Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Experimental Design
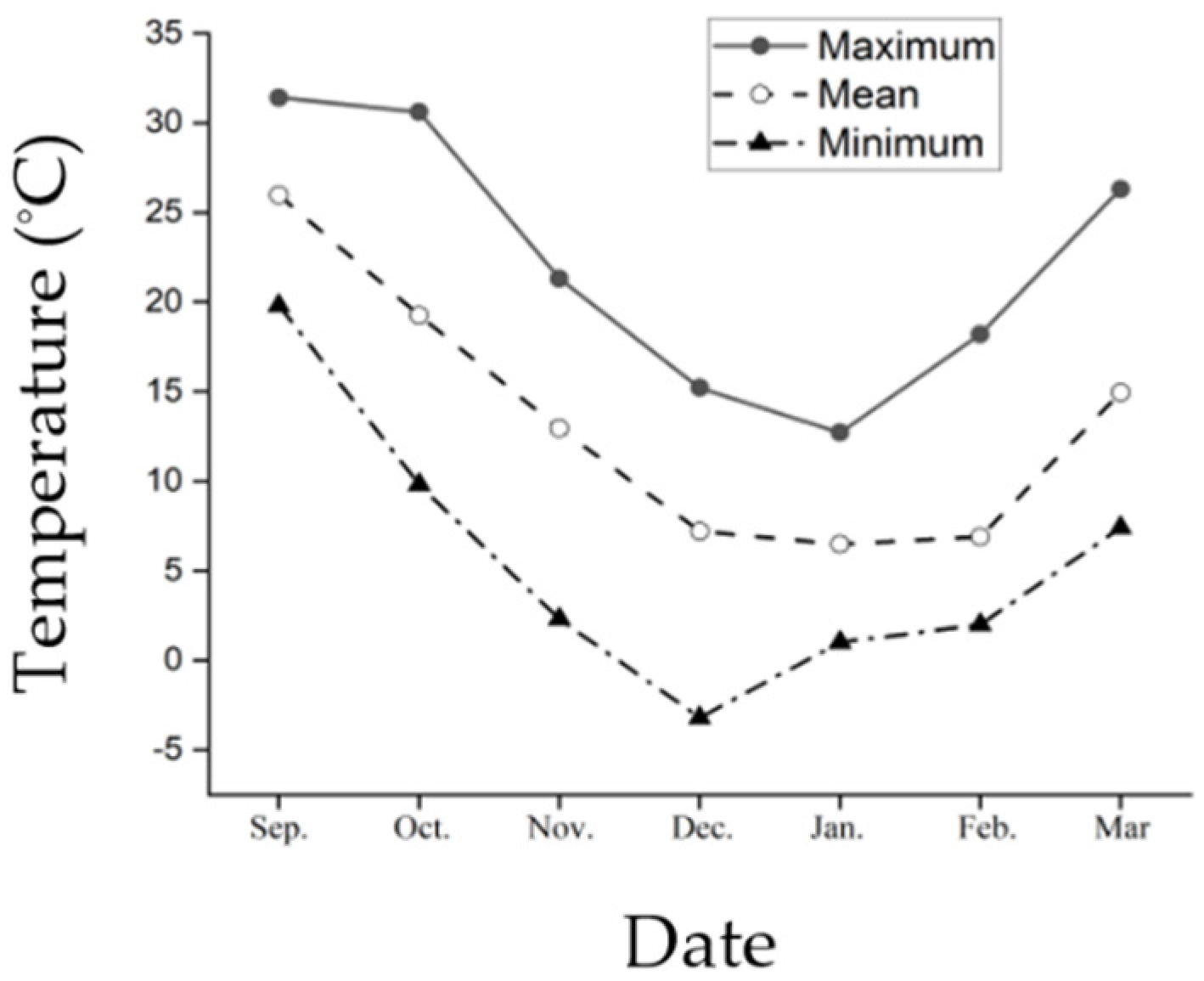
2.3. Ambient Temperature
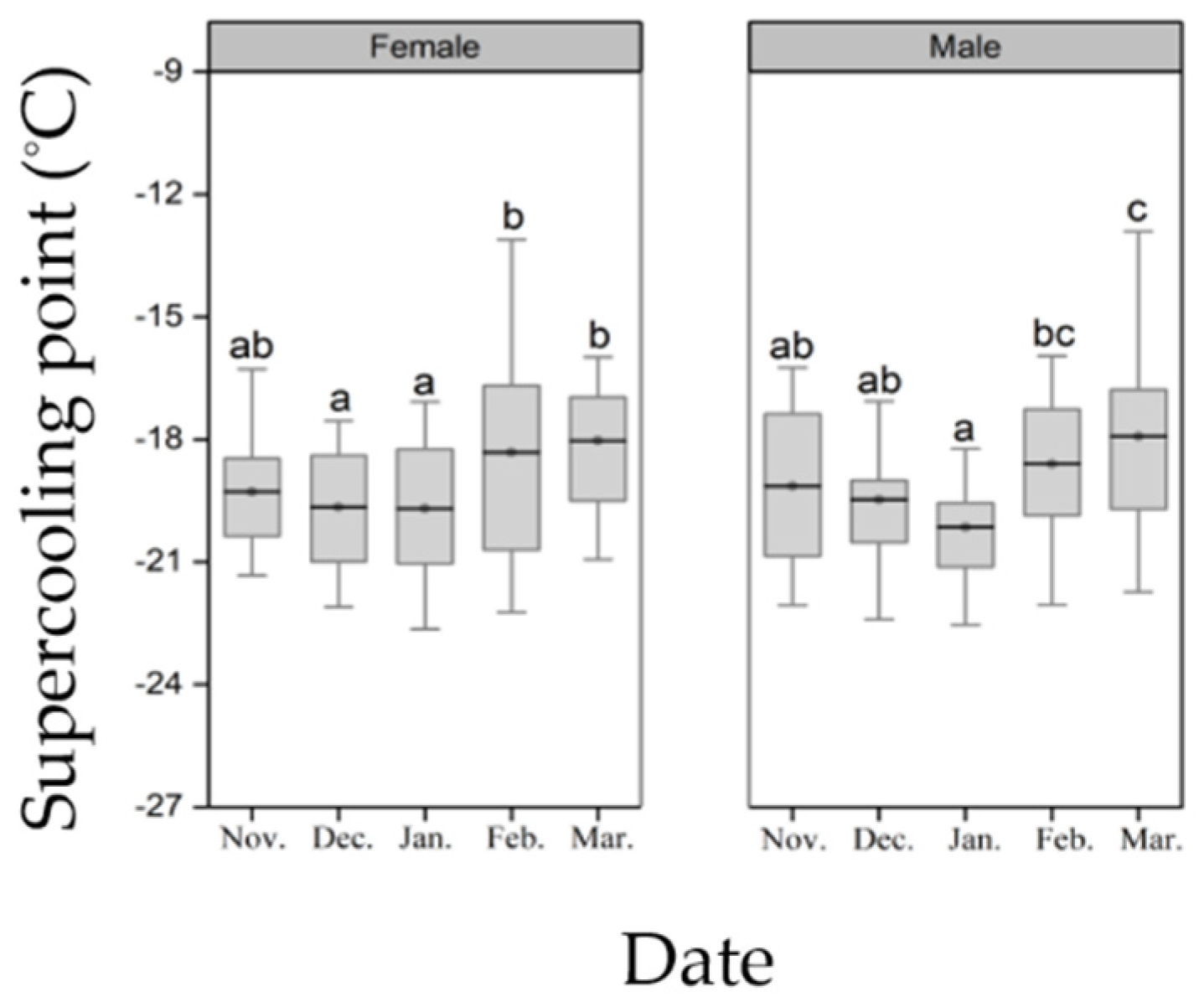
2.4. Supercooling Point
2.5. Biochemical Analysis
2.5.1. Trehalose and Glycogen
2.5.2. Lipid
2.6. Development Time of Diapausing Pupae and Post-Diapause Adult Fitness
2.7. Data Analysis
3. Results
3.1. Ambient Temperature
3.2. Supercooling Point
3.3. Biochemical Analysis
3.4. Pupal Diapause Development Time and Adult Fitness of Post-Diapause
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McDosald, J.R.; Bale, J.S.; Walters, K.F. Temperature development and establishment of Thrips palmi (Thysanoptera: Thripidae) in the United Kingdom. Eur. J. Entomol. 1999, 96, 169–173. [Google Scholar]
- Sage, R.B.; Fell, D.; Tucker, K.; Sotherton, N.W. Post hibernation dispersal of three leaf- eating beetle (Coleoptera: Chysomelidae) colonizing cultivated willows and poplars. Agric. For. Entomol. 1999, 1, 61–70. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986; p. 411. ISBN 0195036352. [Google Scholar]
- Denlinger, D.L. Relationship between cold hardiness and diapause. In Insects at Low Temperature; Lee, R.E., Jr., Denlinger, D.L., Eds.; Chapman & Hall: New York, NY, USA, 1991; pp. 174–198. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Bretman, A.; Tregenza, T.O.M.; Tomkins, J.L.; Hosken, D.J. Metabolic rate does not decrease with starvation in Gryllus bimaculatus when changing fuel use is taken into account. Physiol. Entomol. 2011, 36, 84–89. [Google Scholar] [CrossRef]
- Adedokun, T.A.; Denlinger, D.L. Metabolic reserves associated with pupal diapauses in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 1985, 31, 229–233. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 2004, 79, 207–233. [Google Scholar] [CrossRef]
- Marshall, K.E.; Roe, A.D. Surviving in a frozen forest: The physiology of eastern spruce budworm overwintering. Physiology 2021, 36, 174–182. [Google Scholar] [CrossRef]
- Irwin, J.T.; Lee, R.E., Jr. Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos 2003, 100, 71–78. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Effect of pre-wintering and wintering temperature regimes on weight loss, survival, and emergence time in the mason bee Osmia cornuta (Hymenptera: Megachilidae). Apidologie 2004, 35, 469–479. [Google Scholar] [CrossRef]
- Bosch, J.; Sgolastra, F.; Kemp, W.P. Timing of eclosion affects diapause development, fat body consumption and longevity in Osmia lignaria, a univoltine, adult-wintering solitary bee. J. Insect Physiol. 2010, 56, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Kemp, W.P.; Buckner, J.S.; Pitts-Singer, T.L.; Maini, S.; Bosch, J. The long summer: Pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J. Insect Physiol. 2011, 57, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Arnan, X.; Pitts-Singer, T.L.; Maini, S.; Kemp, W.P.; Bosch, J. Pre-wintering conditions and post-winter performance in a solitary bee: Does diapause impose an energetic cost on reproductive success? Ecol. Entomol. 2016, 41, 201–210. [Google Scholar] [CrossRef]
- Lehmann, P.; Pruisscher, P.; Posledovich, D.; Carlsson, M.; Käkelä, R.; Tang, P.; Nylin, S.; Wheat, C.W.; Wiklund, C.; Gotthard, K. Energy and lipid metabolism during direct and diapauses development in a pierid butterfly. J. Exp. Biol. 2016, 219, 3049–3060. [Google Scholar] [CrossRef]
- Lee, R.E.; Denlinger, D.L. A primer on insect cold tolerance. In Low Temperature Biology of Insects; Cambridge University Press: Cambridge, UK, 2010; pp. 3–35. ISBN 978-05-2188-635-2. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Sinclair, B.J. The role of the midgut in insect chilling injury: Cold-induced disruption of osmoregulation in the fall field cricket, Gryllus pennsylvanicus. J. Exp. Biol. 2011, 214, 726–734. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Wasielewski, O.; Wojciechowicz, T.; Giejdasz, K.; Krishnan, N. Overwintering strategies in the red mason solitary bee-physiological correlates of midgut metabolic activity and turnover of nutrient reserves in females of Osmia bicornis. Apidologie 2013, 44, 642–656. [Google Scholar] [CrossRef]
- Heydari, M.; Izadi, H. Effects of seasonal acclimation on cold tolerance and biochemical status of the carob moth, Ectomyelois ceratoniae Zeller, last instar larvae. Bull. Entomol. Res. 2014, 104, 592–600. [Google Scholar] [CrossRef]
- Li, Y.P.; Oguchi, S.; Goto, M. Physiology of diapause and cold hardiness in overwintering pupae of the apple leaf miner Phyllonorycter ringoniella in Japan. Physiol. Entomol. 2002, 27, 92–96. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, K.; Tang, F.; Xu, M. Activation of the host immune response in Hyphantria cunea (Drury) (Lepidoptera: Noctuidae) induced by Serratia marcescens Bizio. Insects 2021, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Goto, M.; Ito, S.; Sato, Y.; Sasaki, K.; Goto, N. Physiology of diapause and cold hardiness in the overwintering pupae of the fall webworm Hyphantria cunea (Lepidoptera: Arctiidae) in Japan. J. Insect Physiol. 2001, 47, 1181–1187. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhu, M.M.; Li, L.L.; Zhang, G.C.; Zheng, Y.; Li, T.; Sun, S.H. Cold hardiness characteristic of the overwintering pupae of fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) in the northeast of China. J. Asia-Pac. Entomol. 2015, 18, 39–45. [Google Scholar] [CrossRef]
- Liu, J.; Ye, L.Q.; Cheng, C.; Xiong, D.B.; Chen, Z.Y. The occurrence of forestry pests in 2015 and its occurrence trend prediction in 2016 in Jiangsu Province. J. Jinling Inst. Tech. 2016, 32, 64–67. [Google Scholar]
- Zhao, L.; Wang, W.; Qiu, Y.; Torson, A.S. Physiological Mechanisms of Variable Nutrient Accumulation Patterns Between Diapausing and Non- Diapausing Fall Webworm (Lepidoptera: Arctiidae) Pupae. Environ. Entomol. 2021, 50, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Loewy, K.J.; Flansburg, A.L.; Grenis, K.; Kjeldgaard, M.K.; Mccarty, J.; Montesano, L.; Vernick, J.; Murphy, S.M. Life history traits and rearing techniques for fall webworms (Hyphantria cunea Drury) in Colorado. J. Lepid. Soc. 2013, 67, 196–205. [Google Scholar] [CrossRef]
- Williams, C.M.; Chick, W.D.; Sinclair, B.J. A cross-seasonal perspective on local adaptation: Metabolic plasticity mediates responses to winter in a thermal-generalist moth. Funct. Ecol. 2015, 29, 549–561. [Google Scholar] [CrossRef]
- Carroll, N.V.; Longley, R.W.; Roe, J.H. The determination of glycogen in liver and muscle by use of anthrone reagent. J. Biol. Chem. 1956, 220, 583–593. [Google Scholar] [CrossRef]
- Lorenz, M.W. Adipokinetic hormone inhibits the formation of energy stores and egg production in the cricket Gryllus bimaculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 197–206. [Google Scholar] [CrossRef]
- Sinclair, B.J. Linking energetics and overwintering in temperate insects. J. Therm. Biol. 2015, 54, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Marshall, K.E. The many roles of fats in overwintering insects. J. Exp. Biol. 2018, 221, jeb161836. [Google Scholar] [CrossRef] [PubMed]
- Batz, Z.A.; Armbruster, P.A. Diapause-associated changes in the lipid and metabolite profiles of the Asian tiger mosquito, Aedes albopictus. J. Exp. Biol. 1894, 221, jeb189480. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Borzoui, E.; Izadi, H. Physiological and Biochemical Differences in Diapausing and Nondiapausing Larvae of Eurytoma plotnikovi (Hymenoptera: Eurytomidae). Environ. Entomol. 2017, 46, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, W. Effects of autumn warming on energy consumption of diapausing fall webworm (Lepidoptera: Arctiidae) Pupae. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef]
- Cuddington, K.; Sobek-Swant, S.; Crosthwaite, J.C.; Lyons, D.B.; Sinclair, B.J. Probability of emerald ash borer impact for Canadian cities and North America: A mechanistic model. Biol. Invasions 2018, 20, 2661–2677. [Google Scholar] [CrossRef]
- Izadi, H.; Mohammadzadeh, M.; Mehrabian, M. Changes in biochemical contents and survival rates of two stored product moths under different thermal regimes. J. Therm. Biol. 2019, 80, 7–15. [Google Scholar] [CrossRef]
- Hand, S.C.; Moore, D.S.; Patil, Y. Challenges during diapause and anhydrobiosis: Mitochondrial bioenergetics and desiccation tolerance. IUBMB Life 2018, 70, 1251–1259. [Google Scholar] [CrossRef]
- Ellers, J.; Van Alphen, J.J.M. A trade-off between diapause duration and fitness in female parasitoids. Ecol. Entomol. 2002, 27, 279–284. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Lehmann, P.; Gotthard, K. Longer and warmer prewinter periods reduce post- winter fitness in a diapausing insect. Funct. Ecol. 2022, 36, 1151–1162. [Google Scholar] [CrossRef]
- Gomi, T. Effects of timing of diapause induction on winter survival and reproductive success in Hyphantria cunea in a transition area of voltinism. Entomol. Sci. 2000, 3, 433–438. [Google Scholar]
- Chen, C.; Wei, X.T.; Xiao, H.J.; He, H.M.; Xia, Q.W.; Xue, F.S. Diapause induction and termination in Hyphantria cunea (drury) (Lepidoptera: Arctiinae). PLoS ONE 2014, 9, e98145. [Google Scholar] [CrossRef] [PubMed]



| Time of Pupae Removed from Winter Conditions | Time from Pupation to Adult Emergence (d) (Mean ± SE) | Survival Rate % | ||
|---|---|---|---|---|
| Male Pupae | Female Pupae | Male Pupae | Female Pupae | |
| 5 February. | 160.5 ± 4.4a | 161.8 ± 4.4a | 44.3a | 46.2a |
| 25 February | 160.7 ± 3.9a | 162.7 ± 4.6a | 44.4a | 46.1a |
| 17 March | 177.5 ± 4.1b | 178.5 ± 4.1b | 41.9b | 44.6b |
| 6 April | 190.1 ± 4.2c | 191.8 ± 3.8c | 39.2c | 42.0c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Wang, X.; Liu, Z.; Torson, A.S. Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae. Insects 2022, 13, 853. https://doi.org/10.3390/insects13090853
Zhao L, Wang X, Liu Z, Torson AS. Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae. Insects. 2022; 13(9):853. https://doi.org/10.3390/insects13090853
Chicago/Turabian StyleZhao, Lvquan, Xinmei Wang, Zheng Liu, and Alex S. Torson. 2022. "Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae" Insects 13, no. 9: 853. https://doi.org/10.3390/insects13090853
APA StyleZhao, L., Wang, X., Liu, Z., & Torson, A. S. (2022). Energy Consumption and Cold Hardiness of Diapausing Fall Webworm Pupae. Insects, 13(9), 853. https://doi.org/10.3390/insects13090853






