Potential of Entomopathogenic Nematode HbSD as a Candidate Biocontrol Agent against Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. S. frugiperda and EPN Collection and Culturing
2.2. Virulence of HbSD against S. frugiperda Larvae in Petri Plate Bioassay
2.3. Efficacy of HbSD against S. frugiperda Larvae in Potted Soil Bioassay
2.4. Field Trial
2.5. Statistical Analysis
3. Results
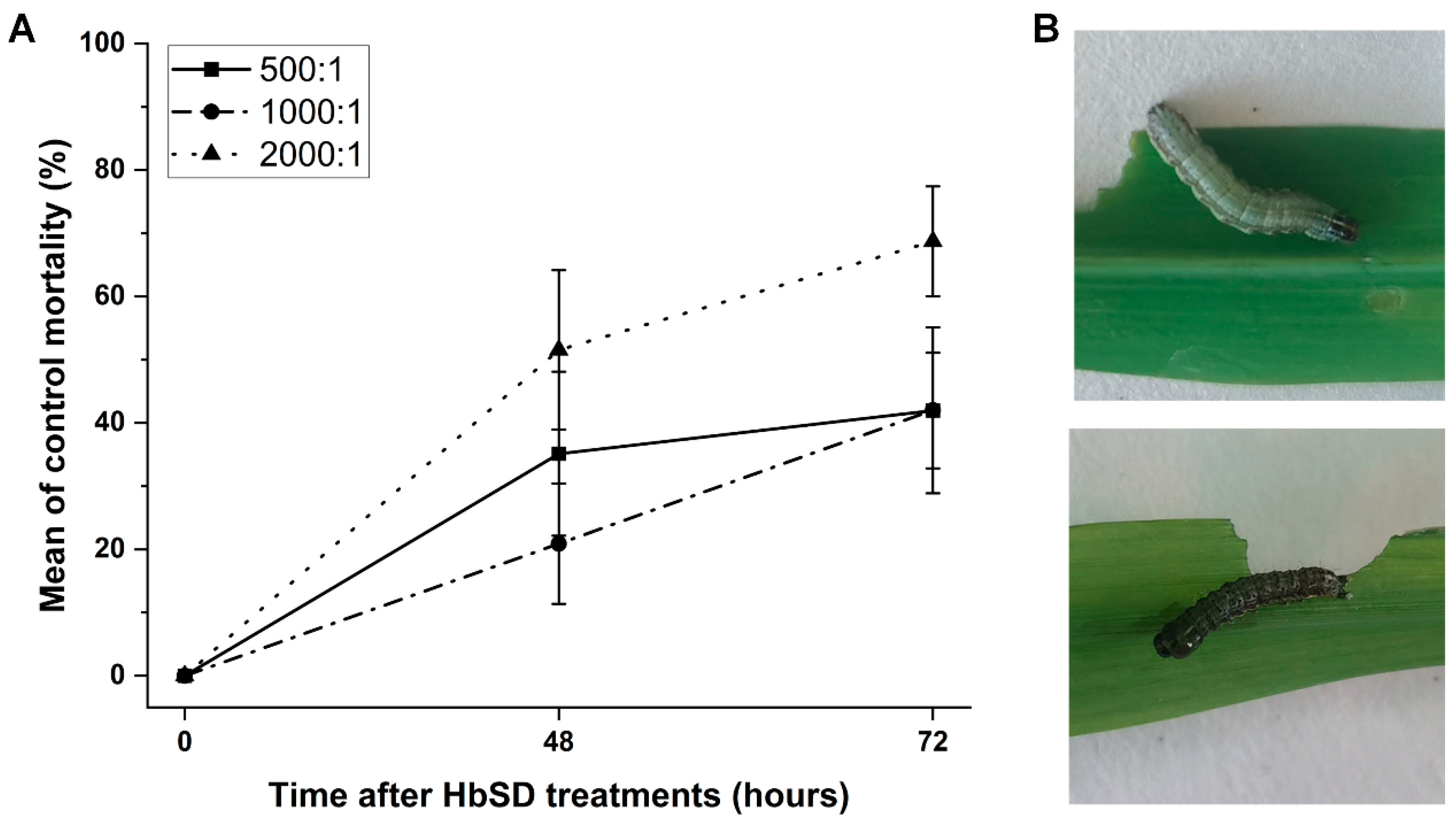
3.1. Pathogenicity of HbSD to S. frugiperda Larvae under Laboratory Conditions
3.2. Effect of HbSD against S. frugiperda Larvae in Potted Soil Bioassays
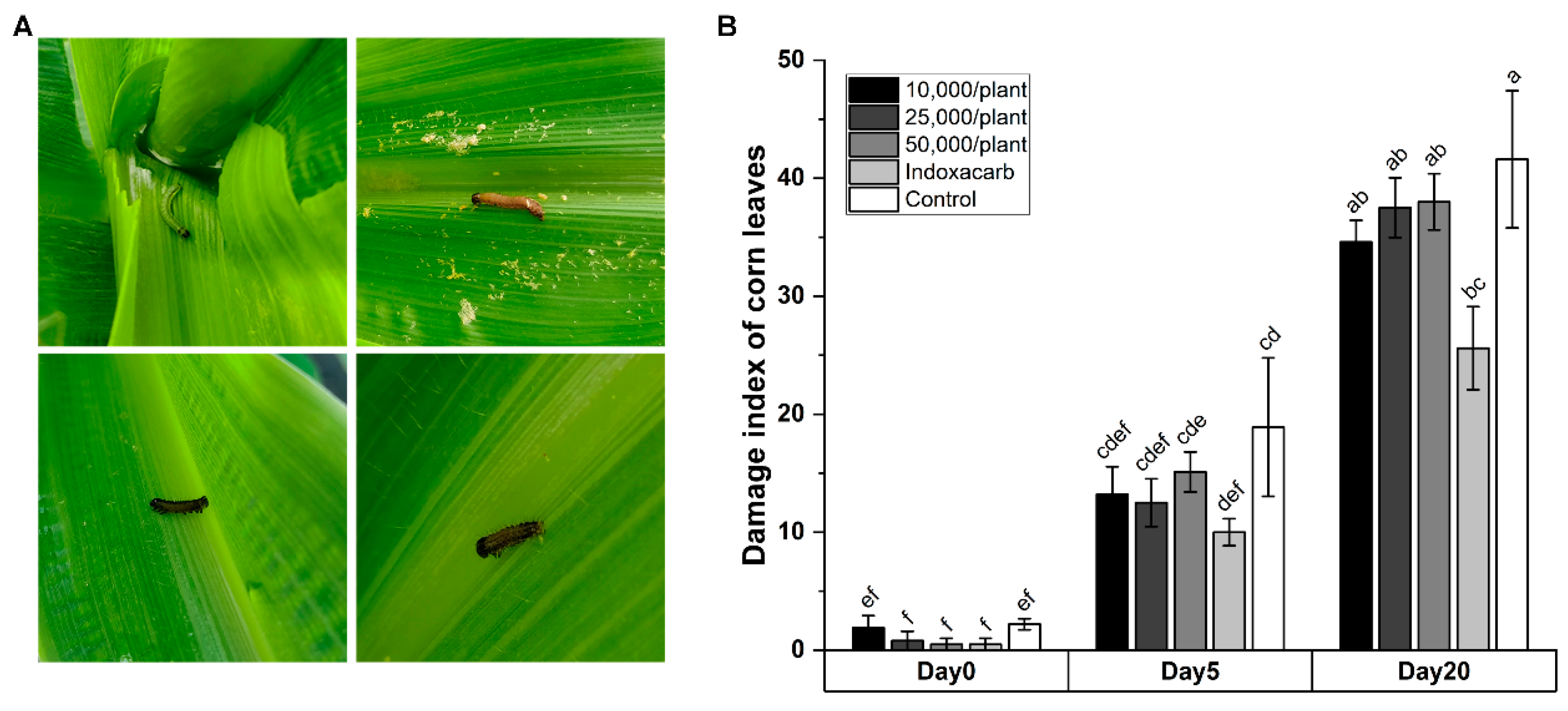
3.3. Efficacy of HbSD against S. frugiperda Applied in Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yainna, S.; Nègre, N.; Silvie, P.J.; Brévault, T.; Tay, W.T.; Gordon, K.; dAlençon, E.; Walsh, T.; Nam, K. Geographic monitoring of insecticide resistance mutations in native and invasive populations of the fall armyworm. Insects 2021, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Cañas-Hoyos, N.; Márquez, E.J.; Saldamando-Benjumea, C.I. Heritability of wing size and shape of the rice and corn strains of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2016, 45, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, Q.; Zhang, H.; Wu, K. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, X.; Xie, W.; Wang, R.; Feng, C.; Ma, L.; Li, Q.; Yang, Q.; Wang, H. Predicting the potential distribution of the fall armyworm Spodoptera frugiperda (J.E. Smith) under climate change in China. Glob. Ecol. Conserv. 2022, 33, e01994. [Google Scholar] [CrossRef]
- Sun, X.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Zhao, S.; Jiang, Y.; Wu, K. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Romeis, J. Managing the invasive fall armyworm through biotech crops: A Chinese perspective. Trends Biotechnol. 2021, 39, 105–107. [Google Scholar] [CrossRef]
- Mitchell, E.R. Fall armyworm symposium: Preface. Fla. Entomol. 1979, 62, 81. [Google Scholar]
- Davis, T.; Day, R.; Early, R.; Godwin, J.; Gonzalez-Moreno, P.; Kansiime, M.; Kenis, M. Fall armyworm: Impacts and implications for Africa. In CABI Evidence Note Update; CABI Publishing: Wallingford, Oxfordshire, UK, 2018; p. 26. [Google Scholar]
- Zhang, L.; Liu, B.; Zheng, W.; Liu, C.; Zhang, D.; Zhao, S.; Li, Z.; Xu, P.; Wilson, K.; Withers, A.; et al. Genetic structure and insecticide resistance characteristics of fall armyworm populations invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, S.; Zhang, F.; Huang, C.; He, K.; Babendreier, D.; Wang, Z. Prospects for microbial control of the fall armyworm Spodoptera frugiperda: A review. BioControl 2020, 65, 647–662. [Google Scholar] [CrossRef]
- Burnell, A.; Stock, S.P. Heterorhabditis, Steinernema and their bacterial symbionts—Lethal pathogens of insects. Nematology 2000, 2, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.J.; Fodor, A.; Koppenhöfer, H.S.; Stackebrandt, E.; Patricia Stock, S.; Klein, M.G. Reprint of “biodiversity and systematics of nematode–bacterium entomopathogens” [Biol. Control 37 (2006) 32–49]. Biol. Control 2006, 38, 4–21. [Google Scholar] [CrossRef]
- Bhat, A.H.; Chaubey, A.K.; Askary, T.H. Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt. J. Biol. Pest Control 2020, 30, 31. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.E.; Campbell, J.; Griffin, C.; Kaya, H.; Peters, A. Behavioral ecology of entomopathogenic nematodes. Biol. Control 2006, 38, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar]
- Patil, J.; Rangasamy, V. Field evaluation of the entomopathogenic nematodes against the white grub, Leucopholis lepidophora blanchard (Coleoptera: Scarabaeidae). Egypt. J. Biol. Pest Control 2018, 28, 41. [Google Scholar] [CrossRef] [Green Version]
- Torrini, G.; Paoli, F.; Mazza, G.; Simoncini, S.; Benvenuti, C.; Strangi, A.; Tarasco, E.; Barzanti, G.P.; Bosio, G.; Cutino, I.; et al. Evaluation of indigenous entomopathogenic nematodes as potential biocontrol agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy. Insects 2020, 11, 804. [Google Scholar] [CrossRef]
- Mokrini, F.; Laasli, S.-E.; Benseddik, Y.; Joutei, A.B.; Blenzar, A.; Lakhal, H.; Sbaghi, M.; Imren, M.; Özer, G.; Paulitz, T.; et al. Potential of Moroccan entomopathogenic nematodes for the control of the mediterranean fruit fly Ceratitis capitata Wiedemann (Diptera: Tephritidae). Sci. Rep. 2020, 10, 19204. [Google Scholar] [CrossRef]
- Platt, T.; Stokwe, N.F.; Malan, A.P. A review of the potential use of entomopathogenic nematodes to control above-ground insect pests in South Africa. S. Afr. J. Enol. Vitic. 2020, 41, 1–16. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Entomopathogenic nematodes in sustainable food production. Front. Sustain. Food Syst. 2020, 4, 125. [Google Scholar] [CrossRef]
- Andaló, V.; Santos, V.; Moreira, G.F.; Moreira, C.C.; Moino, A., Jr. Evaluation of entomopathogenic nematodes under laboratory and greenhouses conditions for the control of Spodoptera frugiperda. Cienc. Rural 2010, 40, 1860–1866. [Google Scholar] [CrossRef] [Green Version]
- Caccia, M.G.; Del Valle, E.; Doucet, M.E.; Lax, P. Susceptibility of Spodoptera frugiperda and Helicoverpa gelotopoeon (Lepidoptera: Noctuidae) to the entomopathogenicnematode Steinernema diaprepesi (Rhabditida: Steinernematidae) under laboratory conditions. Chil. J. Agric. Res. 2014, 74, 123–126. [Google Scholar] [CrossRef]
- Acharya, R.; Hwang, H.S.; Mostafiz, M.M.; Yu, Y.S.; Lee, K.Y. Susceptibility of various developmental stages of the fall armyworm, Spodoptera frugiperda, to entomopathogenic nematodes. Insects 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Bai, C.; Long, H. Laboratory virulence and field efficacy of different Heterorhabditis bacteriophora strains against Tetramoera schistaceana. Russ. J. Nematol. 2016, 24, 111–116. [Google Scholar]
- He, L.; Wang, T.; Chen, Y.; Ge, S.; Wyckhuys, K.A.G.; Wu, K. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 736–744. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology, 1st ed.; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 1997; pp. 281–324. [Google Scholar]
- Shapiro-Ilan, D.I.; Morales-Ramos, J.A.; Rojas, M.G. In vivo production of entomopathogenic nematodes. In Microbial-Based Biopesticides; Glare, T.R., Moran-Diez, M.E., Eds.; Humana Press: New York, NY, USA, 2016; pp. 137–158. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Wing, K.D.; Sacher, M.; Kagaya, Y.; Tsurubuchi, Y.; Mulderig, L.; Connair, M.; Schnee, M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot. 2000, 19, 537–545. [Google Scholar] [CrossRef]
- Bajracharya, A.S.R.; Bhat, B.; Sharma, P. Field efficacy of selected insecticides against fall armyworm, Spodoptera frugiperda (J.E. Smith) in maize. J. Plant Prot. Soc. 2020, 6, 127–133. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Yang, L. Investigation method and grading standard of Spodoptera frugiperda in maize. Yunnan Nongye Keji 2020, 3, 36–37. [Google Scholar]
- Neelima, C.; Nameirakam, L.; Devi, Y.K. The invasive fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in maize, status and infestation taken control under sustainable management: A review. J. Emerg. Technol. Innov. Res. 2020, 7, 1459–1471. [Google Scholar]
- de Waal, J.Y.; Addison, M.F.; Malan, A.P. Potential of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) for the control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in semi-field trials under South African conditions. Int. J. Pest Manag. 2018, 64, 102–109. [Google Scholar] [CrossRef]
- Kamali, S.; Karimi, J.; Koppenhöfer, A.M. New insight into the management of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae) with entomopathogenic nematodes. J. Econ. Entomol. 2018, 111, 112–119. [Google Scholar] [CrossRef]
- Mikaia, N. The susceptibility of mulberry moth to infection by entomopathogenic nematodes, Heterorhabditis bacteriophora and Steinernema carpocapsae. IOBC/WPRS Bull. 2013, 90, 313–316. [Google Scholar]
- Schroer, S.; Ehlers, R.U. Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella). Biol. Control 2005, 33, 81–86. [Google Scholar] [CrossRef]
- Vashisth, S.; Chandel, Y.S.; Chandel, R.S.; Sharma, P.K. Pathogenicity of Heterorhabditis nematodes isolated from north-western Himalaya against the larvae of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Ann. Soc. Entomol. Fr. 2017, 53, 204–210. [Google Scholar] [CrossRef]
- Fallet, P.; De Gianni, L.; Machado, R.A.R.; Bruno, P.; Bernal, J.S.; Karangwa, P.; Kajuga, J.; Waweru, B.; Bazagwira, D.; Degen, T.; et al. Comparative screening of Mexican, Rwandan and commercial entomopathogenic nematodes to be used against invasive fall armyworm, Spodoptera frugiperda. Insects 2022, 13, 205. [Google Scholar] [CrossRef]
- Shinde, S.P.; Ingole, D.B.; Biradar, V.K.; Gokte-Narkhedkar, N.; Lavhe, N.V.; Thube, S.H.; Shah, V.; Prasad, Y.G. Efficacy of native strains of entomopathogenic nematode, Heterorhabditis indica against the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) from India. Egypt. J. Biol. Pest Control 2022, 32, 141. [Google Scholar] [CrossRef]
- Lalramnghaki, H.C.; Lalremsanga, H.T.; Lalramchuani, M. Susceptibility of the fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae), to four species of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from Mizoram, North-Eastern India. Egypt. J. Biol. Pest Control 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, D.J.; Rodgers, A.; Gerard, P.J. Developing a strategy for using entomopathogenic nematodes to control the african black beetle (Heteronychus arator) in New Zealand pastures and investigating temperature constraints. Biol. Control 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Memari, Z.; Karimi, J.; Kamali, S.; Goldansaz, S.H.; Hosseini, M. Are entomopathogenic nematodes effective biological control agents against the carob moth, Ectomyelois ceratoniae? J. Nematol. 2016, 48, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Linden, C.F.; Fatouros, N.E.; Kammenga, J.E. The potential of entomopathogenic nematodes to control moth pests of ornamental plantings. Biol. Control 2022, 165, 104815. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Gouge, D.H.; Piggott, S.J.; Fife, J.P. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol. Control 2006, 38, 124–133. [Google Scholar] [CrossRef]
- Sharma, M.P.; Sharma, A.N.; Hussaini, S.S. Entomopathogenic nematodes, a potential microbial biopesticide: Mass production and commercialisation status—A mini review. Arch. Phytopathol. Plant Prot. 2011, 44, 855–870. [Google Scholar] [CrossRef]
- Garcia, L.C.; Raetano, C.G.; Leite, L.G. Application technology for the entomopathogenic nematodes Heterorhabditis indica and Steinernema sp. (Rhabditida: Heterorhabditidae and Steinernematidae) to Control Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Corn. Neotrop. Entomol. 2008, 37, 305–311. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Dolinski, C. Entomopathogenic nematode application technology. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 231–254. [Google Scholar]

| Grade | Grading Standard |
| 0 | Healthy |
| 1 | Leaves of the whole plant were infected by 0–5% (excluding 0%). The heart leaf, tassel and corn ears were not infected. |
| 2 | Leaves of the whole plant were infected by 5–15% (excluding 5%), or the heart leaf, tassel and corn ears were infected by 0–5% (excluding 0%). |
| 3 | Leaves over the whole plant were infected by 15–25% (excluding 15%), or the heart leaf, tassel and corn ears were infected by 5–15% (excluding 5%). |
| 4 | Leaves over the whole plant were infected by 25–50% (excluding 25%), or the heart leaf, tassel and corn ears were infected by 15–25% (excluding 15%). |
| 5 | Leaves over the whole plant were infected over 50%, or the heart leaf, tassel and corn ears were infected over 25%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Long, H.; Jin, T.; Peng, Z.; Sun, Y.; Feng, T. Potential of Entomopathogenic Nematode HbSD as a Candidate Biocontrol Agent against Spodoptera frugiperda. Insects 2023, 14, 2. https://doi.org/10.3390/insects14010002
Chen Y, Long H, Jin T, Peng Z, Sun Y, Feng T. Potential of Entomopathogenic Nematode HbSD as a Candidate Biocontrol Agent against Spodoptera frugiperda. Insects. 2023; 14(1):2. https://doi.org/10.3390/insects14010002
Chicago/Turabian StyleChen, Yuan, Haibo Long, Tao Jin, Zhengqiang Peng, Yanfang Sun, and Tuizi Feng. 2023. "Potential of Entomopathogenic Nematode HbSD as a Candidate Biocontrol Agent against Spodoptera frugiperda" Insects 14, no. 1: 2. https://doi.org/10.3390/insects14010002
APA StyleChen, Y., Long, H., Jin, T., Peng, Z., Sun, Y., & Feng, T. (2023). Potential of Entomopathogenic Nematode HbSD as a Candidate Biocontrol Agent against Spodoptera frugiperda. Insects, 14(1), 2. https://doi.org/10.3390/insects14010002






