Photosensitivity of Dispersing Cryptic Date Stone Beetles Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae)—A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Origin of Samples
2.2. Set-Up
2.2.1. Experiment #1
2.2.2. Experiment #2
2.2.3. Experiment #3
2.3. Recording
2.4. Illumination Measurements
2.5. Statistics
3. Results
3.1. Experiment #1
3.2. Experiment #2
3.3. Experiment #3
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Schedl, K.E. Scolytidae und Platypodidae Afrikas. Rev. Entomol. Mocamb. 1961, 4, 355–742. [Google Scholar]
- Fabricius, J.C. Systema Eleutheratorum Secundum Ordines, Genera, Species: Adiectis Synonymis, Locis, Observationibus, Descriptionibus; Impensis Bibliopolii Academici Novi Kiliae: Kiel, Germany, 1801; Volume 2, p. 314. [Google Scholar]
- Letzner, C.W. Ueber den Bistrochus dactyliperda und seine frühern Stände. Uebersicht Arb. Veränd. Schles. Ges. Für Vaterl. Cult. Jahre 1839 1840, 17, 116–120. [Google Scholar]
- Eichhoff, W. Ratio, descriptio, emendatio eorum Tomicinorum qui sunt in Dr. Chapuisi et autoris ipsius collection et autoris ipsius collectionibus et quos praeterea recognovit. Mem. Soc. R. Sci. Liege 1879, 8, 1–554. [Google Scholar]
- Spennemann, D.H.R.; Kent, K.; Cook, R. Uninvited Guests: Mass Emergence of Scolytinid Beetles in a Seed Germination Experiment and Its Management; Institute for Land, Water and Society, Charles Sturt University: Albury, NSW, Australia, 2018; p. 33. [Google Scholar]
- Spennemann, D.H.R. Resilience of breeding Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae) to ingestion by vertebrates. Turk. J. Entomol. 2020, 44, 205–217. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. Resilience of the date stone beetle Coccotrypes dactyliperda (Coleoptera, Curculionidae), following periods of exposure to subzero temperature. Turk. J. Entomol. 2019, 43, 379–385. [Google Scholar]
- Spennemann, D.H.R. Global distribution of the date stone beetle, Coccotrypes dactyliperda (Coleoptera: Curculionidae, Scolytinae). J. Insect Biodivers. Syst. 2018, 4, 203–226. [Google Scholar] [CrossRef]
- Blumberg, D. Review: Date Palm Arthropod Pests and Their Management in Israel. Phytoparasitica 2008, 36, 411–448. [Google Scholar] [CrossRef]
- Hussein, A.E. Date varieties and palm height in relation to infestation with date stone beetle, Coccotrypes dactyliperda F. (Coleoptera: Scolytidae). Ann. Agric. Sci. Moshtohor 1990, 28, 2613–2622. [Google Scholar]
- Kehat, M.; Blumberg, D.; Greenberg, S. Fruit drop and damage in dates: The role of Coccotrypes dactyliperda F. & nitidulid beetles, and prevention by mechanical measures. Phytoparasitica 1976, 4, 93–99. [Google Scholar]
- Spennemann, D.H.R. Biology, ecology and distribution of the date stone beetle, Coccotrypes dactyliperda (Scolytinae, Coleoptera). Zool. Middle East 2019, 65, 163–182. [Google Scholar] [CrossRef]
- Blumberg, D.; Kehat, M. Biological studies of the date stone beetle, Coccotrypes dactyliperda. Phytoparasitica 1982, 10, 73–78. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. An Experimental Evaluation of Food Preferences and Associated Hatching Times of the Date Stone Beetle, Coccotrypes dactyliperda (Scolytinae, Coleoptera); Institute for Land, Water and Society, Charles Sturt University: Albury, NSW, Australia, 2018; p. 81. [Google Scholar]
- El-Sufty, R.; Helal, R.M. Studies on the date stone beetle, Coccotrypes dactyliperda F. (Col: Scolytidae) in North of Nile Delta. Menofiya J. Agric. Res. 1998, 23, 1683–1692. [Google Scholar]
- Ramírez, B.H.; Parrado-Rosselli, Á.; Stevenson, P. Seed dispersal of a useful palm (Astrocaryum chambira Burret) in three amazonian forests with different human intervention. Rev. Colomb. For. 2009, 12, 5–16. [Google Scholar]
- Spennemann, D.H.R. Dispersal of the date stone beetle Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae) in a managed rural landscape in Australia. J. Insect Biodivers. Syst. 2022, 8, 191–205. [Google Scholar]
- Herfs, A. Studien an dem Steinnußborkenkäfer Coccotrypes tanganus Eggers. Höfchen-Briefe Wiss. Prax. (Bayer Leverk.) 1948, 2, 22–49. [Google Scholar]
- Herfs, A. Studien an dem Steinnußborkenkäfer Coccotrypes tanganus Eggers. 2. Die Soziologie von Coccotrypes tanganus. Höfchen-Briefe Wiss. Prax. (Bayer Leverk.) 1950, 3, 3–31. [Google Scholar]
- Spennemann, D.H.R. Observations of seed penetration action by the date stone beetle Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae). J. Insect Biodivers. Syst. 2021, 7, 205–214. [Google Scholar] [CrossRef]
- Spennemann, D.H.R. Patterns of seed penetration by the date stone beetle Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae). Insects 2021, 13, 10. [Google Scholar] [CrossRef]
- Tremmel, M.; Steinitz, H.; Kliot, A.; Harari, A.; Lubin, Y. Dispersal, endosymbiont abundance and fitness-related consequences of inbreeding and outbreeding in a social beetle. Biol. J. Linn. Soc. 2020, 129, 717–727. [Google Scholar] [CrossRef]
- Bright, D.E. Eye Reduction in a Cavernicolous Population of Coccotrypes dactyliperda Fabricius (Coleoptera: Scolytidae). Coleopt. Bull. 1981, 35, 117–120. [Google Scholar]
- El-Barbary, N.S.; Donia, A.R.; Mostafa, A.M. Effect of food preference and extracts of the date palm fruits and stones on the activity of the adult females of the date stone beetle, Coccotrypes dactyliperda Fabricus (Coleoptera, Scolytidae). Alex. J. Agric. Res. 2002, 47, 103–107. [Google Scholar]
- Meisner, J.; Weissenberg, M.; Blumberg, D.; Ascher, K.R.S. Date palm fruit stone extracts as phagostimulants for the adult date stone beetle, Coccotrypes dactyliperda F. (Coleoptera: Scolytidae)/Dattelkern-Extrakte als Phagostimulantien für Imagines des Dattelkern-Borkenkäfers, Coccotrypes dactyliperda F. (Coleoptera: Scolytidae). Z. Pflanzenkrankh. Pflanzenschutz 1985, 92, 305–309. [Google Scholar]
- Mayfield, A.E.; Brownie, C. The redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) uses stem silhouette diameter as a visual host-finding cue. Environ. Entomol. 2013, 42, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Goyer, R.A. Effect of silhouette color on trap catches of Dendroctonus frontalis (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 2001, 94, 948–953. [Google Scholar] [CrossRef]
- Goyer, R.; Lenhard, G.; Strom, B.L. The influence of silhouette color and orientation on arrival and emergence of Ips pine engravers and their predators in loblolly pine. For. Ecol. Manag. 2004, 191, 147–155. [Google Scholar] [CrossRef]
- Groberman, L.J.; Borden, J.H. Electrophysiological response of Dendroctonus pseudotsugae and Ips paraconfusus (Coleoptera: Scolytidae) to selected wavelength regions of the visible spectrum. Can. J. Zool. 1982, 60, 2180–2189. [Google Scholar] [CrossRef]
- Briscoe, A.D.; Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 2001, 46, 471–510. [Google Scholar] [CrossRef]
- Mazza, C.A.; Izaguirre, M.M.; Zavala, J.; Scopel, A.L.; Ballaré, C.L. Insect perception of ambient ultraviolet-B radiation. Ecol. Lett. 2002, 5, 722–726. [Google Scholar] [CrossRef]
- Entwistle, P. Some evidence for a colour sensitive phase in the flight period of Scolytidae and Platypodidae. Entomol. Exp. Appl. 1963, 6, 143–148. [Google Scholar] [CrossRef]
- Hanula, J.L.; Ulyshen, M.D.; Horn, S. Effect of trap type, trap position, time of year, and beetle density on captures of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2011, 104, 501–508. [Google Scholar] [CrossRef]
- Strom, B.L.; Roton, L.M.; Goyer, R.A.; Meeker, J.R. Visual and semiochemical disruption of host finding in the Southern Pine Beetle. Ecol. Appl. 1999, 9, 1028–1038. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.H.; Wang, Y.; Liu, G.T.; Zhou, X.; Niu, J.; Schlyter, F. Catching Ips duplicatus (Sahlberg) (Coleoptera: Scolytidae) with pheromone-baited traps: Optimal trap type, colour, height and distance to infestation. Pest Manag. Sci. 2010, 66, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Dubbel, V.; Kerck, K.; Sohrt, M.; Mangold, S. Influence of trap color on the efficiency of bark beetle pheromone traps 1, 2. Z. Angew. Entomol. 1985, 99, 59–64. [Google Scholar] [CrossRef]
- Werle, C.T.; Bray, A.M.; Oliver, J.B.; Blythe, E.K.; Sampson, B.J. Ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) captures using colored traps in southeast Tennessee and south Mississippi. J. Entomol. Sci. 2014, 49, 373–382. [Google Scholar] [CrossRef]
- Campbell, S.A.; Borden, J.H. Bark reflectance spectra of conifers and angiosperms: Implications for host discrimination by coniferophagous bark and timber beetles. Can. Entomol. 2005, 137, 719–722. [Google Scholar] [CrossRef]
- Campbell, S.A.; Borden, J.H. Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol. Entomol. 2006, 31, 437–449. [Google Scholar] [CrossRef]
- Abbasi, Q.; Jan, N.; Mahar, A.; Khuhro, R.; Nizamani, S.; Panhwar, A. Monitoring of ambrosia bark beetle through installation of sticky color traps at different heights in mango trees. Int. J. Fruit Sci. 2008, 7, 65–79. [Google Scholar] [CrossRef]
- Hård, A.; Sivik, L. NCS—Natural Color System: A Swedish Standard for Color Notation. Color Res. Appl. 1981, 6, 129–138. [Google Scholar] [CrossRef]
- MedCalc Software. MEDCALC. Comparison of Proportions Calculator. Available online: https://www.medcalc.org/calc/comparison_of_proportions.php (accessed on 8 August 2022).
- Campbell, I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]
- Richardson, J.T.E. The analysis of 2 × 2 contingency tables—Yet again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef]
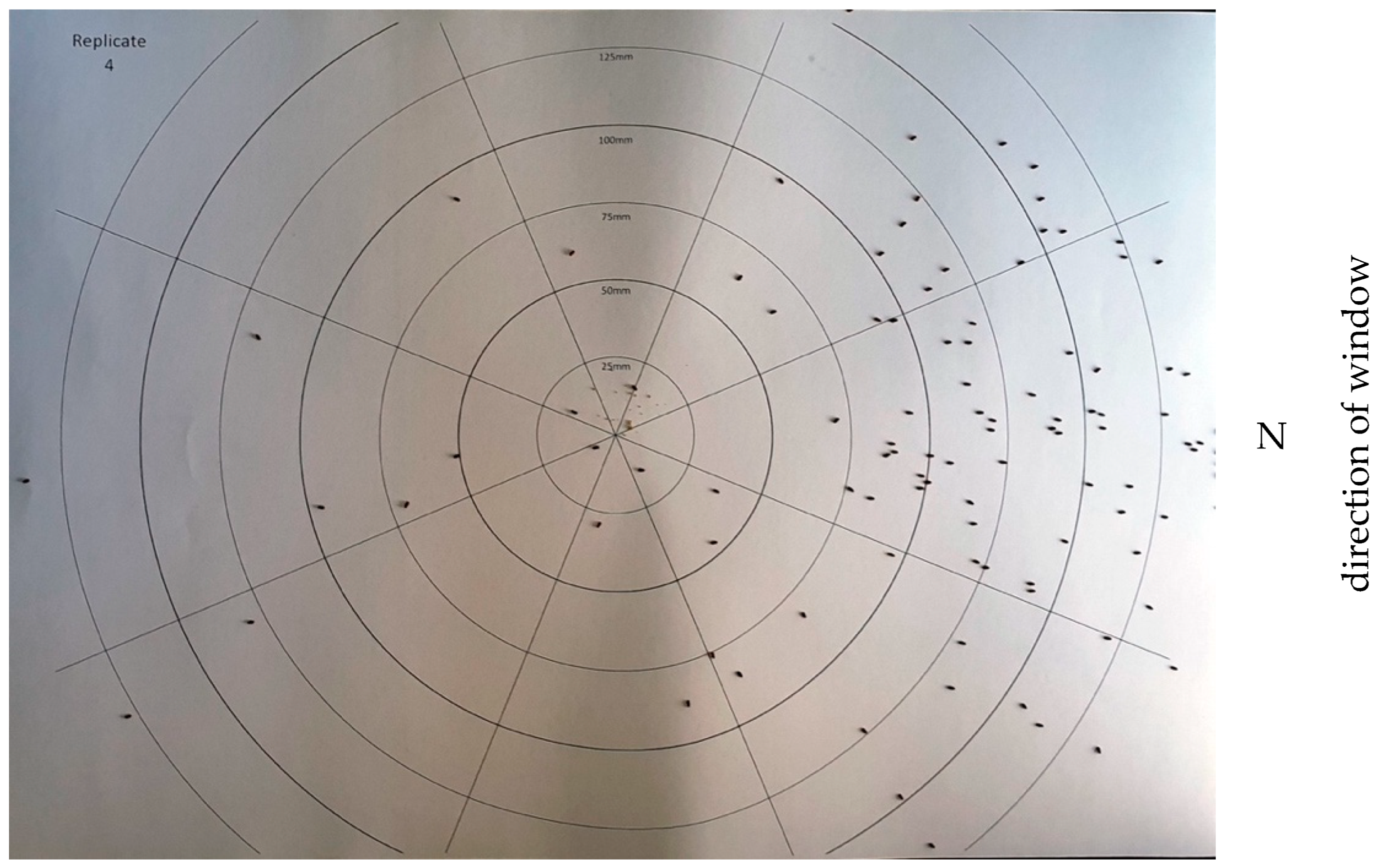
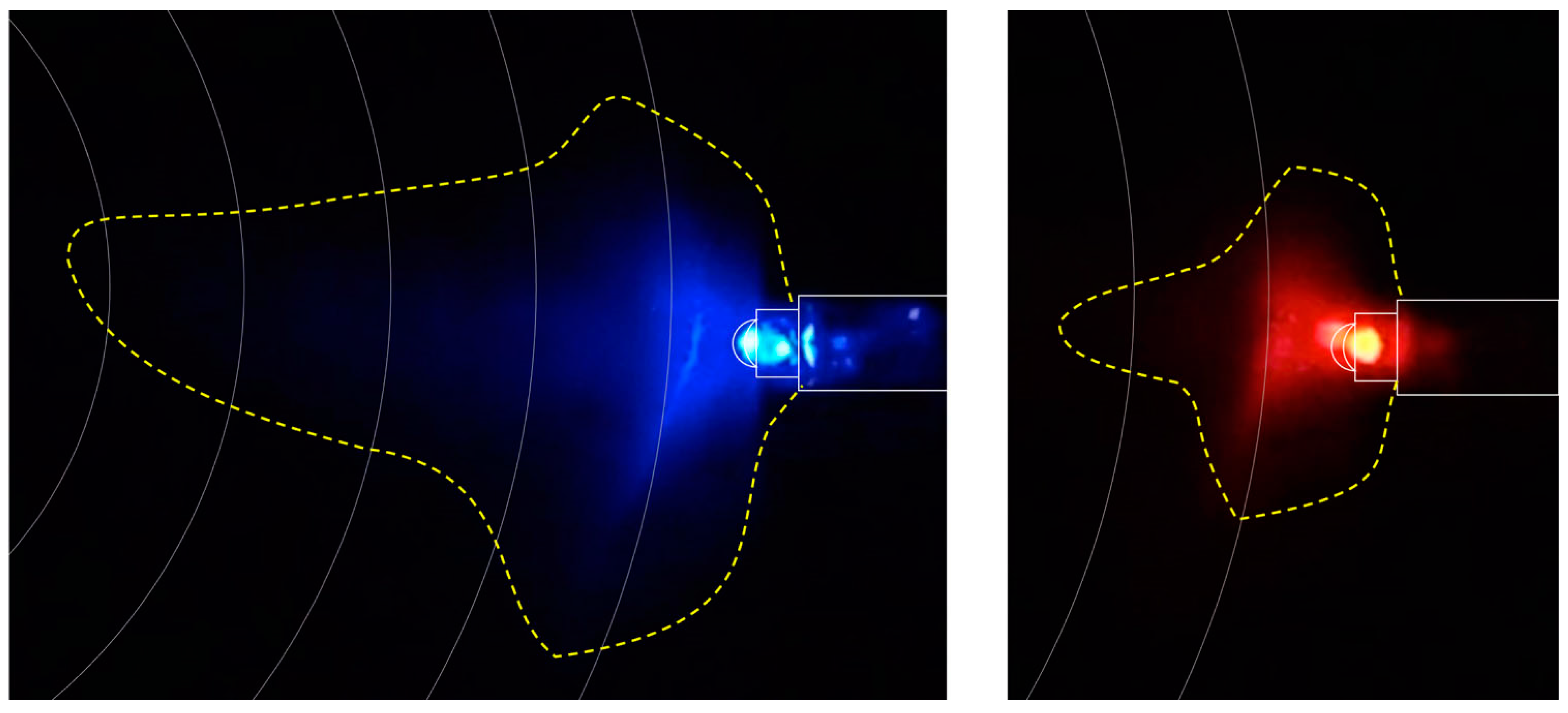
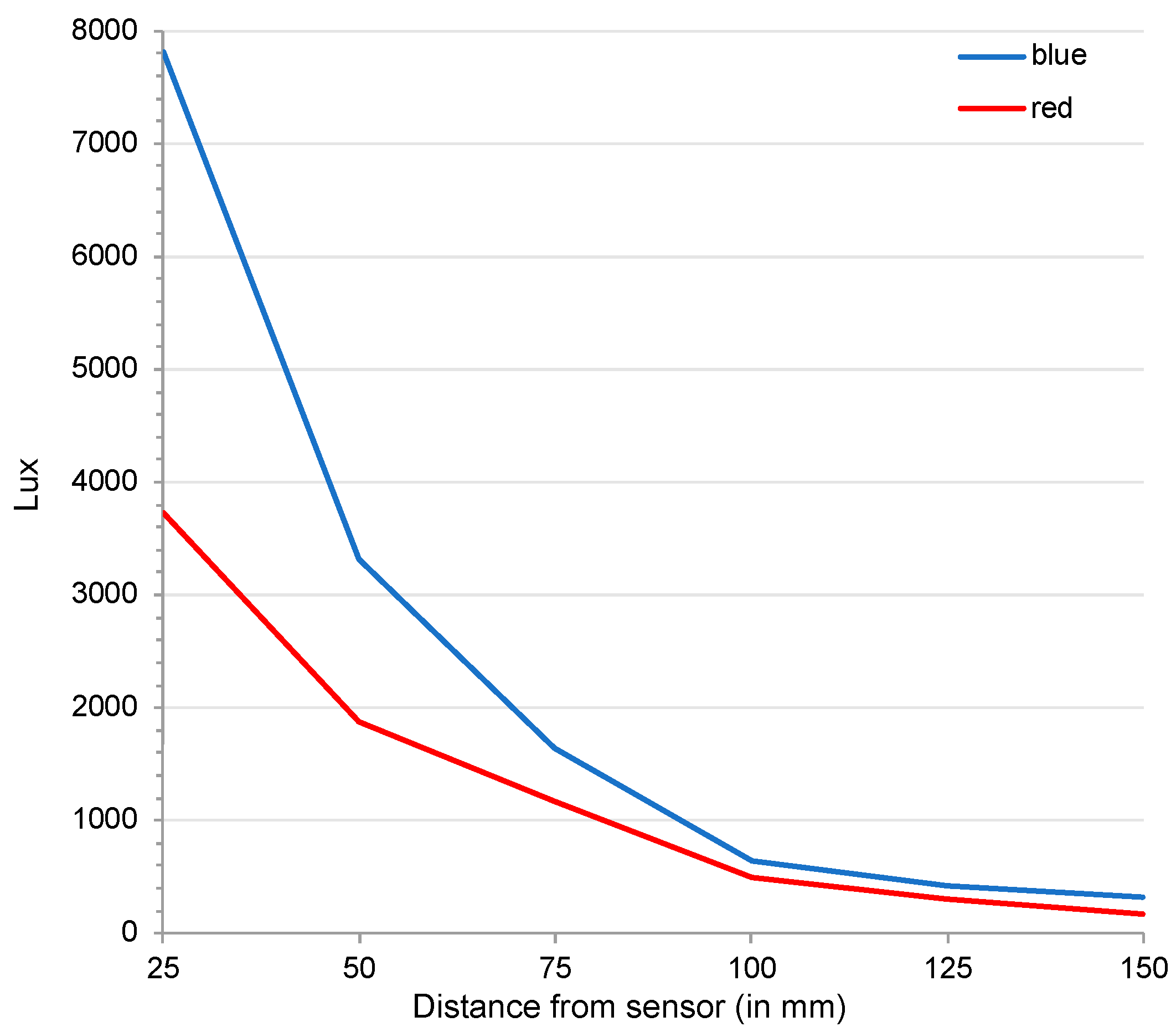
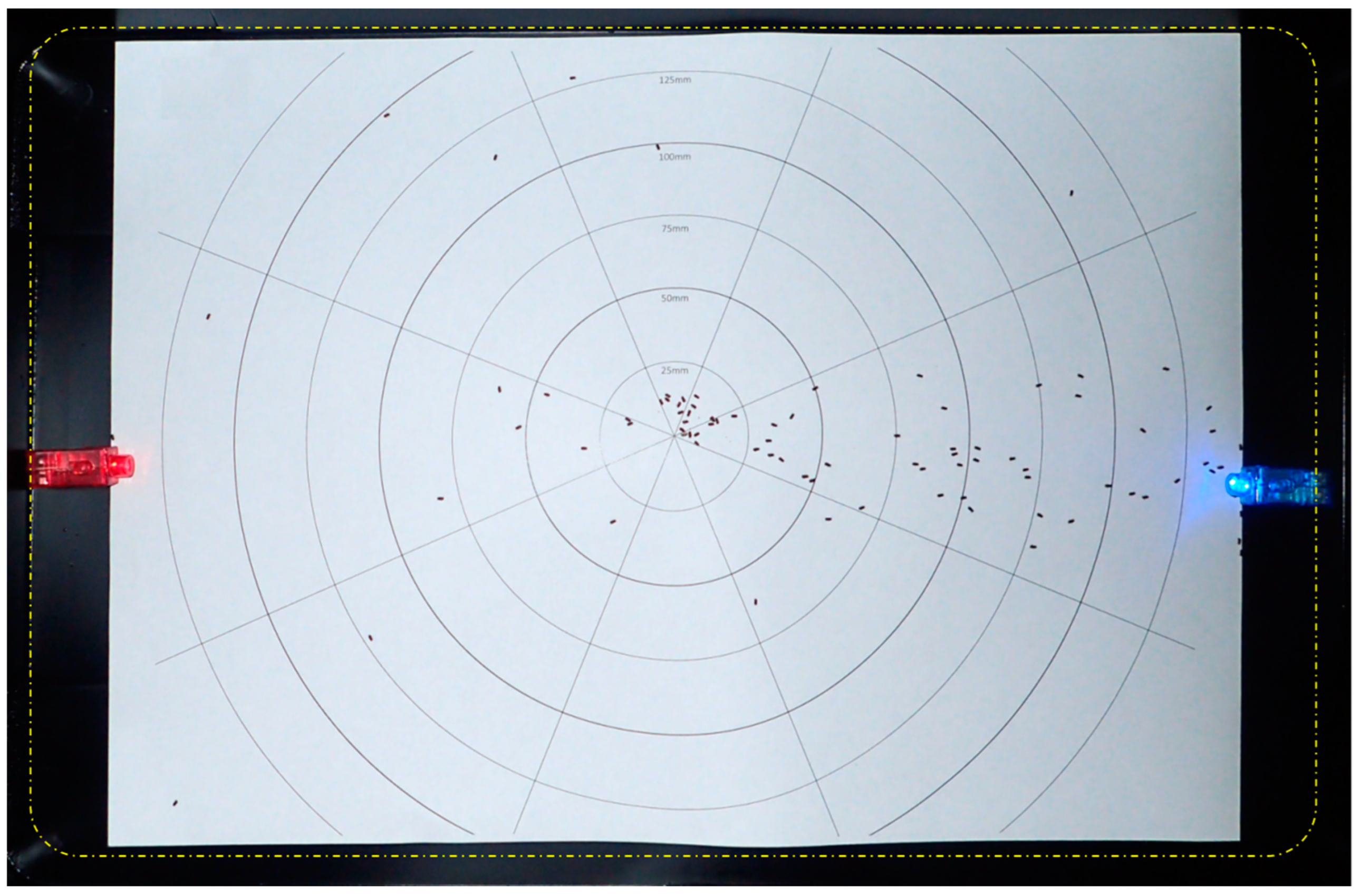
| Replicate | Sector 1 | Sector 2 | Sector 3 | Sector 4 |
|---|---|---|---|---|
| 1 | Blue | Red | Green | Black |
| 2 | Red | Green | Blue | Black |
| 3 | Black | Blue | Red | Green |
| 4 | Green | Blue | Red | Black |
| 5 | Red | Black | Blue | Green |
| 6 | Black | Green | Red | Blue |
| 7 | Red | Green | Black | Blue |
| 8 | Blue | Green | Red | Black |
| 9 | Green | Black | Red | Blue |
| 10 | Red | Black | Blue | Green |
| Replicate | Theoretical | % of Replicates Significantly | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Octant | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | Distribution | Higher | Lower | |
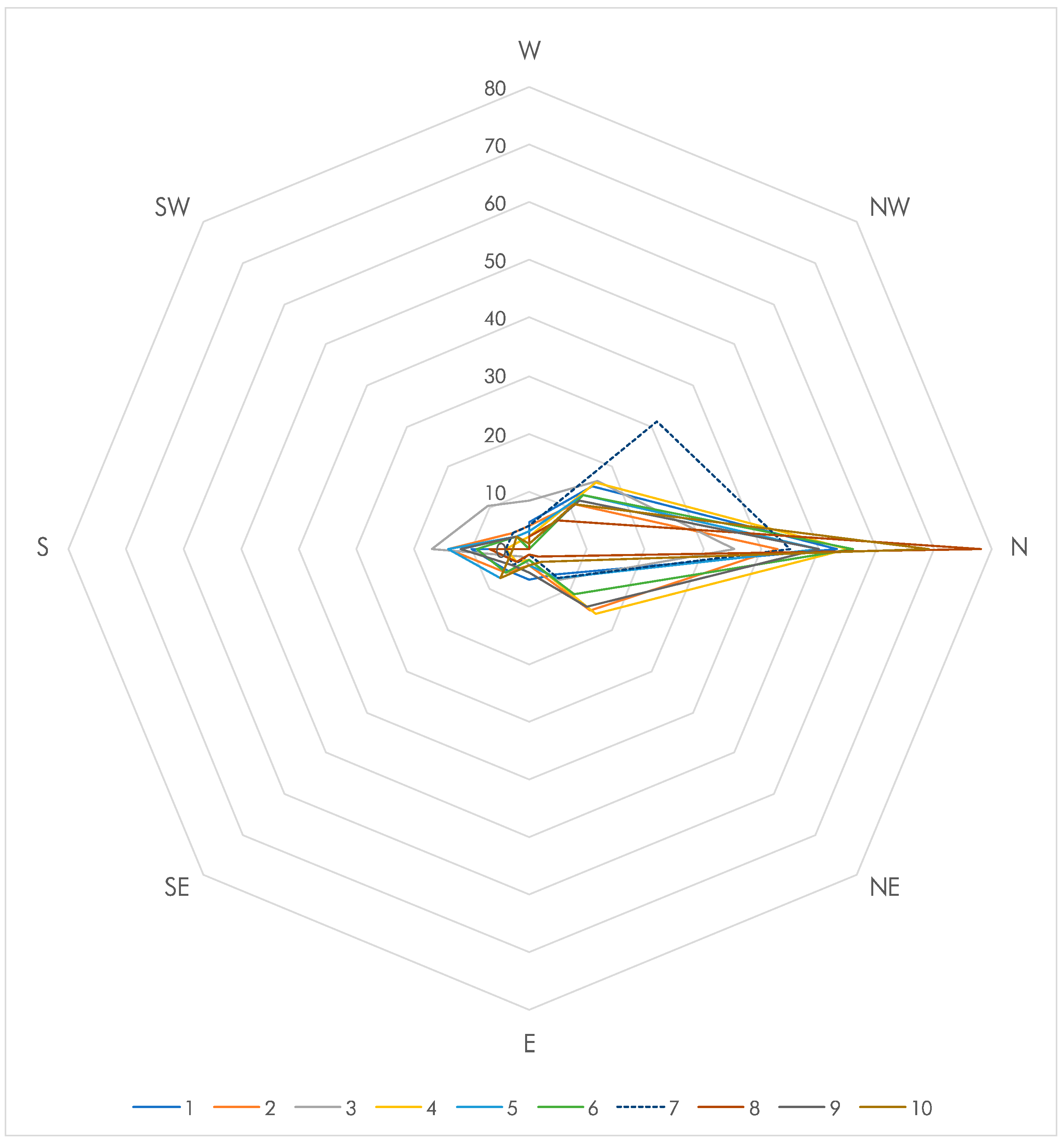
| NW | 15 | 11 | 17 | 16 | 13 | 13 | 31 + + | 7 | 12 | 11 | 12.5 | 10 | — | |
| light | N | 53 + + + | 43 + + + | 36 + + + | 55 + + + | 50 + + + | 56 + + + | 45 + + + | 78 + + + | 50 + + + | 69 + + + | 12.5 | 100 | — |
| NE | 6 | 15 | 7 | 16 | 7 | 11 | 7 | 2 | 14 | 3 | 12.5 | — | — | |
| midpoint | E | 5 | 3 | 3 − | 2 − − | 3 − | 2 − − | 1 − | 1 − | 4 − | 3 − | 12.5 | — | 80 |
| W | 5 | 4 − | 8 | 2 − − | 3 − | 0 − − − | 4 | 2 − − | 1 − | 1 − | 12.5 | — | 80 | |
| SE | 5 | 6 | 2 − − | 3 − | 7 | 6 | 4 − | 3 − | 4 − | 7 | 12.5 | — | 50 | |
| dark | S | 10 | 14 | 17 | 4 − | 14 | 9 | 4 − | 7 | 12 | 3 − | 12.5 | — | 30 |
| SW | 0 − − − | 4 − | 10 | 2 − − | 3 − | 3 − | 4 − | 0 − − − | 3 − | 3 − | 12.5 | — | 90 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||
| Movement to Light Sector (N) | Movement to Dark Sector (S) | Faster | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Repeat | Min | Avg ± StdDev | Max | n | Min | Avg ± StdDev | Max | n | Speed | p= |
| 1 | 2.56 | 3.48 ± 0.55 | 4.17 | 10 | 2.86 | 3.70 ± 0.44 | 4.17 | 10 | Dark | 0.2147 |
| 2 | 2.78 | 3.76 ± 0.43 | 4.17 | 10 | 2.86 | 3.10 ± 0.18 | 3.33 | 10 | Light | 0.0026 |
| 3 | 2.17 | 3.42 ± 0.55 | 4.00 | 10 | 2.38 | 3.24 ± 0.59 | 4.00 | 10 | Light | 0.5253 |
| 4 | 2.63 | 3.34 ± 0.46 | 4.00 | 10 | 2.94 | 3.57 ± 0.51 | 4.55 | 10 | Dark | 0.2877 |
| 5 | 2.94 | 3.55 ± 0.35 | 4.17 | 10 | 2.56 | 3.32 ± 0.44 | 4.00 | 10 | Light | 0.0927 |
| 6 | 2.78 | 4.02 ± 0.62 | 4.55 | 10 | 2.94 | 3.39 ± 0.44 | 4.17 | 10 | Light | 0.0184 |
| 7 | 2.86 | 3.60 ± 0.47 | 4.35 | 10 | 1.82 | 3.24 ± 0.70 | 4.17 | 10 | Light | 0.1531 |
| 8 | 3.13 | 3.83 ± 0.35 | 4.35 | 10 | 2.86 | 3.53 ± 0.39 | 4.17 | 10 | Light | 0.1131 |
| 9 | 2.86 | 3.77 ± 0.42 | 4.17 | 10 | 2.86 | 3.31 ± 0.35 | 3.70 | 10 | Light | 0.0163 |
| 10 | 2.94 | 3.74 ± 0.41 | 4.35 | 10 | 2.22 | 2.99 ± 0.70 | 4.17 | 10 | Light | 0.0036 |
| Total | 2.17 | 3.65 ± 0.49 | 4.55 | 100 | 1.82 | 3.34 ± 0.52 | 4.55 | 100 | Light | 0.0000 |
| Color | Rep #1 | Rep #2 | Rep #3 | Rep #4 | Rep #5 | Rep #6 | Rep #7 | Rep #8 | Rep #9 | Rep #10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Red | 11 | 11 | 10 | 16 | 14 | 17 | 11 | 22 | 17 | 6 |
| Green | 8 | 11 | 14 | 16 | 8 | 14 | 13 | 15 | 6 | 9 |
| Blue | 10 | 14 | 12 | 7 | 18 | 11 | 16 | 10 | 7 | 12 |
| Black | 11 | 14 | 12 | 14 | 14 | 11 | 13 | 16 | 18 | 18 |
| Red-Green | 15 | 12 | 17 | — | 8 | 13 | 16 | 10 | — | 10 |
| Red-Blue | 23 | — | 16 | 19 | — | 3 * | 5 | — | 14 | — |
| Red-Black | — | 15 | — | 8 | 18 | 18 | — | 14 | 19 | 15 |
| Blue-Green | — | 9 | — | 8 | 10 | — | — | 5 | 4 * | 11 |
| Blue-Black | 14 | 15 | 11 | — | 10 | 15 | 8 | 9 | 14 | 18 |
| Green-Black | 8 | — | 8 | 12 | — | — | 18 | — | — | — |
| n | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Color | Min | Avg ± 1σ | Median | Max | n |
|---|---|---|---|---|---|
| Black | 1.61 | 2.82 ± 0.64 | 2.86 | 4.55 | 108 |
| Blue | 1.47 | 2.79 ± 0.60 | 2.86 | 4.17 | 80 |
| Green | 1.52 | 2.76 ± 0.52 | 2.86 | 3.70 | 73 |
| Red | 1.56 | 2.82 ± 0.54 | 2.82 | 4.00 | 96 |
| All | 1.47 | 2.80 ± 0.58 | 2.86 | 4.55 | 357 |
| Mono Color Blue | Mono Color Red | ||||||
|---|---|---|---|---|---|---|---|
| Octant | Rep #1 | Rep #2 | Rep #3 | Rep #1 | Rep #2 | Rep #3 | |
| NW | 15 | 23 | 31 + + | 17 | 22 | 21 | |
| light | BLUE | 31 + + | 22 | 34 + + + | 32 + + + | 23 | 17 |
| NE | 13 | 21 | 12 | 11 | 8 | 13 | |
| midpoint | E | 7 | 6 | 6 | 11 | 0 − − − | 8 |
| W | 6 | 10 | 4 − | 6 | 14 | 16 | |
| SE | 8 | 6 | 2 − − | 5 | 11 | 8 | |
| dark | S | 12 | 8 | 6 | 13 | 10 | 8 |
| SW | 7 | 4 − | 6 | 6 | 13 | 8 | |
| n | 100 | 100 | 100 | 100 | 100 | 100 | |
| Observations | |||
|---|---|---|---|
| Octant | Rep #1 | Rep #2 | Rep #3 |
| NW | 18 | 8 | 10 |
| BLUE | 45 + + + | 38 + + + | 65 + + + |
| NE | 4 | 10 | 4 − |
| E | 5 | 8 | 1 − |
| SE | 6 | 12 | 1 − |
| RED | 9 | 11 | 6 |
| SW | 6 | 9 | 4 − |
| W | 8 | 6 | 10 |
| total | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spennemann, D.H.R. Photosensitivity of Dispersing Cryptic Date Stone Beetles Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae)—A Pilot Study. Insects 2022, 13, 851. https://doi.org/10.3390/insects13090851
Spennemann DHR. Photosensitivity of Dispersing Cryptic Date Stone Beetles Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae)—A Pilot Study. Insects. 2022; 13(9):851. https://doi.org/10.3390/insects13090851
Chicago/Turabian StyleSpennemann, Dirk H. R. 2022. "Photosensitivity of Dispersing Cryptic Date Stone Beetles Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae)—A Pilot Study" Insects 13, no. 9: 851. https://doi.org/10.3390/insects13090851
APA StyleSpennemann, D. H. R. (2022). Photosensitivity of Dispersing Cryptic Date Stone Beetles Coccotrypes dactyliperda (Coleoptera, Curculionidae, Scolytinae)—A Pilot Study. Insects, 13(9), 851. https://doi.org/10.3390/insects13090851






