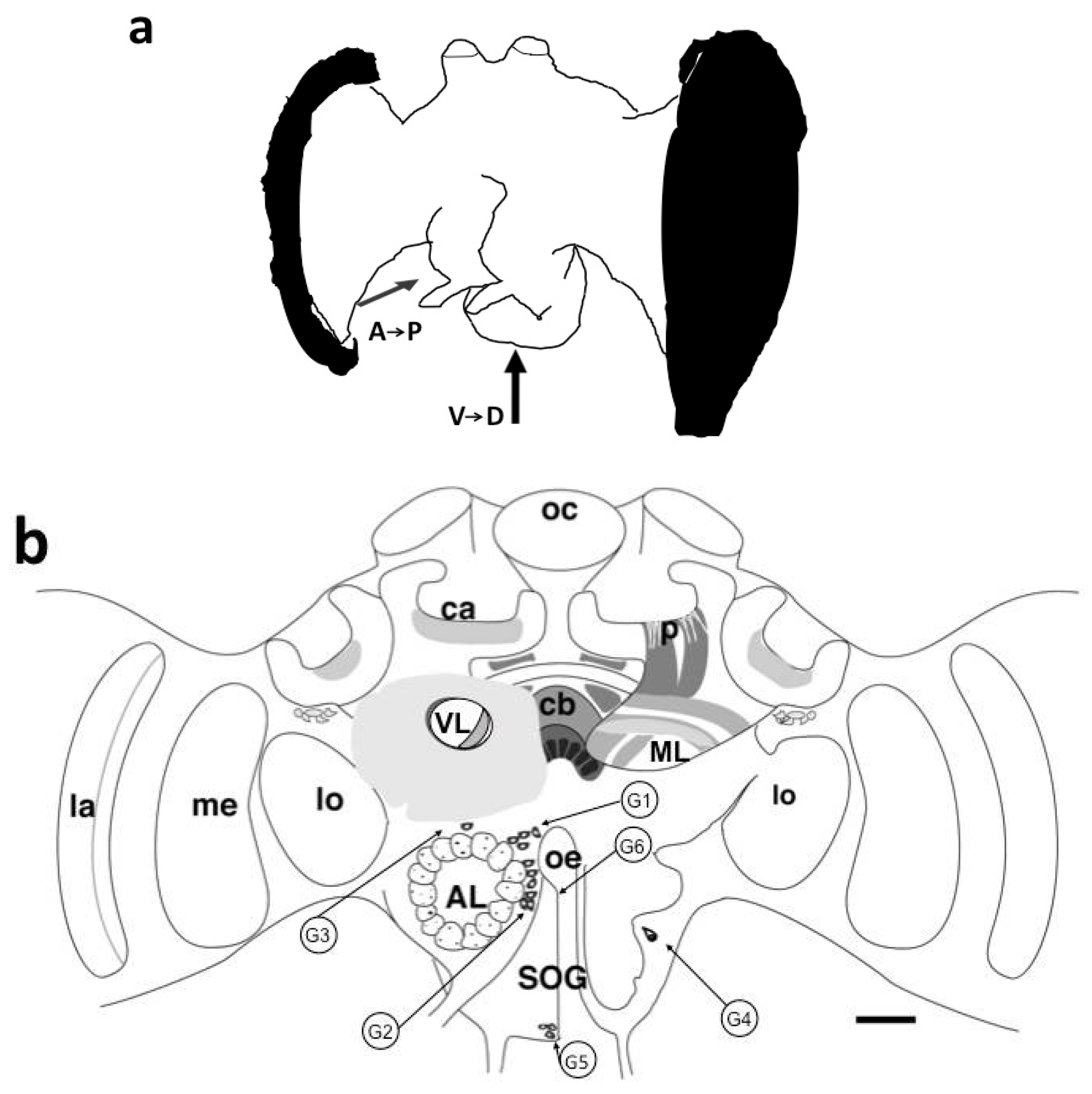
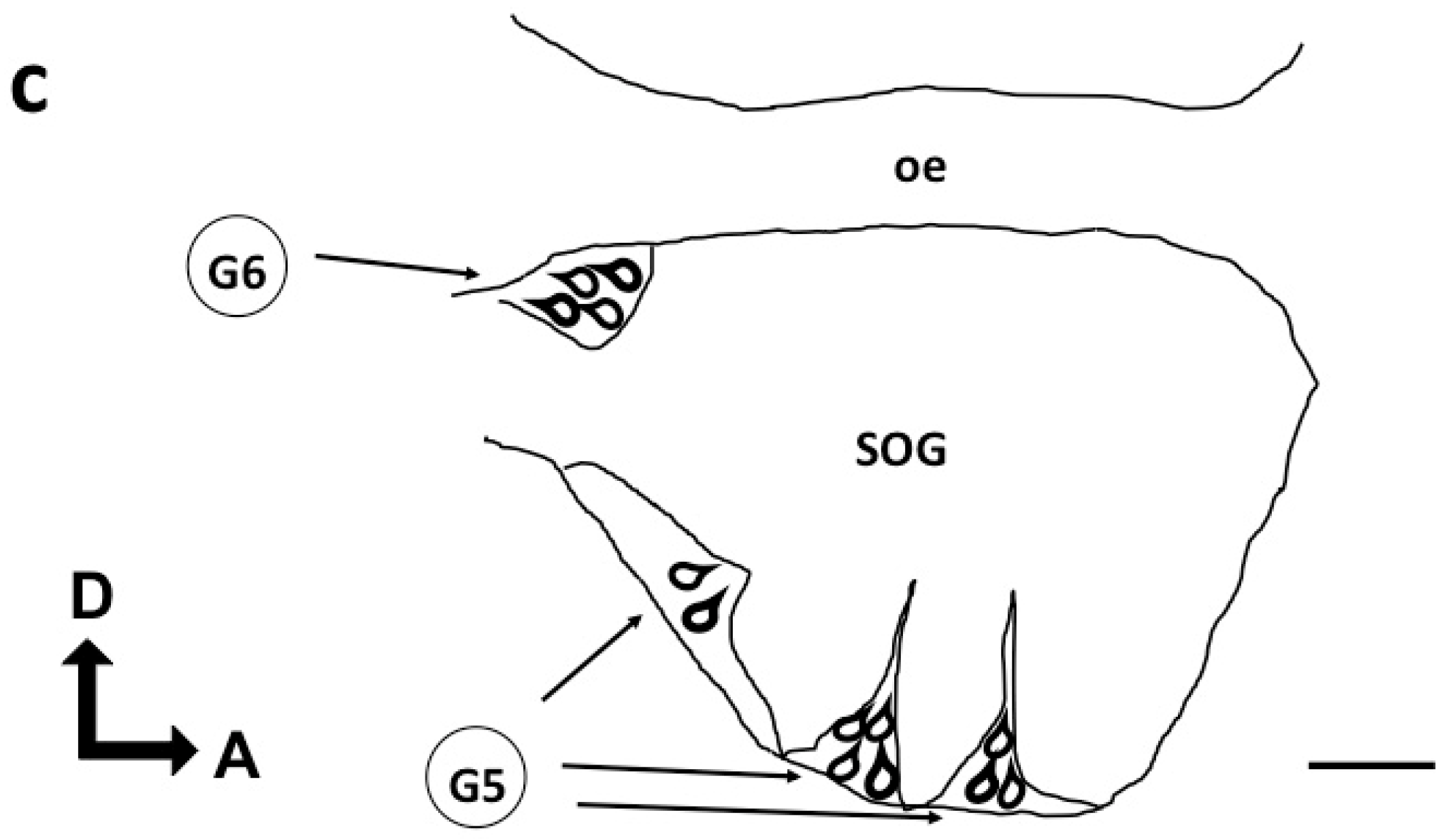
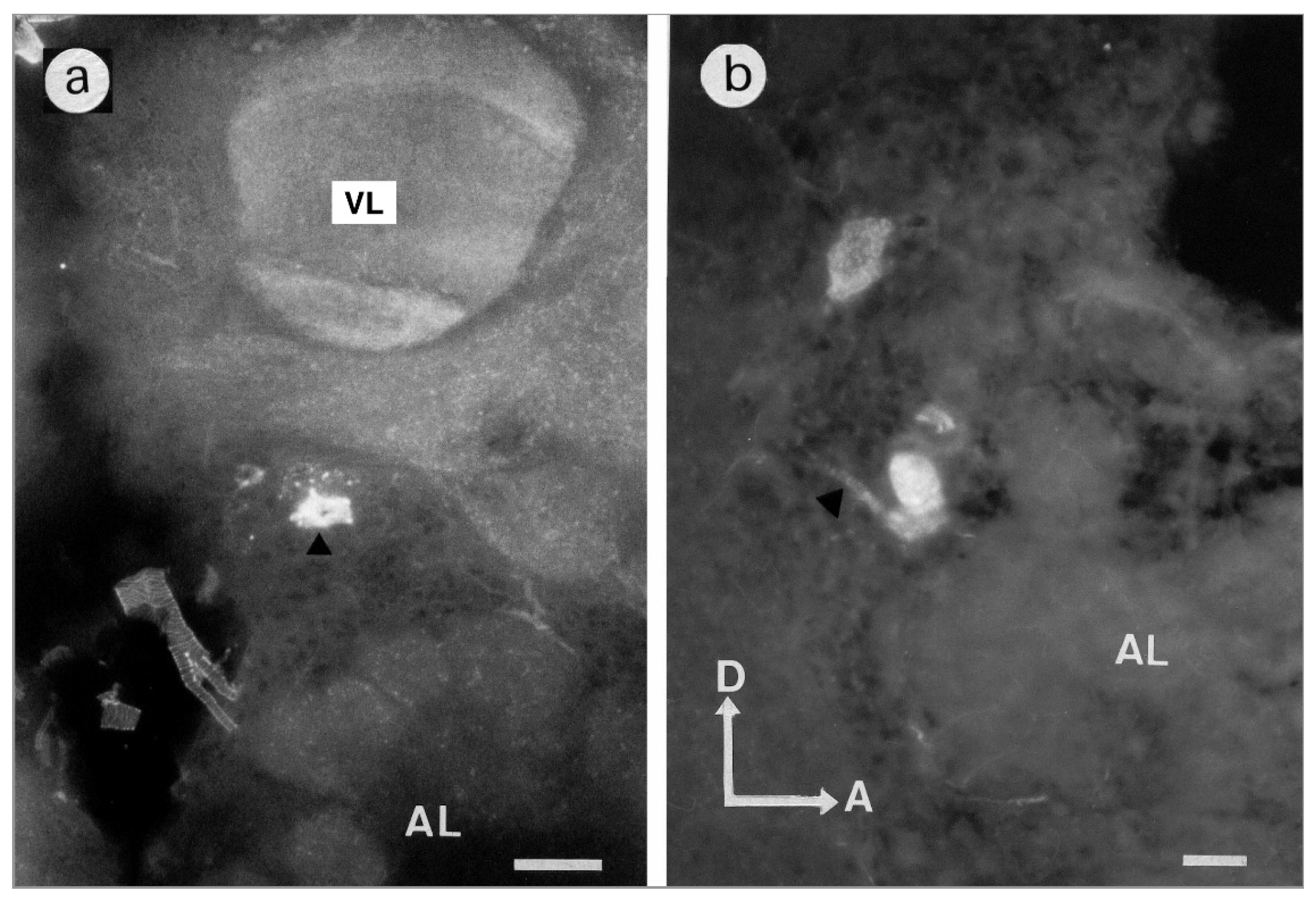
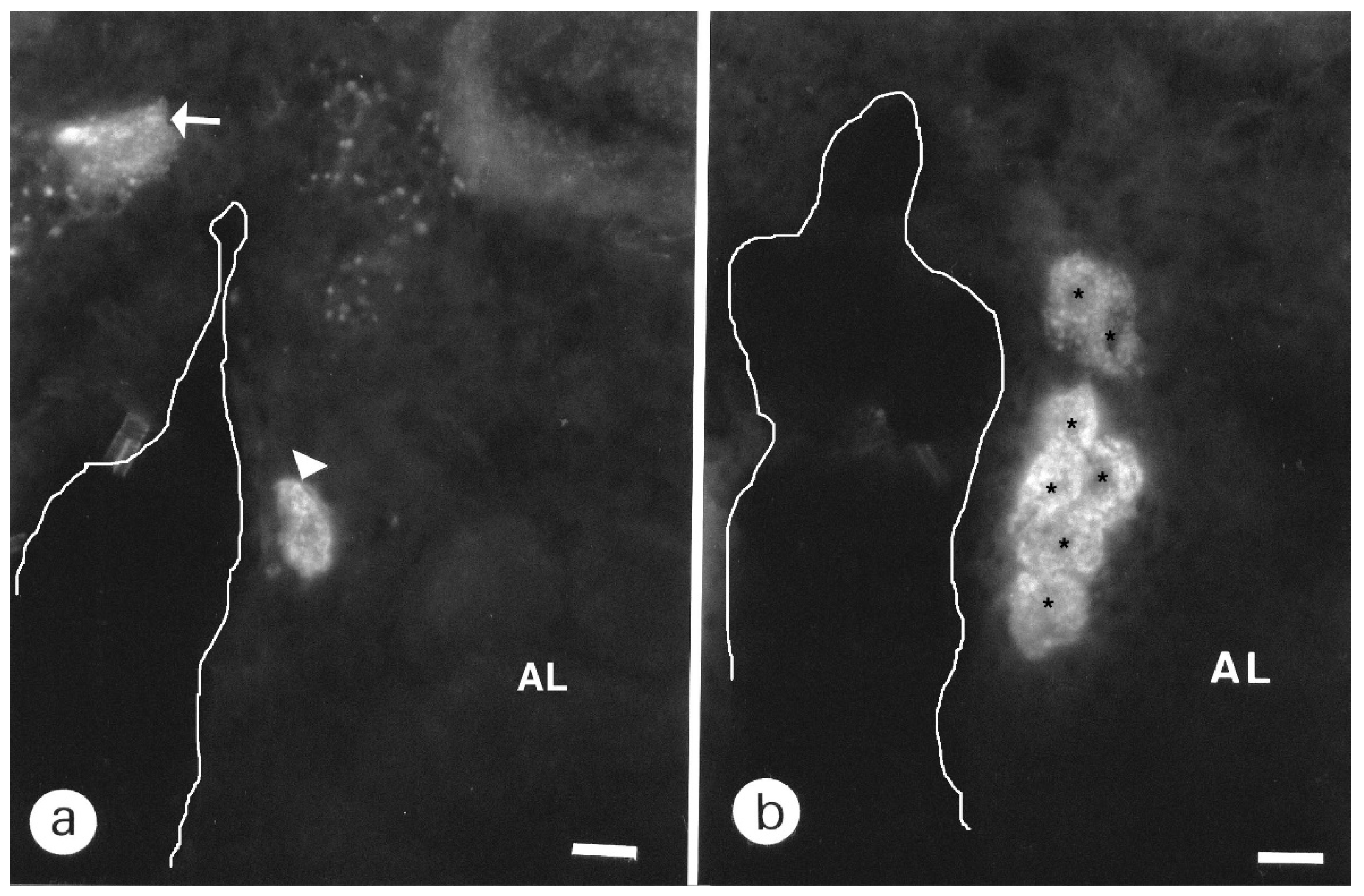




| Antennal Lobe | Subesophageal Ganglion | |||||
|---|---|---|---|---|---|---|
| Group | G1 | G2 | G3 | G4 | G5 | G6 |
| Number of somata | 4 | 7–8 | 1 | 1 | 10–15 | 2 |
| Anterior–posterior: from (μm) | 138 ± 15 | 215 ± 14 | 52 ± 3 | 356 ± 12 | 432 ± 11 | 750 |
| to (μm) | 206 ± 17 | 294 ± 17 | ND | ND | 592 ± 22 | 825 |
| n | 19 | 18 | 7 | 9 | 15 | 2 |
| Ventral–dorsal: from (μm) | 530 | 364 ± 16 | 600 | ND | 56 | 256 |
| to (μm) | 656 | 432 | ND | ND | 160 | 349 |
| n | 3 | 6 | 1 | 9 | 4 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messikh, C.; Gauthier, M.; Armengaud, C. Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning. Insects 2022, 13, 806. https://doi.org/10.3390/insects13090806
Messikh C, Gauthier M, Armengaud C. Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning. Insects. 2022; 13(9):806. https://doi.org/10.3390/insects13090806
Chicago/Turabian StyleMessikh, Chaïma, Monique Gauthier, and Catherine Armengaud. 2022. "Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning" Insects 13, no. 9: 806. https://doi.org/10.3390/insects13090806
APA StyleMessikh, C., Gauthier, M., & Armengaud, C. (2022). Pirenzepine Binding Sites in the Brain of the Honeybee Apis mellifera: Localization and Involvement in Non-Associative Learning. Insects, 13(9), 806. https://doi.org/10.3390/insects13090806






