Chasing Flies: The Use of Wingbeat Frequency as a Communication Cue in Calyptrate Flies (Diptera: Calyptratae)
Abstract
:Simple Summary
Abstract
1. Introduction
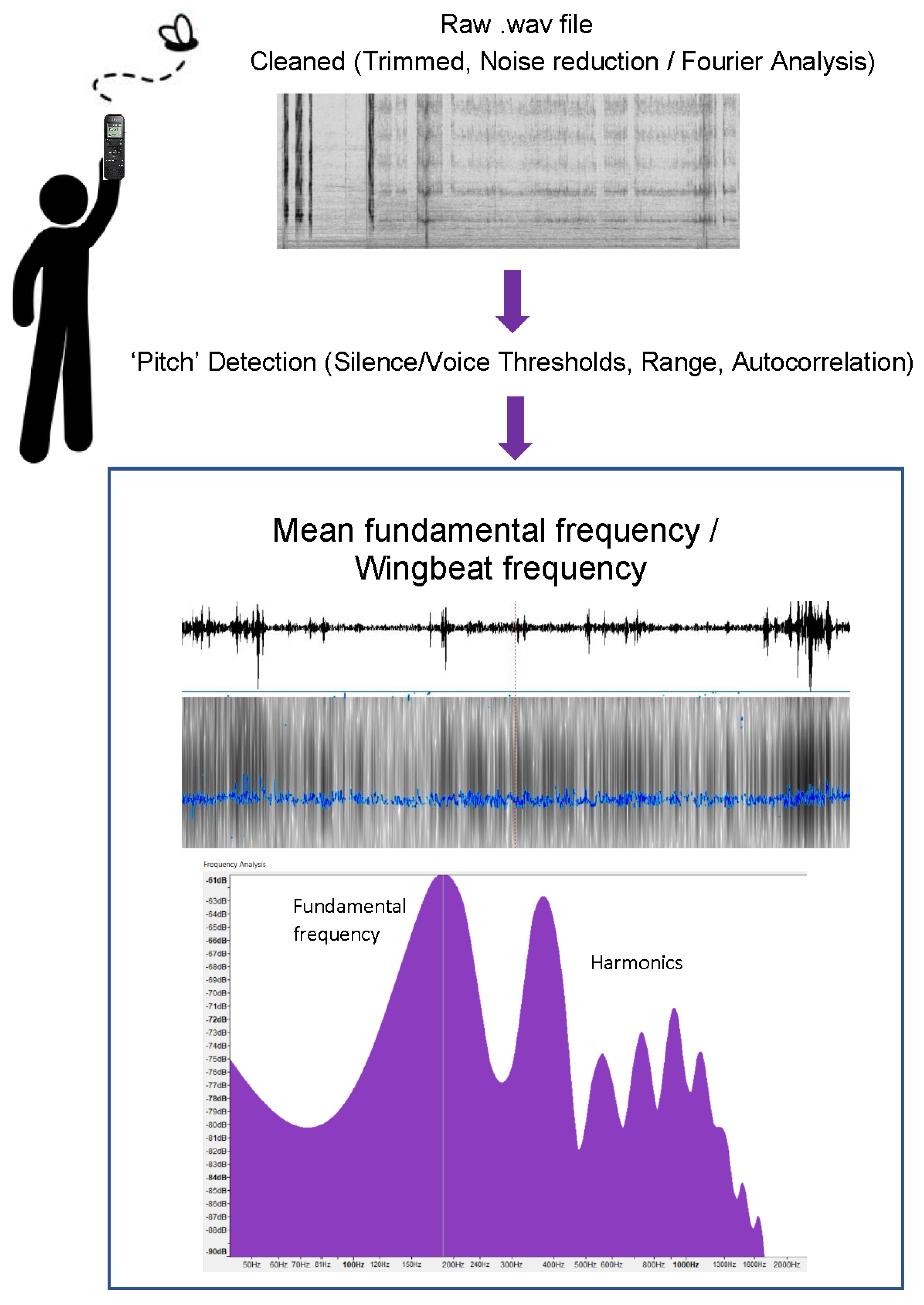
2. Materials and Methods
2.1. Fly Specimens
2.2. Recording Protocol
2.3. Analysis Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sueur, J.; Tuck, E.; Robert, D. Sound radiation around a flying fly. J. Acoust. Soc. Am. 2005, 118, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Galambos, R. Oscilloscopic and stroboscopic analysis of the flight sounds of Drosophila. Biol. Bull. 1950, 99, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mukundarajan, H.; Hol, F.J.H.; Castillo, E.A.; Newby, C.; Prakash, M. Using mobile phones as acoustic sensors for high-throughput mosquito surveillance. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, C.; Fu, X.; Long, T.; Zeng, T. Micro-Doppler measurement of insect wing-beat frequencies with W-band coherent radar. Sci. Rep. 2017, 7, 1396. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; DeBriere, T.J.; Cherukumalli, S.; White, G.S.; Burkett-Cadena, N.D. Infrared light sensors permit rapid recording of wingbeat frequency and bioacoustic species identification of mosquitoes. Sci. Rep. 2021, 11, 10042. [Google Scholar] [CrossRef] [PubMed]
- Landois, H. Die Ton-und Stimmapparate der Insecten in Anatomisch-Physiologischer und Akustischer Beziehung; W. Engelmann: Lemgo, Germany, 1867. [Google Scholar]
- Chadwick, L.E. Some Factors Which Affect the Rate of Movement of the Wings in Drosophila. Physiol. Zool. 1939, 12, 151–160. [Google Scholar] [CrossRef]
- Reed, S.C.; Williams, C.; Chadwick, L.E. Frequency of wing-beat as a character for separating species races and geographic varieties of Drosophila. Genetics 1942, 27, 349. [Google Scholar] [CrossRef]
- Kahn, M.C.; Celestin, W.; Offenhauser, W. Recording of Sounds Produced by Certain Disease-Carrying Mosquitoes. Science 1945, 101, 335–336. [Google Scholar] [CrossRef]
- Sotavalta. The flight-tone (wing-stroke frequency) of insects. Acta Entomol. Fenn. 1947, 4, 1–117. [Google Scholar]
- Roeder, K.D. Movements of the thorax and potential changes in the thoracic muscles of insects during flight. Biol. Bull. 1951, 100, 95–106. [Google Scholar] [CrossRef]
- Sotavalta, O. Flight-Tone and Wing-Stroke Frequency of Insects and the Dynamics of Insect Flight. Nature 1952, 170, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Rockstein, M.; Bhatnagar, P.L. Duration and frequency of wing beat in the aging house fly, Musca domestica L. Biol. Bull. 1966, 131, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.F.; de Almeida Leandro, M.E.D.; Valero, C.; Coronel, L.C.P.; Bazzo, C.O.G. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Jansson, S.; Malmqvist, E.; Mlacha, Y.; Ignell, R.; Okumu, F.; Killeen, G.; Kirkeby, C.; Brydegaard, M. Real-time dispersal of malaria vectors in rural Africa monitored with lidar. PLoS ONE 2021, 16, e0247803. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.; Gebru, A.; Ignell, R.; Abbott, J.; Brydegaard, M. Correlation of Mosquito Wing-Beat Harmonics to Aid in Species Classification and Flight Heading Assessment; SPIE: Bellingham, WA, USA, 2019; Volume 11075. [Google Scholar]
- Santos, D.; Rodrigues, J.; Furtado, V.; Saleem, K.; Korotaev, V. Automated electronic approaches for detecting disease vectors mosquitoes through the wing-beat frequency. J. Clean. Prod. 2019, 217, 767–775. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.A. Automated Surveillance of Fruit Flies. Sensors 2017, 17, 110. [Google Scholar] [CrossRef]
- Moore, A.; Miller, R.H. Automated Identification of Optically Sensed Aphid (Homoptera: Aphidae) Wingbeat Waveforms. Ann. Entomol. Soc. Am. 2002, 95, 1–8. [Google Scholar] [CrossRef]
- Hassall, K.; Dye, A.; Potamitis, I.; Bell, J. Resolving the identification of weak-flying insects during flight: A coupling between rigorous data processing and biology. Agric. For. Entomol. 2021, 23, 489–505. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, Y.; Guo, J.; Wyckhuys, K.A.G.; Shen, X.; Li, X.; Ge, S.; Liu, D.; Wu, K. Interspecific and Seasonal Variation in Wingbeat Frequency Among Migratory Lepidoptera in Northern China. J. Econ. Entomol. 2020, 113, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.H.; Lehmann, F.-O.; Sane, S.P. Wing rotation and the aerodynamic basis of insect flight. Science 1999, 284, 1954–1960. [Google Scholar] [CrossRef]
- Sane, S.P. The aerodynamics of insect flight. J. Exp. Biol. 2003, 206, 4191–4208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, D.N.; Buchmann, S.L.; Spangler, H.G. Relationship between wing loading, wingbeat frequency and body mass in homopterous insects. J. Exp. Biol. 1988, 135, 9–23. [Google Scholar] [CrossRef]
- Ha, N.S.; Truong, Q.T.; Goo, N.S.; Park, H.C. Relationship between wingbeat frequency and resonant frequency of the wing in insects. Bioinspir. Biomim. 2013, 8, 046008. [Google Scholar] [CrossRef] [PubMed]
- Tercel, M.P.; Veronesi, F.; Pope, T.W. Phylogenetic clustering of wingbeat frequency and flight-associated morphometrics across insect orders. Physiol. Entomol. 2018, 43, 149–157. [Google Scholar] [CrossRef]
- Belton, P. Sounds of Insects in Flight; Springer: Berlin/Heidelberg, Germany, 1986; pp. 60–70. [Google Scholar]
- Deora, T.; Gundiah, N.; Sane, S.P. Mechanics of the thorax in flies. J. Exp. Biol. 2017, 220, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.H.; de Souza, A.M.; Huda, A.; Dickinson, M.H. Flies Regulate Wing Motion via Active Control of a Dual-Function Gyroscope. Curr. Biol. CB 2019, 29, 3517–3524.e3513. [Google Scholar] [CrossRef]
- Nachtigall, W.; Wilson, D.M. Neuro-muscular control of dipteran flight. J. Exp. Biol. 1967, 47, 77–97. [Google Scholar] [CrossRef]
- Ellington, C.P.; van den Berg, C.; Willmott, A.P.; Thomas, A.L.R. Leading-edge vortices in insect flight. Nature 1996, 384, 626–630. [Google Scholar] [CrossRef]
- Ewing, A.W. Communication in Diptera. In How Animals Communicate; Indiana University Press Bloomington: Bloomington, IN, USA, 1977; pp. 403–417. [Google Scholar]
- Smith, M.J.; Harper, D.G.C. Animal Signals: Models and Terminology. J. Theor. Biol. 1995, 177, 305–311. [Google Scholar] [CrossRef]
- Higham, J.P.; Hebets, E.A. An introduction to multimodal communication. Behav. Ecol. Sociobiol. 2013, 67, 1381–1388. [Google Scholar] [CrossRef]
- Partan, S.R. Multimodal shifts in noise: Switching channels to communicate through rapid environmental change. Anim. Behav. 2017, 124, 325–337. [Google Scholar] [CrossRef]
- Bayless, K.M.; Trautwein, M.D.; Meusemann, K.; Shin, S.; Petersen, M.; Donath, A.; Podsiadlowski, L.; Mayer, C.; Niehuis, O.; Peters, R.S.; et al. Beyond Drosophila: Resolving the rapid radiation of schizophoran flies with phylotranscriptomics. BMC Biol. 2021, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.N.; Meusemann, K.; Zhou, X.; Yeates, D.; Cerretti, P.; Meier, R.; Pape, T. Phylogenomic analysis of Calyptratae: Resolving the phylogenetic relationships within a major radiation of Diptera. Cladistics 2019, 35, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Bomphrey, R.J.; Walker, S.M.; Taylor, G.K. The typical flight performance of blowflies: Measuring the normal performance envelope of Calliphora vicina using a novel corner-cube arena. PLoS ONE 2009, 4, e7852. [Google Scholar] [CrossRef] [PubMed]
- Ennos, A.R. The kinematics and aerodynamics of the free flight of some diptera. J. Exp. Biol. 1989, 142, 49–85. [Google Scholar] [CrossRef]
- Hedenström, A. How insect flight steering muscles work. PLoS Biol 2014, 12, e1001822. [Google Scholar] [CrossRef]
- Liu, P.; Sane, S.P.; Mongeau, J.-M.; Zhao, J.; Cheng, B. Flies land upside down on a ceiling using rapid visually mediated rotational maneuvers. Sci. Adv. 2019, 5, eaax1877. [Google Scholar] [CrossRef]
- Walker, S.M.; Schwyn, D.A.; Mokso, R.; Wicklein, M.; Muller, T.; Doube, M.; Stampanoni, M.; Krapp, H.G.; Taylor, G.K. In vivo time-resolved microtomography reveals the mechanics of the blowfly flight motor. PLoS Biol. 2014, 12, e1001823. [Google Scholar] [CrossRef]
- Unwin, D.M.; Corbet, S.A. Wingbeat frequency, temperature and body size in bees and flies. Physiol. Entomol. 1984, 9, 115–121. [Google Scholar] [CrossRef]
- Eichorn, C.; Hrabar, M.; Van Ryn, E.C.; Brodie, B.S.; Blake, A.J.; Gries, G. How flies are flirting on the fly. BMC Biol. 2017, 15, 2. [Google Scholar] [CrossRef]
- Levenbook, L.; Williams, C.M. Mitochondria in the flight muscles of insects. III. Mitochondrial cytochrome c in relation to the aging and wing beat frequency of flies. J. Gen. Physiol. 1956, 39, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belyaev, O.A.; Chukanov, V.S.; Farisenkov, S.E. Comparative description of the wing apparatus and flight of some flies (diptera, brachycera). Mosc. Univ. Biol. Sci. Bull. 2012, 67, 117–120. [Google Scholar] [CrossRef]
- Chen, Y.; Why, A.; Batista, G.; Mafra-Neto, A.; Keogh, E. Flying insect detection and classification with inexpensive sensors. J. Vis. Exp. 2014, e52111. [Google Scholar] [CrossRef] [PubMed]
- Mankin, R.; Machan, R.; Jones, R. Field Testing of a Prototype Acoustic Device for Detection of Mediterranean Fruit Flies Flying into a Trap. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil; 2006; p. 165169. [Google Scholar]
- Aubin, T.; Rybak, F.; Moulin, B. A Simple Method for Recording Low-Amplitude Sounds. Application to the Study of the Courtship Song of the Fruit Flydrosophila Melanogaster. Bioacoustics 2000, 11, 51–67. [Google Scholar] [CrossRef]
- Mankin, R.; Hagstrum, D.; Guo, M.; Eliopoulos, P.; Njoroge, A. Automated Applications of Acoustics for Stored Product Insect Detection, Monitoring, and Management. Insects 2021, 12, 259. [Google Scholar] [CrossRef]
- Lehmann, F.O.; Dickinson, M.H. The changes in power requirements and muscle efficiency during elevated force production in the fruit fly Drosophila melanogaster. J. Exp. Biol. 1997, 200, 1133–1143. [Google Scholar] [CrossRef]
- Unwin, D.M.; Ellington, C.P. An Optical Tachometer for Measurement of the Wing-Beat Frequency of Free-Flying Insects. J. Exp. Biol. 1979, 82, 377–378. [Google Scholar] [CrossRef]
- Meiklejohn, K.A. Taxonomy and systematics of the Australian Sarcophaga s.l. (Diptera: Sarcophagidae). Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2012. [Google Scholar]
- Bonacci, T.; Greco, S.; Berardo, C.; Brandmayr, P.; Vercillo, V. The Flesh Fly Sarcophaga (Liopygia) crassipalpis Macquart 1839 as an Invader of a Corpse in Calabria (Southern Italy). J. Forensic Sci. Criminol. 2014, 1, 1–5. [Google Scholar]
- Cook, D.F.; Dadour, I.R. Larviposition in the ovoviviparous blowfly Calliphora dubia. Med. Vet. Entomol. 2011, 25, 53–57. [Google Scholar] [CrossRef]
- Levot, G. Insect Fauna used to Estimate the Post-Mortem Interval of Deceased Persons; Entomological Society of New South Wales: Sydney, Australia, 2003; Volume 32, pp. 31–39. [Google Scholar]
- Pinto, J.; Magni, P.A.; O’Brien, R.C.; Dadour, I.R. Forensically relevant blow flies (Diptera: Calliphoridae) of Central Connecticut, USA. Forensic Sci. Int. 2021, 327, 110940. [Google Scholar] [CrossRef] [PubMed]
- Prado e Castro, C.; García, M.-D.; Arnaldos, M.; González-Mora, D. Sarcophagidae (Diptera) attracted to piglet carcasses including new records for Portuguese fauna. Graellsia 2010, 66, 285–294. [Google Scholar] [CrossRef]
- Shropshire, J.D.; Moore, D.; Seier, E.; Joplin, K.H. Male aggression, limited female choice and the ontogeny of mating behaviour in the flesh fly Sarcophaga crassipalpis. Physiol. Entomol. 2015, 40, 325–335. [Google Scholar] [CrossRef]
- Wallman, J.F.; Adams, M. Molecular Systematics of Australian Carrion-breeding Blowflies of the Genus Calliphora (Diptera: Calliphoridae). Aust. J. Zool. 1997, 45, 337–356. [Google Scholar] [CrossRef]
- Cook, D.; Voss, S.; Finch, J.; Rader, R.; Cook, J.; Spurr, C. The Role of Flies as Pollinators of Horticultural Crops: An Australian Case Study with Worldwide Relevance. Insects 2020, 11, 341. [Google Scholar] [CrossRef]
- Zumpt, F. Myiasis in Man and Animals in the Old World; a Textbook for Physicians, Veterinarians and Zoologists; Butterworth: London, UK, 1965. [Google Scholar]
- Nuorteva, P. Synanthropy of blowflies (Dipt., Calliphoridae) in Finland. Ann. Entomol. Fenn. 1963, 29, 1–49. [Google Scholar]
- Hall, M.J. Traumatic myiasis of sheep in Europe: A review. Parassitologia 1997, 39, 409–413. [Google Scholar]
- Waterhouse, D.; Paramonovo, S. The Status of the Two Species of Lucilia (Diptera, Calliphoridae) Attacking Sheep in Austhalia. Aust. J. Biol. Sci. 1950, 3, 310–336. [Google Scholar] [CrossRef]
- Crisp, P.; Yazdani, M.; Benelli, M.; Mainali, B.; Cunningham, N.; van Helden, M.; Taylor, P. Evaluation of the Sterile Insect Technique for Sheep Blowfly Control; South Australian Research and Development Institute: Meat and Livestock Australia Limited: Sydney, Australia, 2020.
- Hughes, R.D.; Greenham, P.M.; Tyndale-Biscoe, M.; Walker, J.M. A Synopsis of Observations on the Biology of the Australian Bushfly (Musca Vetustissima Walker). Aust. J. Entomol. 1972, 11, 311–331. [Google Scholar] [CrossRef]
- da Cruz, L.; Dadour, I.R.; McAllister, I.L.; Jackson, A.; Isaacs, T. Seasonal variation in trachoma and bush flies in north-western Australian Aboriginal communities. Clin. Exp. Ophthalmol. 2002, 30, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.R. Notes on the ecology of the bushfly, Musca vetustissima Walk. (Diptera: Muscidae), in the Canberra district. Aust. J. Zool. 1966, 14, 1139–1156. [Google Scholar] [CrossRef]
- Wallman, J.F. A key to the adults of species of blowflies in southern Australia known or suspected to breed in carrion. Med. Vet. Entomol. 2001, 15, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Pont, A. Studies on Australian Muscidae (Diptera). IV. A revision of the subfamilies Muscinae and Stomoxyinae. Aust. J. Zool. Suppl. Ser. 1973, 21, 129–296. [Google Scholar] [CrossRef]
- Erzinçlioglu, Z. Blowflies, Naturalists’ Handbooks, 23; The Richmond Publishing Co. Ltd: Slough, UK, 1996. [Google Scholar]
- Crystal, M.M. Observations on the Role of Light, Temperature, Age, and Sex in the Response of Screw-Worm Flies to Attractants. J. Econ. Entomol. 1964, 57, 324–325. [Google Scholar] [CrossRef]
- Bartell, R.J.; Shorey, H.H.; Browne, L.B. Pheromonal stimulation of the sexual activity of males of the sheep blowfly Lucilia cuprina (Calliphoridae) by the female. Anim. Behav. 1969, 17, 576–585. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, M.; Hughes, R.D. Changes in the female reproductive system as age indicators in the bushfly Musca vetustissima Wlk. Bull. Entomol. Res. 1969, 59, 129–141. [Google Scholar] [CrossRef]
- George, K.A.; Archer, M.S.; Toop, T. Abiotic environmental factors influencing blowfly colonisation patterns in the field. Forensic Sci. Int. 2013, 229, 100–107. [Google Scholar] [CrossRef]
- Rusch, T.; Adutwumwaah, A.; Beebe, L.; Tomberlin, J.; Tarone, A. The upper thermal tolerance of the secondary screwworm, Cochliomyia macellaria Fabricius (Diptera: Calliphoridae). J. Therm. Biol. 2019, 85, 102405. [Google Scholar] [CrossRef]
- Traunmüller, H.; Eriksson, A. The Frequency Range of the Voice Fundamental in the Speech of Male and Female Adults, Manuscript; Department of Linguistics, University of Stockholm: Stockholm, Sweden, 1995. [Google Scholar]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 6.1.55. Available online: http://www.praat.org/ (accessed on 25 October 2021).
- Boersma, P. Accurate Short-Term Analysis of the Fundamental Frequency and the Harmonics-to-Noise Ratio of a Sampled Sound. In Proceedings of the Institute of Phonetic Sciences; Citeseer: State College, PA, USA, 1993; pp. 97–110. [Google Scholar]
- Little, M.A.; McSharry, P.E.; Hunter, E.J.; Spielman, J.; Ramig, L.O. Suitability of dysphonia measurements for telemonitoring of Parkinson’s disease. IEEE Trans. Biomed. Eng. 2009, 56, 1015. [Google Scholar] [CrossRef]
- Lovato, A.; De Colle, W.; Giacomelli, L.; Piacente, A.; Righetto, L.; Marioni, G.; de Filippis, C. Multi-Dimensional Voice Program (MDVP) vs Praat for Assessing Euphonic Subjects: A Preliminary Study on the Gender-discriminating Power of Acoustic Analysis Software. J. Voice 2016, 30, 765.e1–765.e5. [Google Scholar] [CrossRef]
- Hoch, H.; Deckert, J.; Wessel, A. Vibrational signalling in a Gondwanan relict insect (Hemiptera: Coleorrhyncha: Peloridiidae). Biol. Lett. 2006, 2, 222–224. [Google Scholar] [CrossRef]
- Casacci, L.P.; Barbero, F.; Ślipiński, P.; Witek, M. The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies. Biology 2021, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Hightower, B.J.; Wijnings, P.W.; Scholte, R.; Ingersoll, R.; Chin, D.D.; Nguyen, J.; Shorr, D.; Lentink, D. How oscillating aerodynamic forces explain the timbre of the hummingbird’s hum and other animals in flapping flight. Elife 2021, 10, e63107. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Warren, B.; Russell, I.J. Humming in tune: Sex and species recognition by mosquitoes on the wing. J. Assoc. Res. Otolaryngol. 2010, 11, 527–540. [Google Scholar] [CrossRef]
- Carmo, R.F.R.; Astúa, D.; Vasconcelos, S.D. Environmental conditions differently affect the wing size and shape of two blow fly species (Calliphoridae) of forensic importance in the Brazilian tropical ecosystems. Int. J. Trop. Insect Sci. 2022, 42, 1903–1911. [Google Scholar] [CrossRef]
- Espra, A.S.; Tabugo, S.R.; Torres, M.A.; Gorospe, J.G.; Manting, M.M.; Demayo, C. Describing dimorphism in wing shapes in the blowfly lucilia sericata meigen (diptera: Calliphoridae) using geometric morphometrics. Adv. Environ. Biol. 2015, 9, 64–70. [Google Scholar]
- Teder, T.; Tammaru, T. Sexual size dimorphism within species increases with body size in insects. Oikos 2005, 108, 321–334. [Google Scholar] [CrossRef]
- Macedo, M.P.; Arantes, L.C.; Tidon, R. Sexual size dimorphism in three species of forensically important blowflies (Diptera: Calliphoridae) and its implications for postmortem interval estimation. Forensic Sci. Int. 2018, 293, 86–90. [Google Scholar] [CrossRef]
- Eberl, D.F.; Boekhoff-Falk, G. Development of Johnston’s organ in Drosophila. Int. J. Dev. Biol. 2007, 51, 679–687. [Google Scholar] [CrossRef]
- Kernan, M.J. Mechanotransduction and auditory transduction in Drosophila. Pflug. Arch. 2007, 454, 703–720. [Google Scholar] [CrossRef]
- Robert, D.; Göpfert, M.C. Acoustic sensitivity of fly antennae. J. Insect Physiol. 2002, 48, 189–196. [Google Scholar] [CrossRef]
- Fuller, S.B.; Straw, A.D.; Peek, M.Y.; Murray, R.M.; Dickinson, M.H. Flying Drosophila stabilize their vision-based velocity controller by sensing wind with their antennae. Proc. Natl. Acad. Sci. USA 2014, 111, E1182–E1191. [Google Scholar] [CrossRef]
- Budick, S.A.; Reiser, M.B.; Dickinson, M.H. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol. 2007, 210, 4092–4103. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Sexual Selection, Social Competition, and Speciation. Q. Rev. Biol. 1983, 58, 155–183. [Google Scholar] [CrossRef]
- Riabinina, O.; Dai, M.; Duke, T.; Albert, J.T. Active Process Mediates Species-Specific Tuning of Drosophila Ears. Curr. Biol. 2011, 21, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Lakes-Harlan, R.; Stolting, H.; Stumpner, A. Convergent Evolution of Insect Hearing Organs from a Preadaptive Structure. Proc. Biol. Sci. 1999, 266, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.; Russell, I. Flying in Tune: Sexual Recognition in Mosquitoes. Curr. Biol. 2006, 16, 1311–1316. [Google Scholar] [CrossRef]
- Cator, L.J.; Arthur, B.J.; Harrington, L.C.; Hoy, R.R. Harmonic convergence in the love songs of the dengue vector mosquito. Science 2009, 323, 1077–1079. [Google Scholar] [CrossRef]
- Warren, B.; Gibson, G.; Russell, I.J. Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr. Biol. 2009, 19, 485–491. [Google Scholar] [CrossRef]
- Pennetier, C.; Warren, B.; Dabiré, K.R.; Russell, I.J.; Gibson, G. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr. Biol. 2010, 20, 131–136. [Google Scholar] [CrossRef]
- Arthur, B.J.; Emr, K.S.; Wyttenbach, R.A.; Hoy, R.R. Mosquito (Aedes aegypti) flight tones: Frequency, harmonicity, spherical spreading, and phase relationships. J. Acoust. Soc. Am. 2014, 135, 933–941. [Google Scholar] [CrossRef]
- von Schilcher, F. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim. Behav. 1976, 24, 18–26. [Google Scholar] [CrossRef]
- Ewing, A.W.; Bennet-Clark, H.C. The Courtship Songs of Drosophila. Behaviour 1968, 31, 288–301. [Google Scholar] [CrossRef]
- Tauber, E.; Eberl, D.F. Acoustic communication in Drosophila. Behav. Processes 2003, 64, 197–210. [Google Scholar] [CrossRef]
- Hall, J.C. The mating of a fly. Science 1994, 264, 1702–1714. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, R.J.; Ferveur, J.F. Courtship in Drosophila. Annu. Rev. Genet. 2000, 34, 205–232. [Google Scholar] [CrossRef]
- Butterworth, N.J. Love at first flight: An investigation of the chemical and visual cues that mediate mate recognition in blowflies (Diptera: Calliphoridae). Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2020. [Google Scholar]
- Jones, S.; Byrne, P.; Wallman, J. Exploring the influence of individual courtship behaviors on male mating success in a blow fly. J. Insect Behav. 2017, 30, 528–543. [Google Scholar] [CrossRef]
- Butterworth, N.J.; Byrne, P.G.; Wallman, J.F. The Blow Fly Waltz: Field and Laboratory Observations of Novel and Complex Dipteran Courtship Behavior. J. Insect Behav. 2019, 32, 109–119. [Google Scholar] [CrossRef]
- Parker, G.A. The Sexual Behaviour of the Blowfly, Protophormia Terrae-Novae R.-D. Behaviour 1968, 32, 291–308. [Google Scholar] [CrossRef]
- Tobin, E.N. The Courtship Behavior of the House Fly, Musca domestica L., and the Face Fly, Musca autumnalis De Geer, with Notes on the Experimental Hybridization of the Two Species; University of Massachusetts: Amherst, MA, USA, 1972; p. 143. [Google Scholar]
- Colwell, A.; Shorey, H. The courtship behavior of the house fly, Musca domestica (Diptera: Muscidae). Ann. Entomol. Soc. Am. 1975, 68, 152–156. [Google Scholar] [CrossRef]
- Thomas, H.T. Field Notes on the Mating Habits of Sarcophaga Meigen (Diptera). Proc. R. Entomol. Soc. Lond. Ser. A Gen. Entomol. 1950, 25, 93–98. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Cruz, M.R.P.; Pontes, W.J.T.; Vasconcelos, S.D. Aspects of the reproductive behaviour and development of two forensically relevant species, Blaesoxipha (Gigantotheca) stallengi (Lahille, 1907) and Sarcophaga (Liopygia) ruficornis (Fabricius, 1794) (Diptera: Sarcophagidae). Rev. Bras. Entomol. 2019, 63, 124–129. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C. Acoustics of Insect Song. Nature 1971, 234, 255–259. [Google Scholar] [CrossRef]
- Bhandawat, V.; Maimon, G.; Dickinson, M.; Wilson, R. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J. Exp. Biol. 2010, 213, 4313. [Google Scholar] [CrossRef]
- Rogoff, W.M.; Gretz, G.H.; Sonnet, P.F.; Schwarz, M. Responses of Male House Flies 1 to Muscalure and to Combinations of Hydrocarbons with and without Muscalure. Environ. Entomol. 1980, 9, 605–606. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Butterworth, N.J.; Wallman, J.F.; Drijfhout, F.P.; Johnston, N.P.; Keller, P.A.; Byrne, P.G. The evolution of sexually dimorphic cuticular hydrocarbons in blowflies (Diptera: Calliphoridae). J. Evol. Biol. 2020, 33, 1468–1486. [Google Scholar] [CrossRef]
- Moore, H.E.; Hall, M.J.R.; Drijfhout, F.P.; Cody, R.B.; Whitmore, D. Cuticular hydrocarbons for identifying Sarcophagidae (Diptera). Sci. Rep. 2021, 11, 7732. [Google Scholar] [CrossRef]
- Butterworth, N.J.; Byrne, P.G.; Keller, P.A.; Wallman, J.F. Body Odor and Sex: Do Cuticular Hydrocarbons Facilitate Sexual Attraction in the Small Hairy Maggot Blowfly? J. Chem. Ecol. 2018, 44, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shorey, H.H.; Bartell, R.J. Role of a volatile female sex pheromone in stimulating male courtship behaviour in Drosophila melanogaster. Anim. Behav. 1970, 18, 159–164. [Google Scholar] [CrossRef]
- Würf, J.; Pokorny, T.; Wittbrodt, J.; Millar, J.G.; Ruther, J. Cuticular Hydrocarbons as Contact Sex Pheromone in the Parasitoid Wasp Urolepis rufipes. Front. Ecol. Evol. 2020, 8, 180. [Google Scholar] [CrossRef]
- Lunau, K. Visual ecology of flies with particular reference to colour vision and colour preferences. J. Comp. Physiol. A 2014, 200, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Burton, B.G.; Laughlin, S.B. Neural images of pursuit targets in the photoreceptor arrays of male and female houseflies Musca domestica. J. Exp. Biol. 2003, 206, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- Stucky, B.J. Eavesdropping to Find Mates: The Function of Male Hearing for a Cicada-Hunting Parasitoid Fly, Emblemasoma erro (Diptera: Sarcophagidae). J. Insect Sci. 2016, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Guillot, F.S.; Brown, H.E.; Broce, A.B. Behavior of Sexually Active Male Screwworm Flies1. Ann. Entomol. Soc. Am. 1978, 71, 199–201. [Google Scholar] [CrossRef]
- Spieth, H.T. Mating behaviour with in genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 1952, 99, 395–474. [Google Scholar]
- Cook, D.F. Influence of previous mating experience on future mating success in male Lucilia cuprina (Diptera: Calliphoridae). J. Insect Behav. 1994, 8, 207–217. [Google Scholar] [CrossRef]
- Bretman, A.; Fricke, C.; Hetherington, P.; Stone, R.; Chapman, T. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 2010, 21, 317–321. [Google Scholar] [CrossRef]
- Butterworth, N.J.; White, T.E.; Byrne, P.G.; Wallman, J.F. Love at first flight: Wing interference patterns are species-specific and sexually dimorphic in blowflies (Diptera: Calliphoridae). J. Evol. Biol. 2021, 34, 558–570. [Google Scholar] [CrossRef]
- Brodie, B.S.; Wong, W.H.L.; VanLaerhoven, S.; Gries, G. Is aggregated oviposition by the blow flies Lucilia sericata and Phormia regina (Diptera: Calliphoridae) really pheromone-mediated? Insect Sci. 2015, 22, 651–660. [Google Scholar] [CrossRef]
- Cragg, J.B.; Cole, P. Laboratory Studies on the Chemo-Sensory Reactions of Blowflies. Ann. Appl. Biol. 1956, 44, 478–491. [Google Scholar] [CrossRef]
- Ashworth, J.R.; Wall, R. Responses of the sheep blowflies Lucilia sericata and L. cuprina to odour and the development of semiochemical baits. Med. Vet. Entomol. 1994, 8, 303–309. [Google Scholar] [CrossRef]
- Diaz, L.A.; Kaufman, P.E. EENY503: Flesh Fly Sarcophaga crassipalpis Macquart (Insects: Diptera: Sarcophagidae). Available online: https://edis.ifas.ufl.edu/publication/IN905 (accessed on 7 June 2022).
- Lodha, K.R.; Treece, R.E.; Koutz, F.R. Studies on the Mating Behavior of the Face Fly12. J. Econ. Entomol. 1970, 63, 207–212. [Google Scholar] [CrossRef]
- Bryant, E.H.; Turner, C.R. Comparative Morphometric Adaptation of the Housefly and the Face Fly in the United States. Evolution 1978, 32, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, J.N. The seasonal distribution and characteristics of bush fly Musca vetustissima Walker populations in south-western Australia. Aust. J. Ecol. 1983, 8, 383–394. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Norris, K. Daily patterns of flight activity of blowflies (Calliphoridae: Diptera) in the Canberra district as indicated by trap catches. Aust. J. Zool. 1966, 14, 853–865. [Google Scholar] [CrossRef]
- Moore, A.; Miller, J.R.; Tabashnik, B.E.; Gage, S.H. Automated identification of flying insects by analysis of wingbeat frequencies. J. Econ. Entomol. 1986, 79, 1703–1706. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I. Novel Noise-Robust Optoacoustic Sensors to Identify Insects Through Wingbeats. IEEE Sens. J. 2015, 15, 4621–4631. [Google Scholar] [CrossRef]
- Silva, D.F.; Souza, V.M.A.; Ellis, D.P.W.; Keogh, E.J.; Batista, G.E.A.P.A. Exploring Low Cost Laser Sensors to Identify Flying Insect Species. J. Intell. Robot. Syst. 2015, 80, 313–330. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C.; Ewing, A.W. The Love Song of the Fruit Fly. Sci. Am. 1970, 223, 84–93. [Google Scholar] [CrossRef]
- Yuval, B. Mating systems of blood-feeding flies. Annu. Rev. Entomol. 2006, 51, 413–440. [Google Scholar] [CrossRef] [PubMed]



| Species | Family | Wingbeat Frequency (Mean Hz) | Method | Reference |
|---|---|---|---|---|
| M. domestica | Muscidae | 130 | free flight in box | [10] |
| M. domestica | Muscidae | 160–162 | tethered, stroboscope | [13] |
| M. domestica | Muscidae | 180 | free flight in box, optical tachometer | [43] |
| L. sericata | Calliphoridae | 190 | tethered, microphone | [1] |
| L. sericata | Calliphoridae | 178 (females) 212 (males) | free flight in box, video-recorded reflected light flashes | [44] |
| P. regina | Calliphoridae | ~150 | oscillograph | [45] |
| L. caesar | Calliphoridae | 205 | free flight in box, high speed camera | [46] |
| C. vicina | Calliphoridae | 145 | tethered, microtomographic imaging | [42] |
| C. vicina | Calliphoridae | 158 | free flight in box, high-speed camera | [39] |
| C. vicina | Calliphoridae | 162 | free flight in box | [10] |
| C. vomitora | Calliphoridae | 215 | free flight in box, high speed camera | [26] |
| Sarcophaga spp. | Sarcophagidae | 150 | free flight in box, high speed camera | [26] |
| S. carnaria | Sarcophagidae | 200 | free flight in box, high speed camera | [46] |
| Species | Sarcophaga crassipalpis | Calliphora dubia | Lucilia sericata | Musca vetustissima | p-Value | |
|---|---|---|---|---|---|---|
| Specimens recorded | 40 20 females 20 males | 40 20 females 20 males | 40 20 females 20 males | 40 20 females 20 males | - | |
| Fundamental frequencies obtained | Total | 230,968 | 206,533 | 132,150 | 103,170 | - |
 | 122,207 | 112,520 | 68,212 | 59,196 | - | |
 | 108,761 | 94,013 | 63,938 | 43,974 | - | |
| Mean wingbeat frequency (Hz) * |  + + | 169 (18) | 186 (14) | 213 (30) | 224 (18) | <0.001 |
 | 161 (14) | 181 (15) | 201 (4) | 229 (16) | <0.01 | |
 | 177 (10) | 191 (15) | 225 (19) | 221 (15) | ||
| Mean variability ** |  + + | 8.87 | 6.19 | 11.36 | 10.32 | <0.001 |
 | 7.82 | 6.21 | 10.39 | 8.74 | <0.05 (except C. dubia) | |
 | 9.91 | 6.17 | 12.33 | 11.90 | ||
| Predicted Group Membership | Classification Results * | ||||
|---|---|---|---|---|---|
| 1 Sarcophaga crassipalpis | 2 Calliphora dubia | 3 Lucilia sericata | Total | ||
| Original Count | 1 | 33 | 7 | 0 | 40 |
| 2 | 12 | 24 | 4 | 40 | |
| 3 | 0 | 4 | 36 | 40 | |
| % | 1 | 82.5 | 17.5 | 0 | 100 |
| 2 | 30 | 60 | 10 | 100 | |
| 3 | 0 | 10 | 90 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Magni, P.A.; O’Brien, R.C.; Dadour, I.R. Chasing Flies: The Use of Wingbeat Frequency as a Communication Cue in Calyptrate Flies (Diptera: Calyptratae). Insects 2022, 13, 822. https://doi.org/10.3390/insects13090822
Pinto J, Magni PA, O’Brien RC, Dadour IR. Chasing Flies: The Use of Wingbeat Frequency as a Communication Cue in Calyptrate Flies (Diptera: Calyptratae). Insects. 2022; 13(9):822. https://doi.org/10.3390/insects13090822
Chicago/Turabian StylePinto, Julie, Paola A. Magni, R. Christopher O’Brien, and Ian R. Dadour. 2022. "Chasing Flies: The Use of Wingbeat Frequency as a Communication Cue in Calyptrate Flies (Diptera: Calyptratae)" Insects 13, no. 9: 822. https://doi.org/10.3390/insects13090822
APA StylePinto, J., Magni, P. A., O’Brien, R. C., & Dadour, I. R. (2022). Chasing Flies: The Use of Wingbeat Frequency as a Communication Cue in Calyptrate Flies (Diptera: Calyptratae). Insects, 13(9), 822. https://doi.org/10.3390/insects13090822








