Simple Summary
Nosema ceranae is a highly prevalent intracellular parasite of honey bees’ midgut worldwide. There is a lack of studies addressing the influence of climatic and beekeeping factors on the dynamics of its infection. A long-term study has been carried out in six apiaries in four Mediterranean countries (France, Israel, Portugal, and Spain), monitoring a total of 103 colonies. The lowest prevalence of infection was observed in mainland France, while the highest percentage of infected honey bees per colony was detected in Israel. The location and beekeeping management were shown to influence the infection levels. The percentage of infected honey bees negatively affected the colony strength in the apiaries located in Spain and mainland Portugal, whereas queen replacement had a positive effect on these same apiaries, reducing infection levels. The highest colony losses occurred in mainland France, which had the lowest levels of N. ceranae. It was followed by Spain, an apiary with high levels of N. ceranae, so no correlation between infection and mortality could be established. These results suggest that complementary studies on interactions with other pathogens and honey bee genetics are needed in order to develop management strategies for its control.
Abstract
Nosema ceranae is a highly prevalent intracellular parasite of honey bees’ midgut worldwide. This Microsporidium was monitored during a long-term study to evaluate the infection at apiary and intra-colony levels in six apiaries in four Mediterranean countries (France, Israel, Portugal, and Spain). Parameters on colony strength, honey production, beekeeping management, and climate were also recorded. Except for São Miguel (Azores, Portugal), all apiaries were positive for N. ceranae, with the lowest prevalence in mainland France and the highest intra-colony infection in Israel. A negative correlation between intra-colony infection and colony strength was observed in Spain and mainland Portugal. In these two apiaries, the queen replacement also influenced the infection levels. The highest colony losses occurred in mainland France and Spain, although they did not correlate with the Nosema infection levels, as parasitism was low in France and high in Spain. These results suggest that both the effects and the level of N. ceranae infection depends on location and beekeeping conditions. Further studies on host-parasite coevolution, and perhaps the interactions with other pathogens and the role of honey bee genetics, could assist in understanding the difference between nosemosis disease and infection, to develop appropriate strategies for its control.
1. Introduction
The conservation of the abundance and diversity of insect pollinators is a decisive action to avoid the negative impact that the lack of these insects can have on agriculture, food production and security, and environmental sustainability. In this regard, managed honey bees are a suitable species that can easily be located in areas where they serve as a central pollination structure for a wide range of crops and a variety of wild flowers, which, in their absence, are not sustainable [1,2].
During the last few decades, there has been an alarming increase in honey bee colony losses where pathogens like Varroa destructor mites, the Microsporidia Nosema spp. and viruses contribute actively [3,4,5,6]. Pathogen spread within honey bee colonies is a dynamic process and is sometimes the result of the invasion of a new virulent pathogen and/or the combination with other pathogens and parasites that may be present in the colony [7,8,9].
Two Nosema species have been identified as honey bee pathogens: Nosema apis and Nosema ceranae, and nowadays, both species infect Apis mellifera colonies worldwide. However, N. ceranae is the species that has become one of the most prevalent honey bee pathogens globally [10,11,12]. This microsporidium is an obligate intracellular parasite of the ventricular cells of honey bees [13] and it is implicated in honey bee colony losses in some regions, especially in warm areas [12,14,15,16,17,18], probably due to the higher resistance of N. ceranae spores to heat and desiccation [19]. In A. mellifera honey bees, infection by this microsporidium induces damage to the ventriculus (midgut), which is the main site of nutrient absorption of the digestive tract and the target tissue of this pathogen. In this tissue, the infection causes degeneration of the epithelial cells, which are full of microsporidia in different stages of development (i.e., meronts, sporonts, sporoblasts, and spores), causing the weakening and death of infected honey bees [13,14]. In fact, the infection has been reported to shorten the lifespan [20,21,22], induce oxidative stress and changes in the metabolism and hormonal regulation of the honey bee host [21,23], or immune modulation [24,25,26,27], among other effects.
The prevalence of N. ceranae varies widely among locations. A common feature is that this pathogen is widely distributed regardless of the climatic conditions, ranging from desert climates [28,29,30] to very cold ones [31,32]. In some areas, this microsporidium is present in more than 50% of the colonies sampled [18,33,34,35,36,37,38,39], whereas in others the prevalence is lower than that percentage [40,41]. However, most studies consist of occasional surveys to determine the prevalence of infected colonies at a specific point in time [18,32,37,38,40,42,43,44,45,46]. Other studies carried out longitudinal surveys to determine the prevalence in selected apiaries and how it fluctuates across the study period. The findings varied among the studies, with countries such as Serbia [35], Germany [47], or New Zealand [48] showing a higher prevalence in spring and Uruguay showing a higher prevalence from the beginning of the winter until the end of the spring [49].
The prevalence of infection varies among colonies, and it is unclear how climate and beekeeping management affect the development of the pathogen and the resultant disease. Although variations at colony or apiary level throughout the year have been reported occasionally [14,50], comparisons among studies are difficult due to the diverse sampling and methodology employed, which hinder understanding of the true impact that climatic factors and beekeeping management have on the infection. Therefore, long-term research conducted using standardized protocols is needed to investigate these issues. To that end, this study aimed to compare Nosema spp. infection and its development in apiaries with very different climates and beekeeping practices during a 2-year period in four Mediterranean countries.
2. Materials and Methods
2.1. Study Design
The study was carried out simultaneously in four Mediterranean countries: Portugal, Spain, France, and Israel. Six apiaries were selected to conduct the survey, namely, four locations where V. destructor is present, including CIAPA (Spain), INRAE (France), ARO (Israel), and CIMO (Portugal), and two others where V. destructor was absent, including the Ouessant (OUE, France) [51,52] and the São Miguel (SMI, Azores, Portugal) [53,54] islands. The exact locations of the apiaries and the total number of colonies involved in the study are shown in Figure 1 and Table 1. All colonies were prescreened to detect N. ceranae as described later. All the apiaries except São Miguel contained positive colonies at the beginning of the study. The São Miguel apiary was negative, although there is a history of N. ceranae presence on the island [55].
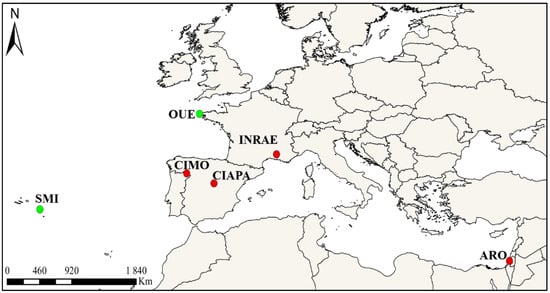
Figure 1.
Apiary locations: Bragança, Portugal (CIMO), Fuente la Higuera, Spain (CIAPA), Avignon, France (INRAE), Zrifin, Israel (ARO), Ouessant Island, France (OUE), and São Miguel Island, Azores, Portugal (SMI). Red—V. destructor present, Green—V. destructor absent.

Table 1.
Location of the apiaries, number of colonies (n = 103) and mitochondrial DNA (MtDNA) lineages of honey bees.
The colonies of the six apiaries were monitored for 20 months. Samplings started in February 2018 and ended in October 2019. On the islands (OUE and SMI), sampling started later in April 2018 due to weather conditions. Samplings were carried out every two months, except for December 2018 in most locations, due to the low temperatures. Colonies that died during the 20-month sampling period were replaced by spare colonies that were kept in the monitored apiaries under identical conditions (except in ARO) from the onset of the study.
2.2. Detection of N. ceranae Infection
In each sampling, adult workers were collected from each colony by brushing off the first comb with no brood surface from the brood chamber. This procedure was done to avoid collecting newborn workers. Samples were taken to the laboratory and kept at −80 °C for further analysis. All the apiaries were sampled using the same standardized protocol to allow comparisons.
The Nosema spp. infection was determined at apiary and intra-colony levels. At the apiary level, the presence of N. ceranae was determined for each colony from 60 adult workers that were processed as a pool. The pools prepared from each colony were placed in a sterile container (tubes or bags with a filter) and 15 mL or 6 mL of RNase-free water were added, respectively. The pools were homogenized using Stomacher® (Qiagen, Hilden, Germany) and 180 µL of the macerates were transferred to 96-well plates (Qiagen, Hilden, Germany). The plates were shaken two times in a Tissuelyser (Qiagen, Hilden, Germany) for 1 min at 30 Hz, changing position between shacking rounds, followed by a short centrifugation. At ARO, the homogenization was performed with 50 mL tubes via Geno-grinder TM at 1550 rpm for 3 min, followed by a spin at 800 rpm 4 °C for 2 min. Afterwards, 50 µL of each homogenate was transferred to a 96-well plate, mixed with 50 µL TE buffer, and incubated at 95 °C for 20 min following the protocol [57] with a slight modification without the addition of Proteinase K [57].
At the intra-colony level, the prevalence of N. ceranae (percentage of bees infected per colony) was determined by individually analyzing 25 workers per colony, as described previously [14], which allowed us to establish the detection threshold at 4% (1 worker positive out of 25). Each worker was placed in a well of a 96-deep well plate with 500 µL of nuclease-free water and two steel beads. The plates were homogenized in a Tissuelyser (Qiagen, Hilden, Germany) for 1 min at 30 Hz four times or spun at 800 rpm 4 °C for 2 min in ARO. DNA extraction was performed in 50 µL of each homogenate, as explained above.
All PCRs were done as multiplex reactions with primers that allow for the detection of N. ceranae, N. apis, and an A. mellifera internal control (COI) in the laboratories of CIAPA (Spanish and Portuguese samples), INRAE (French samples), and ARO (Israeli samples) using a harmonized protocol [33]. PCR amplicons were revealed by agarose gels (French and Israeli samples) or by using the QIAxpert system (Spanish and Portuguese samples). Standard controls (Nosema spp. DNA and spores) were prepared by CIAPA and shared with INRAE and ARO to assure that the same specificity and sensitivity level were reached at the different facilities.
2.3. Beekeeping and Climatic Conditions
On every bimonthly visit to the apiary to collect samples, the colonies were inspected using the same standardized protocol and forms across countries to allow comparisons. Colony mortality, colony strength, colony management, the presence of pathogens (including Varroa levels and brood diseases), and control treatments were recorded. Assessment of colony strength was based on the type of hive, the number of combs covered by bees in the nest and supers, and the number of combs with brood. The percentage of area covered by honey bees was recorded on each side of the combs. Data were converted to the number of honey bees per colony, as indicated in the BEEBOOK [58]. In each brood comb, the area (in percentage) occupied by brood was visually estimated, and the quality of the brood was inspected for the presence of brood diseases. At every sampling date, the presence of the queen (color marked) was checked and, when the queen was not observed, the presence of recently laid eggs was verified. The presence of an unmarked queen in the colony was interpreted as a new queen born after a natural replacement, which was marked in situ according to the accepted international color code. Colony management consisted of recording any activity done. At the ARO apiary, a specific management of colonies was conducted in October–November 2018, in which all the queens were replaced by young mated queens and colonies were balanced through the exchange of brood combs and adults among the colonies of the apiary. Consequently, all colonies in 2019 were considered new ones.
In each apiary, the percentage of V. destructor infestation was determined during each sampling by using the sugar powder test, counting mite falls on 300 honeybees (limit of detection 0.3%) [59]. Additionally, V. destructor was controlled following national regulations (except in OUE and SMI, which were mite-free). In INRAE, two amitraz strips (Apivar®) as active ingredient (a.i.) per hive were used at the end of September each year. In ARO, 2–3 amitraz strips (Galvitraz®) per hive were applied in July and December each year. In CIMO and CIAPA, two amitraz strips (Apivar® in 2018 and Apitraz® in 2019) per hive were used in March and September 2018 and July 2019 (CIMO) or September 2019 (CIAPA); in March 2019, colonies were treated with thymol as a.i. using ApiLifeVar® (CIMO) or Apiguard® (CIAPA).
The presence of other pathogens in the colonies was inspected to detect any clinical signs of adult or brood diseases and was recorded in case of detection.
In addition, honey production was recorded annually by differences in the weight of honey combs from supers before and after honey extraction. The weather conditions were also recorded throughout the study by using meteorological stations in the mainland apiaries. Parameters recorded were mean, maximum, and minimum temperatures (°C); mean, maximum, and minimum relative humidity (%); mean wind speed (m/s); days with mean wind speed ≥6.4 m/s; and height of precipitation (mm).
2.4. Statistical Analysis
To determine whether there were any significant differences in the number of colonies infected by N. ceranae per apiary, all data from each site were analyzed together (cross-tabs, chi-square, with Monte Carlo correction, p < 0.0001). Differences in the intra-colony prevalence (percentage of honey bees infected per colony) among apiaries were analyzed by ANOVA. Homogeneity of variances was determined with a Levene test, and a post-hoc Games Howell or a Bonferroni test (depending on whether the variances were homogeneous or not) were used to compare among apiaries and/or sampling dates within the apiary. A Rho Spearman test was used to determine the correlation between the intra-colony infection level and the Varroa levels, the meteorological data, the colony strength data, and the honey production. The relationship between the levels of N. ceranae intra-colony infection in the colonies that replaced the queen or not was assessed using a Mann–Whitney U test. All p-values < 0.05 were considered significant, and all statistical analyses were carried out using the IBM SPSS Statistics V24 software by the Statistics Unit of the Scientific Computing Area at the SGAI-CSIC (Madrid, Spain).
3. Results
3.1. Nosema spp. Infection at the Apiary Level
Data on Nosema spp. infection was obtained from 103 colonies established at the beginning of the study (Table 1). The total number of colonies analyzed for each sampling round is shown in Table 2. As stated above, some colonies were added to replace the losses in order to monitor a sufficient number of colonies. Both N. ceranae and N. apis were analyzed in all the apiaries, but the latter was rarely found. Only one colony was positive for N. apis in April 2018 at the CIAPA, and therefore the following analyses were only performed on N. ceranae.

Table 2.
Percentage of colonies positive for N. ceranae per sampling and apiary and number of positive colonies out of sampled colonies in parenthesis. SMI had 10 colonies where Nosema spp. were never detected. ND: Not detected.
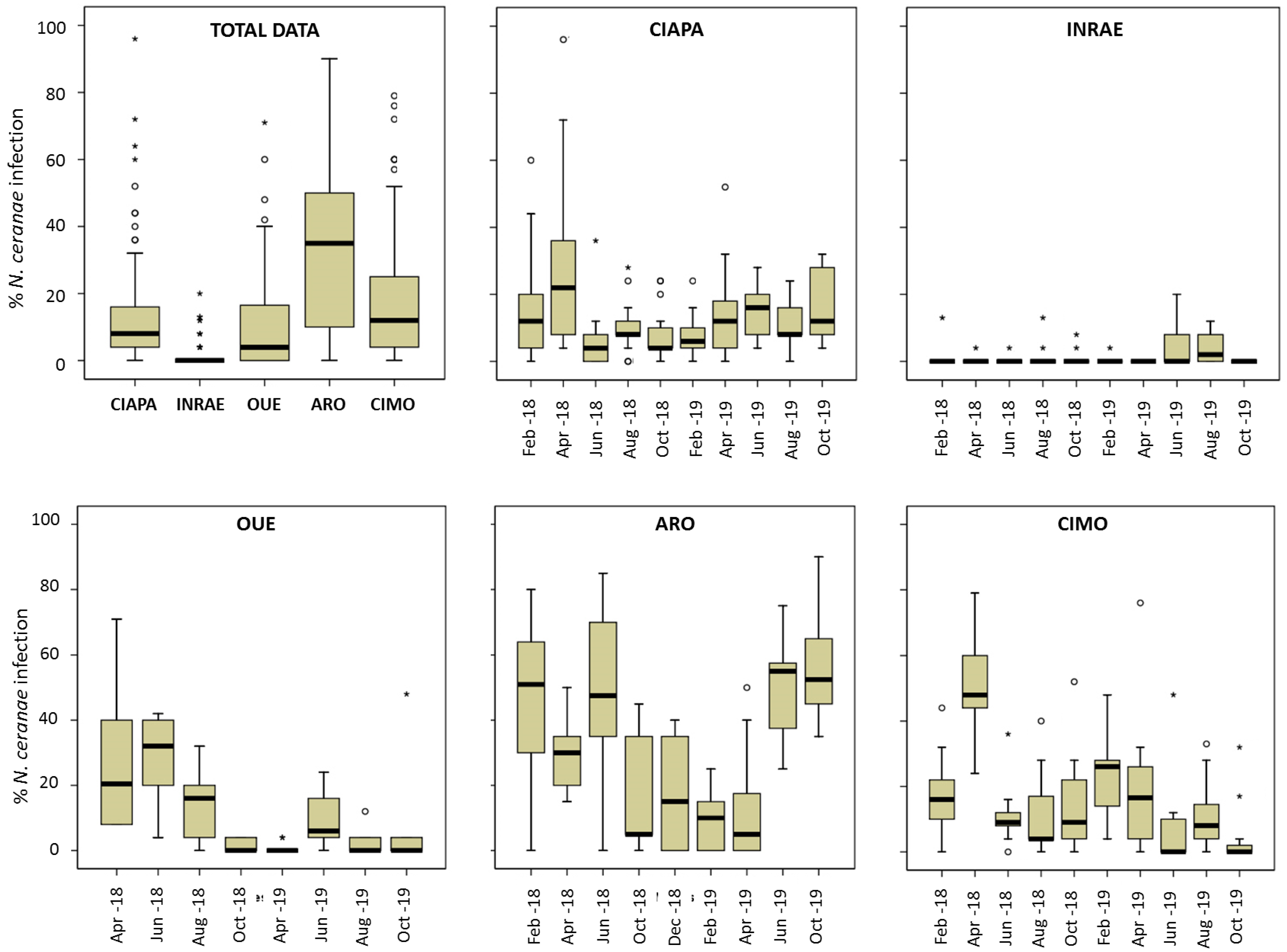
Most colonies were positive for N. ceranae in all apiaries throughout the study except on SMI, where no colony was positive. When we jointly analyzed all the data of infected colonies per apiary, the number of N. ceranae-positive colonies varied significantly among the apiaries (chi-square with Monte Carlo correction, p < 0.0001). All the colonies located in the CIAPA, ARO, and CIMO apiaries were positive for N. ceranae at the onset of the study and remained so nearly across the entire study period (February 2018–October 2019), and only on rare occasions, the microsporidium was not detected (Table 2). In contrast, N. ceranae was not detected in most of the INRAE colonies at the onset of the study, although the number of positive colonies increased in the following samplings, up to 66.67% in April 2018. From this moment on, the number of positive colonies was below 23%, except in August in both sampling years (50% and 60%, respectively). In the case of the OUE apiary, an intermediate pattern was observed, as all the colonies were positive at the beginning of the study and this was maintained during the first year with a low decrease (90% in October 2018), while in the second year, the number of N. ceranae-negative colonies decreased.
3.2. N. ceranae Infection at Intra-Colony Level
A total of 13,907 individual honey bees were screened for N. ceranae during the study (Table 3). The percentage of infected honey bees per colony (intra-colony prevalence) was also significantly (ANOVA; p < 0.0001) different among apiaries (Figure 2) and it varied across time (Figure 2), especially in ARO and CIMO. ARO had the highest mean prevalence (Table 3; Figure 2), with 32.39%, and it was significantly different from the other apiaries (Games–Howell test; p < 0.0001). ARO was followed by CIMO (17.17%), CIAPA (13.30%), and OUE (11.11%), and these three apiaries were not significantly different from each other (Games–Howell test; p > 0.05). Finally, INRAE had the significantly (Games–Howell test; p < 0.0001) lowest mean level of intra-colony infection (1.37%).

Table 3.
Intra-colony prevalence at each apiary. Percentage of N. ceranae infected honey bees per colony during the study. ND: Not detected (<4%).
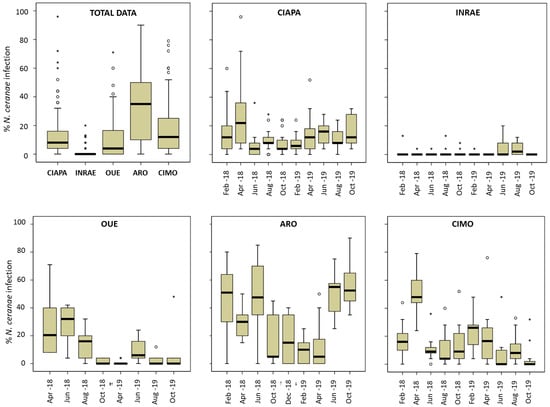
Figure 2.
N. ceranae intra-colony level of infection. Total data per apiary and data per each apiary and sampling round. Data shows percentage of infected workers. Black line represents the median, while the box represents 50% of observations. Outliers are shown as dots and asterisks.
Regarding N. apis, only 14 out of the 13,907 honey bees analyzed were positive (two honey bees in CIAPA and 12 in CIMO), mostly found in coinfection with N. ceranae (12 honey bees coinfected). Thus, the percentage of infection by this species is not included.
In all apiaries, there were colonies with no infected honey bees at some point in time (Supplementary Table S1). On the other hand, the highest level of infection was detected in one colony from CIAPA (96%) in April 2018. In this apiary, April was the month with the highest mean intra-colony prevalence (27.64%; Figure 2, Supplementary Table S1) and the mean values kept similar and below 16% from that moment on until the end of the study (October 2019). The colonies of CIMO showed a similar pattern. The maximum level of infection was also found in April 2018 (50%) and it decreased afterwards, maintaining prevalence values below 20% until February 2019 (24.35%), decreasing again (circa 10%) until the end of the study. The ARO apiary exhibited the highest mean intra-colony prevalence, which was above 30% in all sampling rounds, except between October 2018 and April 2019. From that moment on, the prevalence increased gradually, reaching the highest levels of infection in October 2019 (56%). The colonies of the OUE apiary started with a relatively high percentage of infected honey bees (April and June 2018, >20%), decreasing thereafter to remain at levels below 10% until the end of the study. The intra-colony prevalence in INRAE showed the lowest levels of infection throughout the entire study period, with no significant differences among the samplings, as the mean values were below 2% in most of the samplings, and only in June and August 2019 did it increase to 4%. The highest value (20%) was found in one colony in June 2019 and it became under the detection level in the following sampling round. The SMI was in stark contrast to the remaining apiaries, as no honey bees were detected as positive. The full set of results on the significant differences in the percentage of infected bees for each sampling round and apiary can be found in the Supplementary Table S2.
3.3. Varroa Destructor Levels
A Varroa test was performed only in the apiaries of CIMO, CIAPA, INRAE, and ARO as the islands of SMI and OUE were mite free. The infestation levels differed among apiaries, with the highest mean percentage found in CIMO (1.09%) and ARO (0.9%), and the lowest in CIAPA (0.18%; Table 4). It should be noted that, except for CIAPA, the maximum levels of Varroa were over 9% at INRA, ARO, and CIMO but in just one colony at each apiary. However, only 36 samples, out of the 419 analyzed, exceeded 2% (11 from INRA, 12 from ARO, and 13 from CIMO).

Table 4.
Statistics for the percentage of Varroa destructor in 300 honey bees per apiary across sampling rounds (n = 419). Islands are not included as they were the mite free.
There was a positive correlation between the percentage of Varroa in the colony and the N. ceranae intra-colony infection level (Spearman’s Rho, 2-tailed; p < 0.005), when all the data were analyzed together. This correlation was maintained in the INRAE and ARO apiaries when analyzed separately (Spearman’s Rho, 2-tailed; p < 0.005) whereas in the CIMO and CIAPA apiaries, there was no correlation.
3.4. Climatic Conditions and N. ceranae Infection
The ARO apiary was located in the warmest region with the highest mean temperature (22.16 °C) and mean relative humidity (67.17%). The INRAE apiary exhibited the highest mean precipitation (1.82 mm), whereas CIMO showed the highest mean wind speed (6.25 m/s) of all the studied apiaries (Figure 3; Supplementary Table S3).
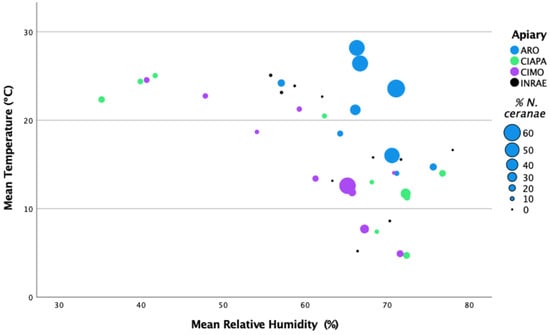
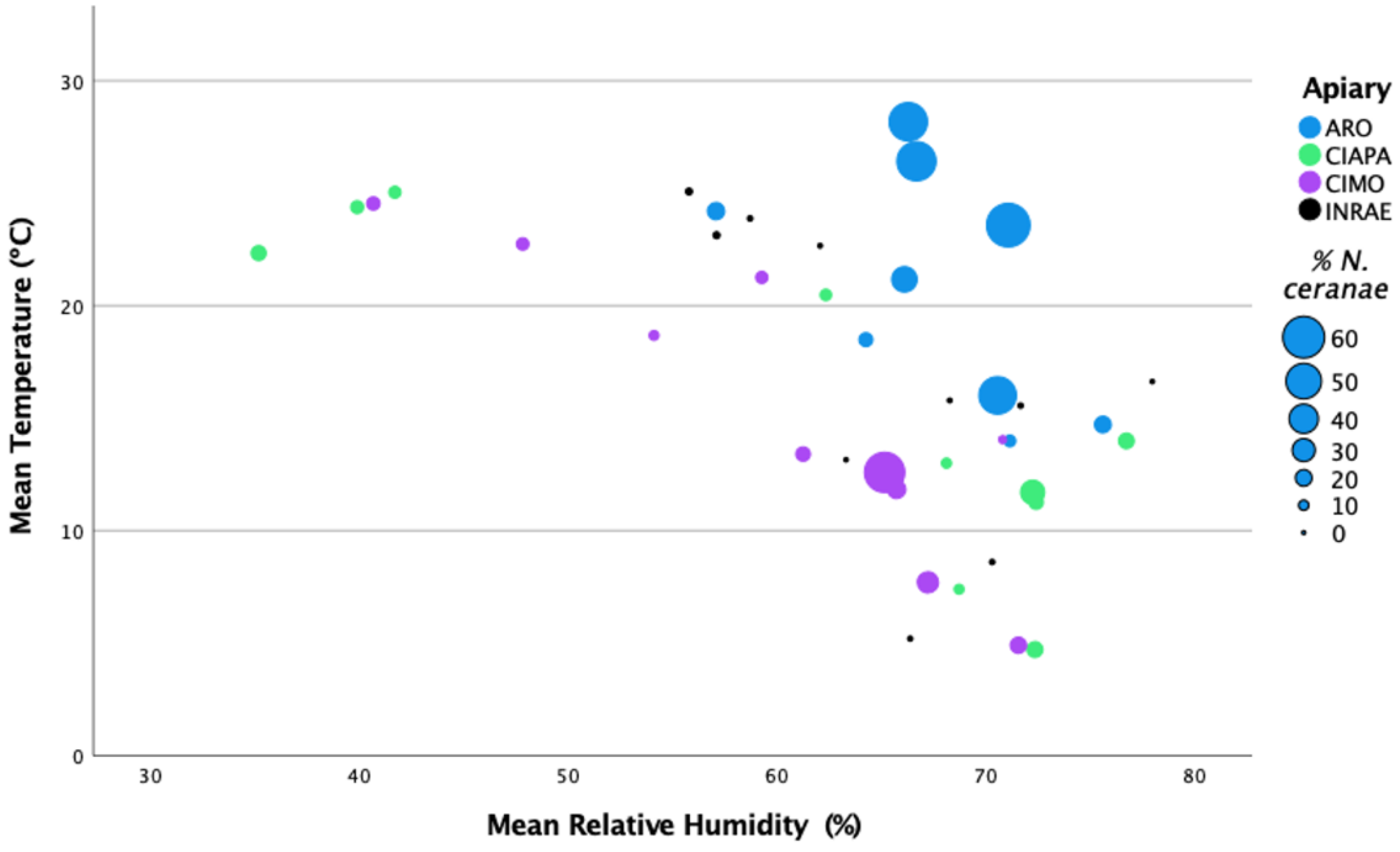
Figure 3.
Representation of N. ceranae infection levels (percentage of infected honey bees) per apiary and sampling round in relation to the mean temperature (°C) and the mean relative humidity (%).
The analysis between the mean percentage of intra-colony infection per apiary across samplings and the climatic conditions (monthly means) did not show significant correlations with the recorded parameters (Spearman’s Rho, 2-tailed; p > 0.05). Only the number of days with a wind speed higher than 6.4 m/s was positively correlated with N. ceranae levels (Spearman’s Rho, 2-tailed; p < 0.005), which could be related with to number of days that honey bees are inside the colony. However, these data were only recorded for the CIMO and INRAE apiaries, and this correlation could not be confirmed at the other locations.
When the mean temperature and mean relative humidity were represented together in relation to the N. ceranae infection levels, the highest levels were found at the highest mean temperature and mean relative humidity of over 65% (Figure 3). These values match the ARO apiary, which exhibited N. ceranae infection levels significantly higher than in the other apiaries (Games–Howell test; p < 0.0001) (see Section 3.2: N. ceranae infection at intra-colony level).
3.5. Colony Strenght and N. ceranae Infection Levels
There was considerable variation in colony strength (number of adult honey bees per colony) both among and within the apiaries (Figure 4). CIAPA exhibited the highest mean (23,124.16) of colony strength and INRAE, the lowest (10,453.83; Table 5). ARO was the first apiary where the population began to increase in spring, reaching maximum values in April in both years. This pattern contrasted with that of the other apiaries, where colony strength peaked in the summer (Figure 4).
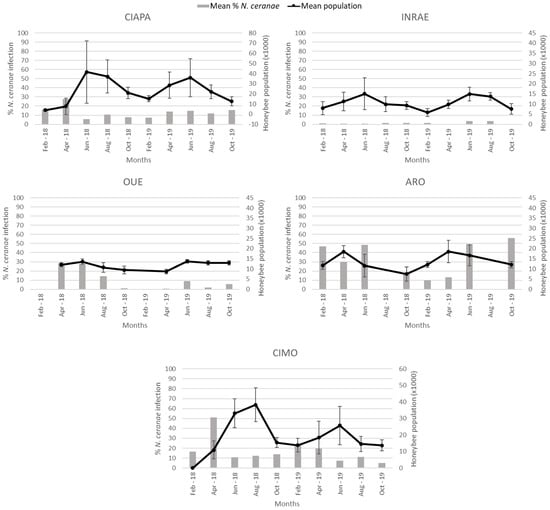
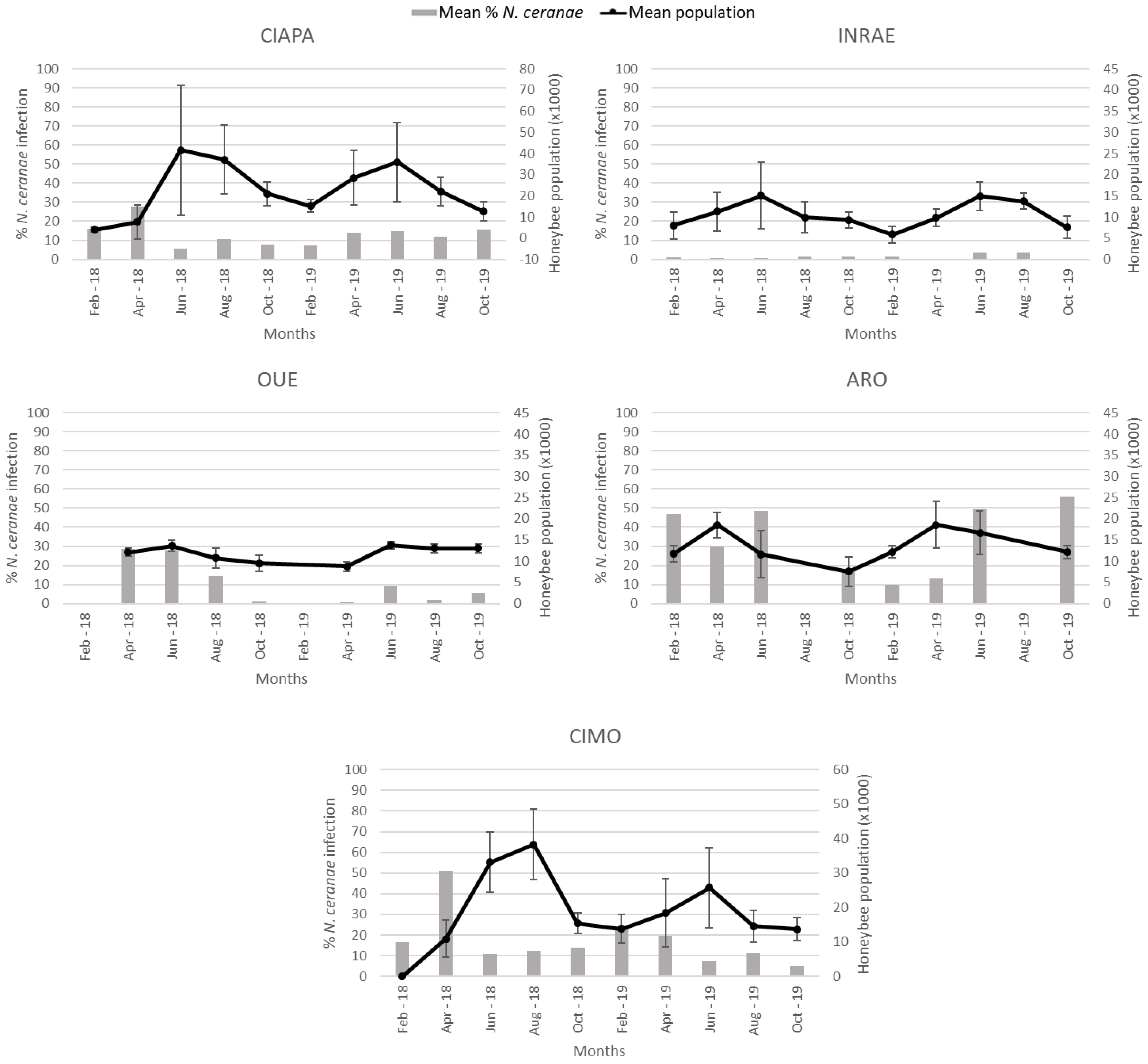
Figure 4.
Mean percentage of N. ceranae infection (grey columns) and mean colony strength and standard deviation (black line) per apiary during the study period. Colony strength is represented in number of honeybees (thousands). In OUE and ARO, there was no data collection in February and August, respectively, due to weather conditions.

Table 5.
Estimation of colony strength for each apiary. Values represent an estimation of the number of honey bee adults.
Colony strength, estimated at each sampling round, was analyzed to determine if there was a relationship with the percentage of honey bees infected by N. ceranae (Figure 4). When the data generated for each apiary were analyzed altogether, there was a positive correlation (r = 0.179) between the two variables (Spearman’s Rho; p < 0.0001). However, when the analysis was done individually for each apiary, a different pattern was observed. While in the CIAPA and CIMO apiaries, a significant negative correlation (r = −0.299 and r = −0.257, respectively) between the percentage of N. ceranae infection and colony strength was observed (Spearman’s Rho; p < 0.01), and in INRAE and OUE, the correlation was positive (Spearman Rho; r = 0.252 and r = 0.299, respectively; p < 0.01). In ARO, the correlation was not significant, although the trend was negative as in CIAPA and CIMO (Spearman’s Rho; p > 0.05). SMI was not included in the analysis because N. ceranae was not detected in any colony across time.
3.6. Honey Production and N. ceranae Infection Levels
The honey production (Kg) per colony was recorded only in the continental apiaries (Table 6). CIMO and CIAPA had the highest mean of honey production (29.30 Kg and 27.64 Kg, respectively), followed by ARO (18.15 Kg) and INRAE (9.16 Kg, data available only for 2019), which is consistent with colony strength. There was no significant correlation between N. ceranae intra-colony infection and honey production (Spearman’s Rho; p > 0.05).

Table 6.
Average honey production per colony (in Kg) at each apiary during the study period. Islands are not included. Data not available.
3.7. Queen Replacement and N. ceranae Infection Levels
In each sampling round, the presence of the queen was checked. Thus, every queen replacement was recorded except on the islands, in which those data were not available.
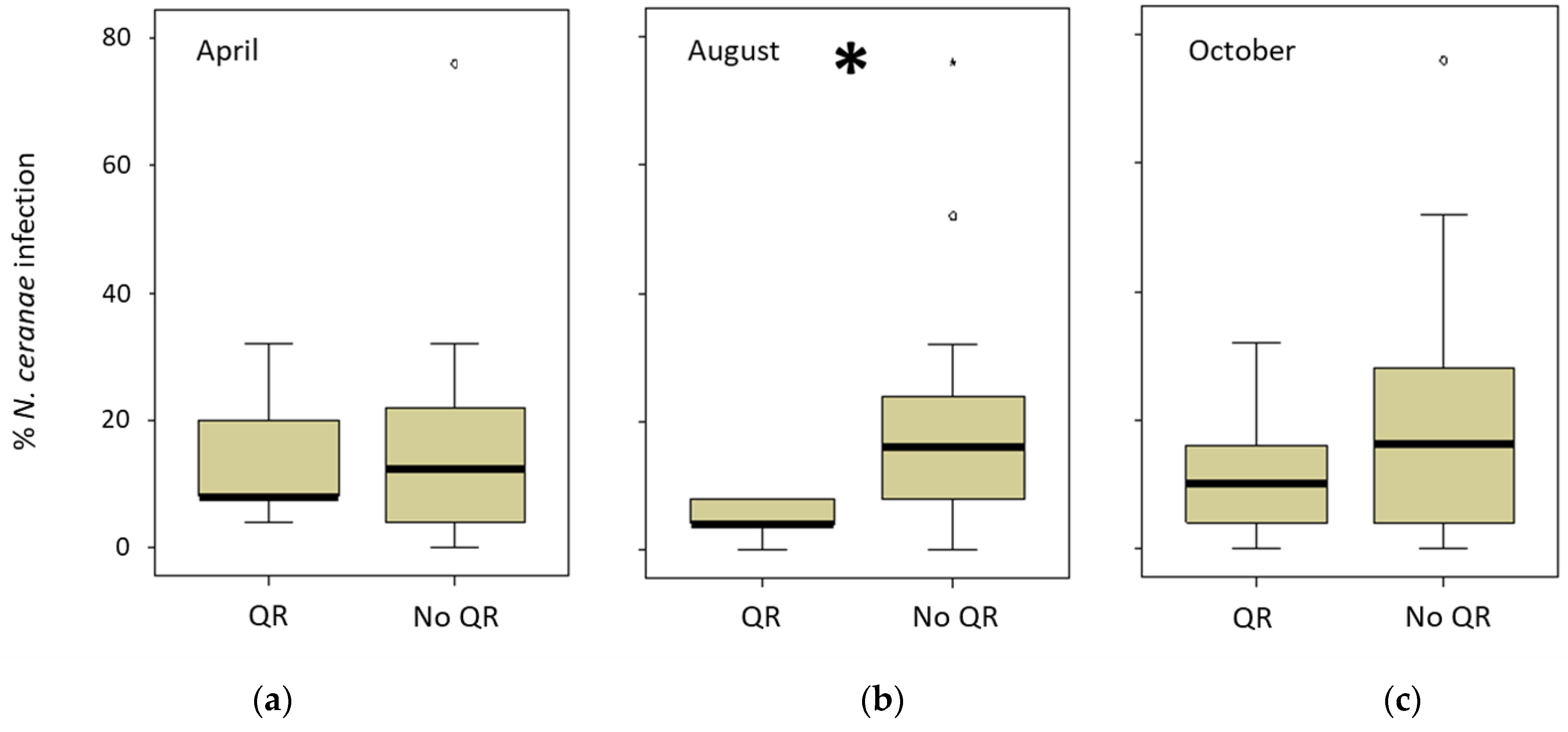
The ARO apiary had a special colony management in autumn in which comb number was equalized and the queens were artificially replaced. INRAE only registered one natural queen replacement (June 2018), whereas CIMO registered seven (three colonies out of 15 in 2018 and four out of 12 in 2019) during the study period. However, in the CIAPA apiary, all the colonies (n = 22) replaced their queen (naturally) at least once between March and September 2018, and two colonies even did it three times. The queen replacement was lower in the following year (five colonies out of 16). Therefore, the relationship between the queen replacement and the percentage of infected honeybees per colony was analyzed only for the CIAPA and CIMO apiaries (Supplementary Table S4). To do this, the percentage of honeybees infected in the sampling previous to the queen replacement (detected in April, August, or October 2018) was compared to the percentage of honey bees infected in the following spring (April 2019). The comparisons were made between two groups: one including the colonies that changed the queen and another group of colonies that did not change the queen. The only significant (Mann–Whitney test; p < 0.05) difference was found in the levels of intra-colony infection that were lower in the colonies that had replaced the queen in the previous summer (detected in August 2018) as opposed to those that had not changed it (Figure 5).
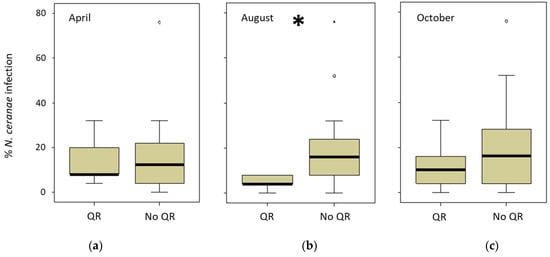
Figure 5.
Percentage of intra-colony infection in CIAPA and CIMO colonies in April 2019. The comparison was made between colonies when the queen replacement (QR) was detected in (a) April 2019 (n = 5), (b) August (n = 5), or (c) October (n = 14) versus the colonies that did not replace the queen (No QR) for the same periods (n = 16; n = 23; n = 14, respectively). * Denotes significant differences (p < 0.05).
3.8. Colony Mortality and N. ceranae Infection Levels
The mortality of colonies was recorded across the 20-month period (Table 7). The highest percentage of losses was observed in INRAE (47.6%) and CIAPA (40.9%), and the lowest on the islands, with one colony lost in OUE and none in SMI. The level of N. ceranae infection of a colony could not be correlated with its mortality. Only one colony (in CIAPA) had 96% of honey bees infected (Supplementary Table S1), which died a month later after infection assessment. When N. ceranae intra-colony infection in the two months prior to colony death was analyzed, a large proportion of the dead colonies were found to have a level of infection greater than or equal to 20% (Supplementary Table S4). Studying all the data together, 46% of the deceased colonies exceeded this value. However, this condition was not fulfilled in OUE or INRAE, so the data were analyzed by grouping the dead colonies from CIAPA, ARO, and CIMO. Thus, 76.5% of the colonies had ≥20% of honey bees infected in the previous two months (CIAPA: 77%, ARO and CIMO: 75%, respectively). Still, some colonies in CIMO or ARO reached over 75% of infection and did not die, having a remarkable decrease of infected honey bees at the following sampling round (below 40%; Supplementary Table S4).

Table 7.
Total number of colonies studied per apiary and colony mortality.
4. Discussion
The prevalence of N. ceranae infection was determined at the apiary and intra-colony levels in six apiaries, covering a wide range of Mediterranean environmental and beekeeping conditions in a long-term study conducted on a large batch of data (103 colonies across 20 months; 13,907 individual worker honey bees). This allowed us to determine the differences in N. ceranae infection among the different environments, confirming that the epidemiology of the infection by the microsporidium varies geographically and temporally. Nosema ceranae infection was confirmed in all the apiaries studied except in SMI, indicating that the microsporidium is widely spread.
The number of colonies infected by N. ceranae in the INRAE (France) apiary was significantly lower than that of the other apiaries. In OUE (France), ARO (Israel), CIMO (Portugal), and CIAPA (Spain), a high number of positive colonies were detected in the four apiaries throughout the sampling rounds, although the intra-colony infection levels varied greatly among apiaries, with ARO exhibiting the highest percentage of infected honey bees. This finding could be partly explained by the climatic conditions at each site, which were different. However, in our study, there was no correlation between the climatic parameters alone and the infection levels, and just the number of days with high wind speed increased the percentage of bees infected per colony. Only a visual and non-significant correlation of the combined effect of high mean temperature and high mean relative humidity with higher levels of infection could be intuited. As opposed to our results, temperature and humidity were correlated positively with N. ceranae incidence (spore density) but negatively to N. apis incidence in Turkey [60] and the levels of N. ceranae were negatively correlated with high temperatures in Serbia, [61]. Another study in China also showed higher N. ceranae prevalence in the more humid regions (South) when compared with apiaries in dryer areas (North) [45]. Moreover, in a previous study carried out in CIAPA [14], monthly rainfall was positively correlated with the percentage of interior honey bees infected by N. ceranae and the percentage of foragers infected was negatively correlated with the mean maximum temperature. Therefore, our results suggest that other factors than climatic conditions could play a role in the prevalence of the N. ceranae infection.
A higher level of N. ceranae infection found in CIAPA, CIMO, and ARO (not statistically significant in the latter) was correlated to a lower adult honey bee population, as opposed to the positive correlation found in INRAE and OUE apiaries. The different relationships between those two groups in colony strength and the percentage of honey bees infected by N. ceranae could be explained by the low level of infection in INRAE (for all samplings) and OUE (2019). On the other hand, the relationship observed in Iberia (CIAPA and CIMO) and Israel (ARO) between the N. ceranae infection level and colony strength seem to confirm previous findings in Spain [14,62,63], where infection has been shown to be detrimental to honey bee colonies.
Beekeeping management in ARO was different from that of the other apiaries. Given that this apiary had the highest levels of infection but had only 20.1% colony mortality, it could indicate that the management was able to control the mortality associated with the high percentage of infected honey bees per colony [64]. Beekeeping management, and, in particular, queen replacement with a younger queen, has been identified as a biotechnical method to control nosemosis [65,66]. This recommendation is consistent with our results as the levels of intra-colony infection in spring were lower in colonies that had replaced the queen in the previous summer. Other authors found that one-year-old queens are able to compensate for the effects of Nosema infection, with this ability gradually decreasing in subsequent years [66]. As well, the infection by N. ceranae has been reported to reduce honey production [67], although in this work we could not establish any correlation between the intra-colony infection level and the honey produced.
The highest colony mortality was recorded in INRAE, which had the apiary with the lowest N. ceranae infection levels. It is very likely that the microsporidium infection was not related to the mortality in this apiary and that other biotic and abiotic stressors contributed to colony mortality and possibly to the lower honey production. One of these biotic stressors could be Varroa. However, this trial was not designed to monitor Varroa levels but only to detect high infestations so that control measures could be taken. Moreover, infestation levels were below 2% in most of the cases, so their influence on the colony mortality seems to be limited. Despite this, a significant correlation between the percentage of honey bees infected by N. ceranae and V. destructor levels was found in two apiaries (INRAE and ARO) out of four analyzed. The correlation between the two pathogens is unclear as, such as in this study, there are studies that both confirm [68] and fail to find a correlation [15,69]. In addition, the Varroa treatments made in each country could have any influence, as oxalic acid has shown an effect on N. ceranae infection [70] and, conversely, infection by Microsporidium could reduce the efficacy of the results [71]. Hence, it is possible that there are factors yet to be determined that may influence the interaction between them. It is well known that V. destructor is an effective vector for Deformed wing virus (DWV), which was frequently detected in INRAE (unpublished data) and probably impacted colony losses in this apiary. On the other hand, the apiaries located on the V. destructor-free islands registered the lowest mortality levels. Thus, the presence of the mite seems to complicate the pathological consequences caused by other pathogens [68,72], as the intensity of these pathogens (N. ceranae and viruses) seems to increase when they appear together [73]. The overall mortality at the CIAPA (40.9%), CIMO (26.7%), and ARO (20.1%) apiaries could be considered high, when compared to the percentage of winter mortality rate of 10.7% reported in 35 countries for the same time frame (2018–2019) [74], although overall mortality is expected to be higher than during overwintering. In this way, although no infection rate could be established as a marker for colony mortality, it is possible that the N. ceranae infection plays a role in the losses, as this microsporidium can cause the death of the infected honey bees [14], impacting on the viability of the colonies [14,15,18,29,75,76,77]. High levels of infection have been reported as a cause of colony losses [14,62]. In our study, the 76.5% of dead colonies from CIAPA, ARO, and CIMO had ≥20% of honey bees infected in the previous two months. These high levels recorded in our study in CIAPA, ARO, and CIMO imply a sustained stress on the colonies. It has been published that an infectious disease causing mortality in foraging bees is very dangerous for the survival of a honey bee colony [78]. In addition, Nosema ceranae infection decreases the energy status of honey bees, leading to changes in their foraging behavior, which have a strong adverse effect on energetic gain efficiency [79]. This will have an effect on food availability and it could lead to colony failure when the forager mortality rate reaches a critical threshold [80].
In addition to the presence of N. ceranae infection, other stressors such as pathogens and pollutants have been shown to impact colony losses [81,82,83,84]. In fact, coinfection of microsporidia with very common viral pathogens might contribute to the death of the colony, even for asymptomatic infections [9,85]. Moreover, stress factors affecting honey bee immunity may trigger latent viral infections to become overt infections. Thus, insecticides [83,86,87] or infestation with other pathogens or parasites like V. destructor may activate dormant virus infections in the colony and even promote selection of virulent strains, such as in the case of DWV [88,89,90,91]. Moreover, the host genetics may influence the development (or the consequences) of the infection since the levels of N. ceranae infection have been noticed to be significantly different between lineages and colonies for both Russian and Italian honey bees, and in the case of the Russian lineage, the patriline-based variance was also found to be significant [92]. Therefore, the differences in the dynamics and the levels of N. ceranae infection here observed could also be related to the different honey bee lineages studied. Nevertheless, it has been suggested that genetic diversity cannot compensate and reduce alone the levels of infection [61].
Our results obtained from apiaries set in several Mediterranean countries confirm the high variability of N. ceranae prevalence. Nosema ceranae has been shown to be a very adaptable parasite and the effects of beekeeping management (such as queen replacement) were found to influence the prevalence, probably more linked with the colony biology adaptations than with the parasite itself. Clinical signs related to N. ceranae’s high prevalence and climatic conditions, such as lower honey production rate, depopulation, and death, were detected in some Mediterranean countries during initial reports [14,15,29,62,93] when the epidemic wave was at its highest. The comparison among different countries after several years in similar conditions, together with the fact that some highly infected colonies did not die, seems to indicate the initial lethal effect of the new parasite for Apis mellifera is downgrading, as coevolution contributes to establishing host–parasite equilibrium. Further studies on this topic, including the role of honey bee genetics, could assist in understanding the difference between nosemosis disease and infection, in a rather similar way to varroosis and Varroa detection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13090844/s1, Table S1: Mean levels of infection per month in each apiary; Table S2: Statistical differences of infected honey bees for each sampling and apiary; Table S3: Climatic conditions recorded per in mainland apiaries; Table S4: Colony losses and queen replacement per apiary.
Author Contributions
Conceptualization, R.M.-H., A.B.-D., N.C., Y.L.C., M.A.P. and V.S.; methodology, all authors; formal analysis, C.J.-U., L.B., M.H. and R.M.-H.; investigation, all authors; data curation, all authors; writing—original draft preparation, C.J.-U. and R.M.-H.; writing—review and editing, all authors; visualization, C.J.-U. and R.M.-H.; project administration and supervision, R.M.-H.; funding acquisition, R.M.-H., A.B.-D., N.C., Y.L.C., M.A.P. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been developed under the BEEHEAL project. The project was funded by ARIMNet2 2016 Call by the following funding agencies: INIA (CSIC; Spain), MOARD (Israel), ANR (France), and FCT (Portugal). ARIMNet2 (ERA-NET) has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement number 618127. C.J.-U. contract was funded by the Ministerio de Asuntos Económicos y Transformación Digital (grant no. BES-2017-080176, RTA-2015-0003-CO3-01). D.H. was supported by the project MEDIBEES—Monitoring the Mediterranean honey bee subspecies and their resilience to climate change for the improvement of sustainable agro-ecosystems. MEDIBEES is part of the PRIMA program supported by the European Union. A.R.L. was supported by a Ph.D. scholarship (SFRH/BD/143627/2019) from the FCT (Fundação Ciência e Tecnologia). FCT provided financial support by national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2021). R.M.-H. contract is co-funded by European Social funds (EU) by the INCRECYT program.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The CIAPA authors wish to thank V. Albendea, J. Almagro, E. Alonso-Prados, M. Benito, C. Botías, T. Corrales, M. Gajero, J. García, C. Uceta, and D. Plaza of the Honey Bee Pathology Laboratory for their technical support. INRAE authors thank beekeepers, Didier Crauser at INRAE and the ACANB in Ouessant (Association Conservatoire de l’Abeille Noire Bretonne).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAO; IZSLT; Apimondia; CAAS. Good Beekeeping Practices for Sustainable Apiculture; FAO: Rome, Italy, 2021. [Google Scholar]
- Bishop, J.; Garratt, M.P.D.; Nakagawa, S. Animal Pollination Increases Stability of Crop Yield across Spatial Scales. Ecol. Lett. 2022, 25, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- vanEngelsdorp, D.; Meixner, M.D. A Historical Review of Managed Honey Bee Populations in Europe and the United States and the Factors That May Affect Them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Meixner, M.D.; Costa, C.; Kryger, P.; Hatjina, F.; Bouga, M.; Ivanova, E.; Büchler, R. Conserving Diversity and Vitality for Honey Bee Breeding. J. Apic. Res. 2010, 49, 85–92. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Carpentier, P.; Madec, F.; Bougeard, S.; Cougoule, N.; Drajnudel, P.; Clément, M.C.; Aubert, M.; Faucon, J.P. The Role of Infectious Agents and Parasites in the Health of Honey Bee Colonies in France. J. Apic. Res. 2010, 49, 31–39. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honey Bee (Apis Mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Mcmahon, D.P.; Fürst, M.A.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R.J. A Sting in the Spit: Widespread Cross-Infection of Multiple RNA Viruses across Wild and Managed Bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Lopez, D.; Grubbs, K.; Posada-Florez, F.; Weaver, D.; Girten, W.; vanEngelsdorp, D.; Chen, Y.; Evans, J.D. Dynamic Evolution in the Key Honey Bee Pathogen Deformed Wing Virus: Novel Insights into Virulence and Competition Using Reverse Genetics. PLoS Biol. 2019, 17, e3000502. [Google Scholar] [CrossRef]
- Borba, R.S.; Hoover, S.E.; Currie, R.W.; Giovenazzo, P.; Guarna, M.M.; Foster, L.J.; Zayed, A.; Pernal, S.F. Phenomic Analysis of the Honey Bee Pathogen-Web and Its Dynamics on Colony Productivity, Health and Social Immunity Behaviors. PLoS ONE 2022, 17, e0263273. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema Ceranae in Apis Mellifera: A 12 Years Postdetection Perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef]
- Grupe, A.C.; Alisha Quandt, C. A Growing Pandemic: A Review of Nosema Parasites in Globally Distributed Domesticated and Native Bees. PLoS Pathog. 2020, 16, e1008580. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Peyre, Y.; Ahuir-Baraja, A.E.; Garijo, M.M.; Llobat, L. The Role of Nosema Ceranae (Microsporidia: Nosematidae) in Honey Bee Colony Losses and Current Insights on Treatment. Vet. Sci. 2022, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental Infection of Apis Mellifera Honeybees with Nosema Ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How Natural Infection by Nosema Ceranae Causes Honeybee Colony Collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Soroker, V.; Hetzroni, A.; Yakobson, B.; David, D.; David, A.; Voet, H.; Slabezki, Y.; Efrat, H.; Levski, S.; Kamer, Y.; et al. Evaluation of Colony Losses in Israel in Relation to the Incidence of Pathogens and Pests. Apidologie 2011, 42, 192–199. [Google Scholar] [CrossRef]
- Bacandritsos, N.; Granato, A.; Budge, G.; Papanastasiou, I.; Roinioti, E.; Caldon, M.; Falcaro, C.; Gallina, A.; Mutinelli, F. Sudden Deaths and Colony Population Decline in Greek Honey Bee Colonies. J. Invertebr. Pathol. 2010, 105, 335–340. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Bryden, J.; Paxton, R.J. Interspecific Competition in Honeybee Intracellular Gut Parasites Is Asymmetric and Favours the Spread of an Emerging Infectious Disease. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141896. [Google Scholar] [CrossRef]
- Moeini, S.; Malekifard, F.; Tavassoli, M. Identification of the Nosema Spp., a Microsporidian Parasite Isolated from the Honey Bees (Apis Mellifera) and Its Association with Honey Bee Colony Losses in Apiaries of Iran. J. Hell. Vet. Med. Soc. 2022, 73, 3667–3672. [Google Scholar] [CrossRef]
- Fenoy, S.; Rueda, C.; Higes, M.; Martín-Hernández, R.; Del Aguila, C. High-Level Resistance of Nosema Ceranae, a Parasite of the Honeybee, to Temperature and Desiccation. Appl. Environ. Microbiol. 2009, 75, 6886–6889. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema Microspores and a Neonicotinoid Weaken Honeybees (Apis Mellifera). Environ. Microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.H.; Martín-Hernández, R.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut Pathology and Responses to the Microsporidium Nosema Ceranae in the Honey Bee Apis Mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the Energetic Stress Associated with Experimental Nosema Ceranae and Nosema Apis Infection of Honeybees (Apis Mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; Alaoui, H.; et al. Exposure to Sublethal Doses of Fipronil and Thiacloprid Highly Increases Mortality of Honeybees Previously Infected by Nosema Ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune Suppression in the Honey Bee (Apis Mellifera) Following Infection by Nosema Ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential Expression of Immune Genes of Adult Honey Bee (Apis Mellifera) after Inoculated by Nosema Ceranae. J. Insect Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.S.; Evans, J.D. Single and Mixed-Species Trypanosome and Microsporidia Infections Elicit Distinct, Ephemeral Cellular and Humoral Immune Responses in Honey Bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Guidugli-Lazzarini, K.R.; de Freitas, N.H.A.; Message, D.; Bitondi, M.M.G.; Simões, Z.L.P.; Teixeira, É.W. Immunity and Physiological Changes in Adult Honey Bees (Apis Mellifera) Infected with Nosema Ceranae: The Natural Colony Environment. J. Insect Physiol. 2021, 131, 104237. [Google Scholar] [CrossRef]
- Haddad, N.J. First Detection of Nosema Ceranae in Jordan. Eur. Sci. J. 2014, 10, 1857–7881. [Google Scholar]
- Adjlane, N.; Haddad, N. Effect of Some Honeybee Diseases on Seasonal Mortality of Apis Mellifera Intermissa in Algeria Apiaries. Proc. Zool. Soc. 2016, 71, 83–87. [Google Scholar] [CrossRef]
- Ansari, M.J.; Al-Ghamdi, A.; Nuru, A.; Khan, K.A.; Alattal, Y. Geographical Distribution and Molecular Detection of Nosema Ceranae from Indigenous Honey Bees of Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 983–991. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Zinatullina, Z.Y.; Ignatieva, A.N.; Zhigileva, O.N.; Malysh, J.M.; Sokolova, Y.Y. Detection of Two Microsporidia Pathogens of the European Honey Bee Apis Mellifera (Insecta: Apidae) in Western Siberia. Acta Parasitol. 2018, 63, 728–732. [Google Scholar] [CrossRef]
- Ostroverkhova, N.V. Prevalence Of Nosema Ceranae (Microsporidia) In The Apis Mellifera Mellifera Bee Colonies From Long Time Isolated Apiaries Of Siberia. Far East. Entomol. 2020, 407, 8–20. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Bailón, E.G.; Martínez-Salvador, A.; Prieto, L.; Meana, A.; Higes, M. Microsporidia Infecting Apis Mellifera: Coexistence or Competition. Is Nosema Ceranae Replacing Nosema Apis? Environ. Microbiol. 2012, 14, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Cepero, A.; Martín-Hernández, R.; Bartolomé, C.; Gómez-Moracho, T.; Barrios, L.; Bernal, J.; Teresa Martín, M.; Meana, A.; Higes, M. Passive Laboratory Surveillance in Spain: Pathogens as Risk Factors for Honey Bee Colony Collapse. J. Apic. Res. 2015, 54, 525–531. [Google Scholar] [CrossRef]
- Stevanovic, J.; Simeunovic, P.; Gajic, B.; Lakic, N.; Radovic, D.; Fries, I.; Stanimirovic, Z. Characteristics of Nosema Ceranae Infection in Serbian Honey Bee Colonies. Apidologie 2013, 44, 522–536. [Google Scholar] [CrossRef]
- Stevanovic, J.; Schwarz, R.S.; Vejnovic, B.; Evans, J.D.; Irwin, R.E.; Glavinic, U.; Stanimirovic, Z. Species-Specific Diagnostics of Apis Mellifera Trypanosomatids: A Nine-Year Survey (2007–2015) for Trypanosomatids and Microsporidians in Serbian Honey Bees. J. Invertebr. Pathol. 2016, 139, 6–11. [Google Scholar] [CrossRef]
- Matthijs, S.; Waele, D.; Vandenberge, V.; Evers, J.; Brunain, M.; Saegerman, C.; De Winter, P.J.J.; Roels, S.; Graaf, D.C.; De Regge, N. Nationwide Screening for Bee Viruses and Parasites in Belgian Honey Bees. Viruses 2020, 12, 890. [Google Scholar] [CrossRef]
- Lage, V.M.G.B.; Santana, C.D.; Patrocínio, E.; Noronha, R.P.; de Melo, R.L.; de Jesus Barbosa, C.; da Cunha Lima, S.T. Prevalence of Nosema Ceranae in Apiculture Regions of Bahia State, Brazil. Ciência Rural 2022, 52, e20210473. [Google Scholar] [CrossRef]
- Bordin, F.; Zulian, L.; Granato, A.; Caldon, M.; Colamonico, R.; Toson, M.; Trevisan, L.; Biasion, L.; Mutinelli, F. Presence of Known and Emerging Honey Bee Pathogens in Apiaries of Veneto Region (Northeast of Italy) during Spring 2020 and 2021. Appl. Sci. 2022, 12, 2134. [Google Scholar] [CrossRef]
- Naudi, S.; Šteiselis, J.; Jürison, M.; Raimets, R.; Tummeleht, L.; Praakle, K.; Raie, A.; Karise, R. Variation in the Distribution of Nosema Species in Honeybees (Apis Mellifera Linnaeus) between the Neighboring Countries Estonia and Latvia. Vet. Sci. 2021, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Imani Baran, A.; Kalami, H.; Mazaheri, J.; Hamidian, G. Vairimorpha Ceranae Was the Only Detected Microsporidian Species from Iranian Honey Bee Colonies: A Molecular and Phylogenetic Study. Parasitol. Res. 2022, 121, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of Colonization of Apis Mellifera by Nosema Ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread Dispersal of the Microsporidian Nosema Ceranae, an Emergent Pathogen of the Western Honey Bee, Apis Mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mederle, N.; Lobo, M.L.; Morariu, S.; Morariu, F.; Darabus, G.; Mederle, O.; Matos, O. Microscopic and Molecular Detection of Nosema Ceranae in Honeybee Apis Mellifera L. from Romania. Rev. Chim. 2018, 69, 3761–3772. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, P.; Guzman-Novoa, E.; Wu, Y.; Hou, C.; Diao, Q. Nosema Ceranae, the Most Common Microsporidium Infecting Apis Mellifera in the Main Beekeeping Regions of China since at Least 2005. J. Apic. Res. 2019, 58, 562–566. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. Prevalence and Infection Intensity of Nosema in Honey Bee (Apis Mellifera L.) Colonies in Virginia. J. Invertebr. Pathol. 2011, 107, 43–49. [Google Scholar] [CrossRef]
- Gisder, S.; Schüler, V.; Horchler, L.L.; Groth, D.; Genersch, E. Long-Term Temporal Trends of Nosema Spp. Infection Prevalence in Northeast Germany: Continuous Spread of Nosema Ceranae, an Emerging Pathogen of Honey Bees (Apis Mellifera), but No General Replacement of Nosema Apis. Front. Cell. Infect. Microbiol. 2017, 7, 301. [Google Scholar] [CrossRef]
- Hall, R.J.; Pragert, H.; Phiri, B.J.; Fan, Q.H.; Li, X.; Parnell, A.; Stanislawek, W.L.; McDonald, C.M.; Ha, H.J.; McDonald, W.; et al. Apicultural Practice and Disease Prevalence in Apis Mellifera, New Zealand: A Longitudinal Study. J. Apic. Res. 2021, 60, 644–658. [Google Scholar] [CrossRef]
- Antúnez, K.; Anido, M.; Branchiccela, B.; Harriet, J.; Campa, J.; Invernizzi, C.; Santos, E.; Higes, M.; Martín-Hernández, R.; Zunino, P. Seasonal Variation of Honeybee Pathogens and Its Association with Pollen Diversity in Uruguay. Microb. Ecol. 2015, 70, 522–533. [Google Scholar] [CrossRef]
- Traver, B.E.; Williams, M.R.; Fell, R.D. Comparison of within Hive Sampling and Seasonal Activity of Nosema Ceranae in Honey Bee Colonies. J. Invertebr. Pathol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef]
- Gauthier, L.; Tentcheva, D.; Tournaire, M.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Viral load estimation in asymptomatic honey bee colonies using the quantitative RT-PCR technique. Apidologie 2007, 38, 426–435. [Google Scholar] [CrossRef]
- Direção Regional da Agricultura (DRA). Programa Sanitário Apícola—Região Autónoma dos Açores; Secretaria Regional da Agricultura e Ambiente: Angra do Heroísmo, Portugal, 2022.
- Direção Regional da Agricultura (DRA). Anexo I—PSA 2021—Situação Epidemiológica (2016–2021); Secretaria Regional da Agricultura e Ambiente: Angra Heroísmo, Portugal, 2021.
- Lopes, A.R.; Martín-Hernández, R.; Higes, M.; Segura, S.K.; Henriques, D.; Pinto, M.A. Colonisation Patterns of Nosema Ceranae in the Azores Archipelago. Vet. Sci. 2022, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Henriques, D.; Lopes, A.R.; Chejanovsky, N.; Dalmon, A.; Higes, M.; Jabal-Uriel, C.; Le Conte, Y.; Reyes-Carreño, M.; Soroker, V.; Martín-Hernández, R.; et al. Mitochondrial and Nuclear Diversity of Colonies of Varying Origins: Contrasting Patterns Inferred from the Intergenic TRNAleu-Cox2 Region and Immune SNPs. J. Apic. Res. 2022, 61, 305–308. [Google Scholar] [CrossRef]
- Urbieta-Magro, A.; Higes, M.; Meana, A.; Gómez-Moracho, T.; Rodríguez-García, C.; Barrios, L.; Martín-Hernández, R. The Levels of Natural Nosema Spp. Infection in Apis Mellifera Iberiensis Brood Stages. Int. J. Parasitol. 2019, 49, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard Methods for Estimating Strength Parameters of Apis Mellifera Colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard Methods for Varroa Research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Ozgor, E.; Guzerin, E.; Keskin, N. Determination and Comparison of Nosema Apis and Nosema Ceranae in Terms of Geographic and Climatic Factors. Hacet. J. Biol. Chem. 2015, 43, 9–15. [Google Scholar] [CrossRef]
- Vejnovic, B.; Stevanovic, J.; Schwarz, R.S.; Aleksic, N.; Mirilovic, M.; Jovanovic, N.M.; Stanimirovic, Z. Quantitative PCR Assessment of Lotmaria Passim in Apis Mellifera Colonies Co-Infected Naturally with Nosema Ceranae. J. Invertebr. Pathol. 2017, 151, 76–81. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema Spp. Infection and Its Negative Effects on Honey Bees (Apis Mellifera Iberiensis) at the Colony Level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and Behavioral Changes in Honey Bees (Apis Mellifera) Induced by Nosema Ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Muñoz, I.; Cepero, A.; Pinto, M.A.; Martín-Hernández, R.; Higes, M.; De la Rúa, P. Presence of Nosema Ceranae Associated with Honeybee Queen Introductions. Infect. Genet. Evol. 2014, 23, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Días, J.; García-Palencia, P.; Matabuena, M.; Juarranz, Á.; Barrios, L.; Meana, A.; Nanetti, A.; Higes, M. The Effect of Induced Queen Replacement on Nosema Spp. Infection in Honey Bee (Apis Mellifera Iberiensis) Colonies. Environ. Microbiol. 2012, 14, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, P.; Stevanovic, J.; Cirkovic, D.; Radojicic, S.; Lakic, N.; Stanisic, L.; Stanimirovic, Z. Nosema Ceranae and Queen Age Influence the Reproduction and Productivity of the Honey Bee Colony. J. Apic. Res. 2014, 53, 545–554. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Meana, A.; Higes, M. Screening Alternative Therapies to Control Nosemosis Type C in Honey Bee (Apis Mellifera Iberiensis) Colonies. Res. Vet. Sci. 2013, 95, 1041–1045. [Google Scholar] [CrossRef]
- Mariani, F.; Maggi, M.; Porrini, M.; Fuselli, S.; Caraballo, G.; Brasesco, C.; Barrios, C.; Principal, J.; Martin, E. Parasitic Interactions between Nosema Spp. and Varroa Destructor in Apis Mellifera Colonies. Zootec. Trop. 2012, 30, 81–90. [Google Scholar]
- Flores, J.M.; Gámiz, V.; Jiménez-Marín, Á.; Flores-Cortés, A.; Gil-Lebrero, S.; Garrido, J.J.; Hernando, M.D. Impact of Varroa Destructor and Associated Pathologies on the Colony Collapse Disorder Affecting Honey Bees. Res. Vet. Sci. 2021, 135, 85–95. [Google Scholar] [CrossRef]
- Nanetti, A.; Rodriguez-García, C.; Meana, A.; Martín-Hernández, R.; Higes, M. Effect of Oxalic Acid on Nosema Ceranae Infection. Res. Vet. Sci. 2015, 102, 167–172. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Garrido-Bailón, E.; Nanetti, A.; Meana, A.; Higes, M. Nosema Spp. Parasitization Decreases the Effectiveness of Acaricide Strips (Apivar ®) in Treating Varroosis of Honey Bee (Apis Mellifera Iberiensis) Colonies. Environ. Microbiol. Rep. 2012, 4, 57–65. [Google Scholar] [CrossRef]
- Doublet, V.; Natsopoulou, M.E.; Zschiesche, L.; Paxton, R.J. Within-Host Competition among the Honey Bees Pathogens Nosema Ceranae and Deformed Wing Virus Is Asymmetric and to the Disadvantage of the Virus. J. Invertebr. Pathol. 2015, 124, 31–34. [Google Scholar] [CrossRef]
- Little, C.M.; Shutler, D.; Williams, G.R. Associations among Nosema Spp. Fungi, Varroa Destructor Mites and Chemical Treatments in Honey Bees, Apis Mellifera. J. Apic. Res. 2016, 54, 378–385. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Charrière, J.D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; Amaro da Costa, C.; et al. Honey Bee Colony Winter Loss Rates for 35 Countries Participating in the COLOSS Survey for Winter 2018–2019, and the Effects of a New Queen on the Risk of Colony Winter Loss. J. Apic. Res. 2020, 59, 744–751. [Google Scholar] [CrossRef]
- Villa, J.D.; Bourgeois, A.L.; Danka, R.G. Negative Evidence for Effects of Genetic Origin of Bees on Nosema Ceranae, Positive Evidence for Effects of Nosema Ceranae on Bees. Apidologie 2013, 44, 511–518. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Mor, S.K.; Phelps, N.B.D.; Goyal, S.M.; Armién, A.G. A Case Report of Nosema Ceranae Infection in Honey Bees in Minnesota, USA. Vet. Q. 2015, 35, 48–50. [Google Scholar] [CrossRef]
- Hatjina, F.; Tsoktouridis, G.; Bouga, M.; Charistos, L.; Evangelou, V.; Avtzis, D.; Meeus, I.; Brunain, M.; Smagghe, G.; De Graaf, D.C. Polar Tube Protein Gene Diversity among Nosema Ceranae Strains Derived from a Greek Honey Bee Health Study. J. Invertebr. Pathol. 2011, 108, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.I.; Wahl, L.M.; Zamir, M. Effects of Infection on Honey Bee Population Dynamics: A Model. PLoS ONE 2014, 9, e110237. [Google Scholar] [CrossRef]
- Naug, D. Infected Honeybee Foragers Incur a Higher Loss in Efficiency than in the Rate of Energetic Gain. Biol. Lett. 2014, 10, 20140731. [Google Scholar] [CrossRef]
- Khoury, D.S.; Barron, A.B.; Myerscough, M.R. Modelling Food and Population Dynamics in Honey Bee Colonies. PLoS ONE 2013, 8, e59084. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Alonso-Prados, E.; González-Porto, A.V.; Bernal, J.L.; Bernal, J.; Martín-Hernández, R.; Higes, M. A Case Report of Chronic Stress in Honey Bee Colonies Induced by Pathogens and Acaricide Residues. Pathogens 2021, 10, 955. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-Insecticide Interactions: A Case Study of Nosema Ceranae and Fipronil Synergy on Honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Tchamitchian, S.; Bonnet, M.; Cousin, M.; Kretzschmar, A.; Brunet, J.L.; Le Conte, Y. Combined Neonicotinoid Pesticide and Parasite Stress Alter Honeybee Queens’ Physiology and Survival. Sci. Rep. 2016, 6, 31430. [Google Scholar] [CrossRef] [PubMed]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis Mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- Coulon, M.; Dalmon, A.; Di Prisco, G.; Prado, A.; Arban, F.; Dubois, E.; Ribière-Chabert, M.; Alaux, C.; Thiéry, R.; Le Conte, Y. Interactions Between Thiamethoxam and Deformed Wing Virus Can Drastically Impair Flight Behavior of Honey Bees. Front. Microbiol. 2020, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Martin, S.J.; Hardy, J.; Villalobos, E.; Martín-Hernández, R.; Nikaido, S.; Higes, M. Do the Honeybee Pathogens Nosema Ceranae and Deformed Wing Virus Act Synergistically? Environ. Microbiol. Rep. 2013, 5, 506–510. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis Mellifera) Prevails after Varroa Destructor-Mediated, or In Vitro, Transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef]
- Moore, J.; Jironkin, A.; Chandler, D.; Burroughs, N.; Evans, D.J.; Ryabov, E.V. Recombinants between Deformed Wing Virus and Varroa Destructor Virus-1 May Prevail in Varroa Destructor-Infested Honeybee Colonies. J. Gen. Virol. 2011, 92, 156–161. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Rinderer, T.E.; Sylvester, H.A.; Holloway, B.; Oldroyd, B.P. Patterns of Apis Mellifera Infestation by Nosema Ceranae Support the Parasite Hypothesis for the Evolution of Extreme Polyandry in Eusocial Insects. Apidologie 2012, 43, 539–548. [Google Scholar] [CrossRef]
- Lodesani, M.; Costa, C.; Besana, A.; Dall’Olio, R.; Franceschetti, S.; Tesoriero, D.; Vaccari, G. Impact of Control Strategies for Varroa Destructor on Colony Survival and Health in Northern and Central Regions of Italy. J. Apic. Res. 2014, 53, 155–164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).