Development of Spodoptera exigua Population: Does the Nutritional Status Matter?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collecting and Rearing of Test Insects
2.2. Experimental Method
2.3. Statistical Analysis of Data
3. Results
3.1. Developmental Duration of S. exigua under Different Nutritional Conditions
3.2. The Fecundity Parameters of S. exigua under Different Nutritional Conditions
3.3. Life Table Parameters of S. exigua Population under Different Nutritional Conditions
3.4. Age-Stage Survival Rate and Fecundity of S. exigua under Different Nutritional Conditions
3.5. The Age-Specific Life Expectancy of S. exigua Population under Different Nutritional Conditions
3.6. The Reproductive Value of S. exigua under Different Nutritional Conditions
3.7. The Prediction of S. exigua Population Size under Different Nutritional Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauerfeind, S.S.; Fischer, K. Testing the plant stress hypothesis: Stressed plants offer better food to an insect herbivore. Entomol. Exp. Appl. 2013, 149, 148–158. [Google Scholar] [CrossRef]
- Hunter, M.D.; McNeil, J.N. Host-plant quality influences diapause and voltinism in a polyphagous insect herbivore. Ecology 1997, 78, 977–986. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Behmer, S.T.; Joern, A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc. Natl. Acad. Sci. USA 2008, 105, 1977–1982. [Google Scholar] [CrossRef]
- Coley, P.D.; Bateman, M.L.; Kursar, T.A. The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 2006, 115, 219–228. [Google Scholar] [CrossRef]
- Sznajder, B.; Harvey, J.A. Second and third trophic level effects of differences in plant species reflect dietary specialisation of herbivores and their endoparasitoids. Entomol. Exp. Appl. 2003, 109, 73–82. [Google Scholar] [CrossRef]
- Denno, R.F.; Peterson, M.A.; Gratton, C.; Cheng, J.; Langellotto, G.A.; Huberty, A.F.; Finke, D.L. Feeding-induced changes in plant quality mediate interspecific competition between sap-feeding herbivores. Ecology 2000, 81, 1814–1827. [Google Scholar] [CrossRef]
- Joern, A.; Provin, T.; Behmer, S.T. Not just the usual suspects: Insect herbivore populations and communities are associated with multiple plant nutrients. Ecology 2012, 93, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Javed, M.; Sohail, M.; Sagheer, M. Environmental effects on insects and their population dynamics. J. Entomol. Zool. Stud. 2014, 2, 1–7. [Google Scholar]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Alejandro, R.D.; María Teresa, P.A.R.; Juan Rafael, R.E. Carbohydrate metabolism in drosophila: Reliance on the disaccharide trehalose. In Carbohydrates; Chuan-Fa, C., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 14. [Google Scholar]
- Wang, S.; Ding, T.; Xu, M.; Zhang, B. Bidirectional interactions between beet armyworm and its host in response to different fertilization conditions. PLoS ONE 2018, 13, e0190502. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Huang, Y.B. Life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae): With a mathematical invalidation for applying the jackknife technique to the net reproductive rate. Nat. Preced. 2012, 1–31. [Google Scholar] [CrossRef]
- Kashyap, R.K.; Banerjee, M.K.; Kalloo; Verma, A.N. Survival and development of fruit borer, Heliothis armigera (Hübner), (Lepidoptera: Noctuidae) on Lycopersicon spp. Int. J. Trop. Insect Sci. 1990, 11, 877–881. [Google Scholar] [CrossRef]
- Kumar, Y.; Pandey, N.; Bhatnagar, S. Bio-efficacy of plant extracts on growth, development and survival of tobacco caterpillar (Spodoptera litura (Fab.)) larvae. Bioinfolet-A Q. J. Life Sci. 2012, 9, 667–669. [Google Scholar]
- Friend, W.G. Nutritional requirements of phytophagous insects. Annu. Rev. Entomol. 1958, 3, 57–74. [Google Scholar] [CrossRef]
- Waldbauer, G.P.; Friedman, S. Self-selection of optimal diets by insects. Annu. Rev. Entomol. 1991, 36, 43–63. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D.; Bone, Q. A multi-level analysis of feeding behaviour: The geometry of nutritional decisions. Philos. Trans. R. Soc. B Biol. Sci. 1993, 342, 381–402. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and foraging theory. Curr. Opin. Insect Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Kwon, M.; Cho, H.-M.; Ahn, Y.-J. Relationship between feeding damage by Beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae) and leaf trichome density of potato. J. Asia-Pac. Entomol. 2006, 9, 361–367. [Google Scholar] [CrossRef]
- Xia-lin, Z.; Cong, X.; Wang, X.; Lei, C.l. A review of geographic distribution, overwintering and migration in Spodoptera exigua Hübner (Lepidoptera: Noctuidae). J. Entomol. Res. Soc. 2011, 13, 39–48. [Google Scholar]
- Saeed, S.; Sayyed, A.H.; Ahmad, I. Effect of host plants on life-history traits of Spodoptera exigua (Lepidoptera: Noctuidae). J. Pest Sci. 2010, 83, 165–172. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wang, J.; Zhou, X. Advance in the research on Spodoptera exigua (Hübner)(Lepidoptera: Noctuidae). Chin. Agric. Sci. Bull. 2008, 24, 427–433. [Google Scholar]
- Ali, A.; Gaylor, M.J. Effects of temperature and larval diet on development of the beet armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 1992, 21, 780–786. [Google Scholar] [CrossRef]
- Estiarte, M.; Filella, I.; Serra, J.; Peñuelas, J. Effects of nutrient and water stress on leaf phenolic content of peppers and susceptibility to generalist herbivore Helicoverpa armigera (Hübner). Oecologia 1994, 99, 387–391. [Google Scholar] [CrossRef]
- Hervet, V.A.D.; Laird, R.A.; Floate, K.D. A review of the mcmorran diet for rearing lepidoptera species with addition of a further 39 species. J. Insect Sci. 2016, 16, 19. [Google Scholar] [CrossRef]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent advances in the integrative nutrition of arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef]
- Xiao, W.; Deng, X.P.; Liu, H. An improved method of rearing Spodoptera exigua lavae. Chin. J. Appl. Entomol. 2005, 5, 581–583. [Google Scholar]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 224–240. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2018. Available online: http://140.120.197.173/Ecology/ (accessed on 20 October 2018).
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Birch, L. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Merkx-Jacques, M.; Despland, E.; Bede, J.C. Nutrient utilization by caterpillars of the generalist beet armyworm, Spodoptera exigua. Physiol. Entomol. 2008, 33, 51–61. [Google Scholar] [CrossRef]
- Simpson, S.J.; Simpson, C.L. The mechanisms of nutritional compensation by phytophagous insects. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 1990; p. 50. [Google Scholar]
- Lee, K.P.; Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J. Insect Physiol. 2002, 48, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ruberson, J.R.; Olson, D.M. Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 2008, 126, 244–255. [Google Scholar] [CrossRef]
- Wang, P.; Furlong, M.J.; Walsh, T.K.; Zalucki, M.P. Moving to keep fit: Feeding behavior and movement of Helicoverpa armigera (Lepidoptera: Noctuidae) on artificial diet with different protein: Carbohydrate ratios. J. Insect Sci. 2019, 19, 20. [Google Scholar] [CrossRef]
- Qiao, F.J.; Xing, L.S.; Li, C.Y.; Zheng, G.L.; Zhang, B. Sensitivity of Spodoptera exigua larvae to nucleopolyhedrovirus and their feeding preference under different nutritional conditions. Environ. Entomol. 2021, 43, 1122–1128. [Google Scholar]
- Al Baki, M.A.; Jung, J.K.; Kim, Y. Regulation of hemolymph trehalose titers by insulin signaling in the legume pod borer, Maruca vitrata (Lepidoptera: Crambidae). Peptides 2018, 106, 28–36. [Google Scholar] [CrossRef]

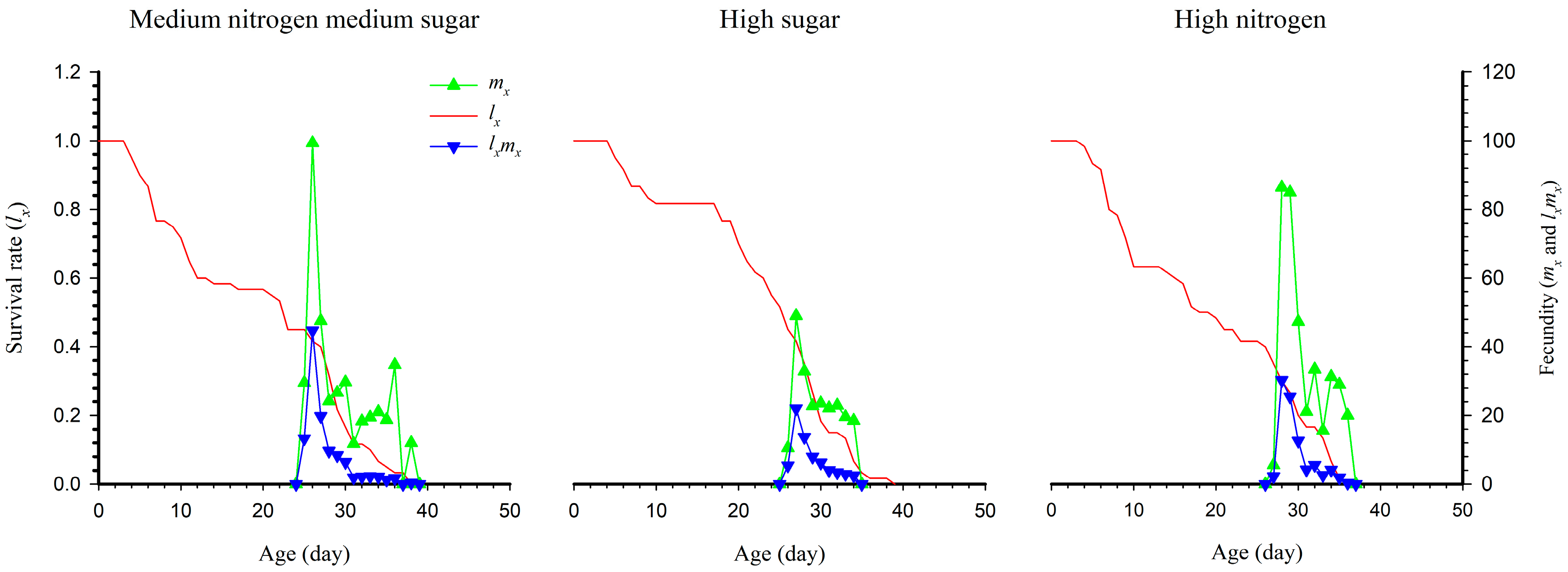
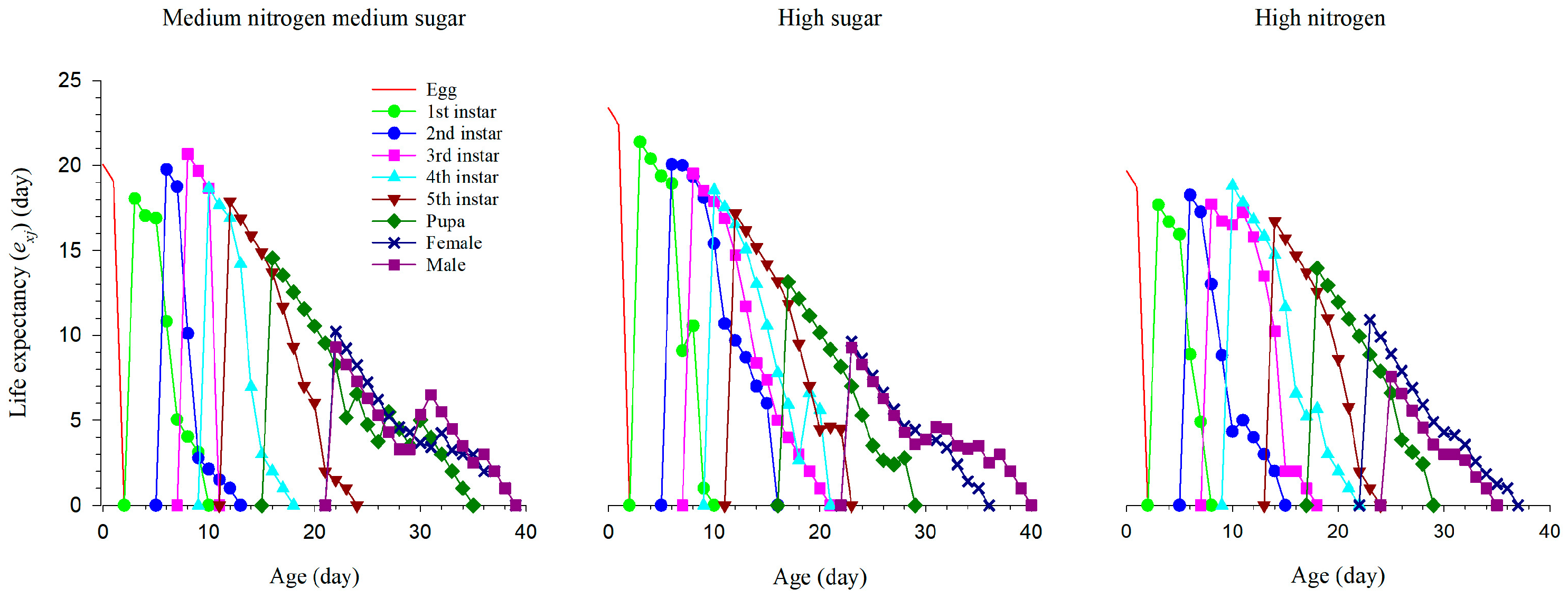


| Developmental Stage (d) | Medium Nitrogen, Medium Sugar | High Sugar | High Nitrogen |
|---|---|---|---|
| First-instar larva | 3.53 ± 0.16 a,b | 3.66 ± 0.08 a | 3.21 ± 0.08 b |
| Second-instar larva | 1.97 ± 0.03 b | 2.65 ± 0.16 a | 2.40 ± 0.16 a |
| Third-instar larva | 2.22 ± 0.08 b | 2.23 ± 0.10 b | 4.34 ± 0.17 a |
| Fourth-instar larva | 2.79 ± 0.08 b | 3.00 ± 0.14 a,b | 3.17 ± 0.14 a |
| Fifth-instar larva | 4.39 ± 0.15 a | 4.63 ± 0.17 a | 4.48 ± 0.17 a |
| Pupa | 6.13 ± 0.14 a | 6.50 ± 0.15 a | 6.22 ± 0.19 a |
| Adult lifespan | 8.78 ± 0.63 a | 7.96 ± 0.63 a,b | 7.22 ± 0.70 b |
| Preadult stage | 22.04 ± 0.18 b | 23.50 ± 0.28 a,b | 25.17 ± 0.35 a |
| Parameters | Medium Nitrogen, Medium Sugar | High Sugar | High Nitrogen |
|---|---|---|---|
| Oviposition days (d) | 6.00 ± 0.55 a | 4.75 ± 0.56 a | 5.19 ± 0.64 a |
| TPOP (d) | 24.38 ± 0.35 b | 26.75 ± 0.53 a | 27.55 ± 0.31 a |
| APOP (d) | 3.23 ± 0.20 a | 3.88 ± 0.61 a | 2.36 ± 0.15 b |
| Fecundity (eggs/female) | 605.42 ± 36.33 a | 358.90 ± 94.50 b | 486.89 ± 64.82 a,b |
| Parameters | Medium Nitrogen, Medium Sugar | High Sugar | High Nitrogen |
|---|---|---|---|
| Intrinsic rate of increase (r) | 0.18 ± 0.01 a | 0.15 ± 0.02 b | 0.15 ± 0.01 b |
| Finite rate of increase (λ) | 1.20 ± 0.01 a | 1.16 ± 0.02 b | 1.16 ± 0.01 b |
| Net reproductive rate (R0) | 3.79 ± 0.24 a | 4.83 ± 1.35 a | 4.62 ± 1.89 a |
| Mean generation time | 26.38 ± 0.54 b | 28.30 ± 0.62 a | 29.50 ± 0.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Zhang, B.; Li, J.; Qiao, F.; Ma, Q.; Wan, X.; Jiang, Z.; Li, C. Development of Spodoptera exigua Population: Does the Nutritional Status Matter? Insects 2023, 14, 13. https://doi.org/10.3390/insects14010013
Ma H, Zhang B, Li J, Qiao F, Ma Q, Wan X, Jiang Z, Li C. Development of Spodoptera exigua Population: Does the Nutritional Status Matter? Insects. 2023; 14(1):13. https://doi.org/10.3390/insects14010013
Chicago/Turabian StyleMa, Hancheng, Bin Zhang, Jiangjie Li, Fengjiao Qiao, Qihong Ma, Xuanwu Wan, Zhufeng Jiang, and Changyou Li. 2023. "Development of Spodoptera exigua Population: Does the Nutritional Status Matter?" Insects 14, no. 1: 13. https://doi.org/10.3390/insects14010013
APA StyleMa, H., Zhang, B., Li, J., Qiao, F., Ma, Q., Wan, X., Jiang, Z., & Li, C. (2023). Development of Spodoptera exigua Population: Does the Nutritional Status Matter? Insects, 14(1), 13. https://doi.org/10.3390/insects14010013






