Meta-Analysis of Herbicide Non-Target Effects on Pest Natural Enemies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Data Analysis
3. Results and Discussion
3.1. General Trends in Herbicide Non-Target Research
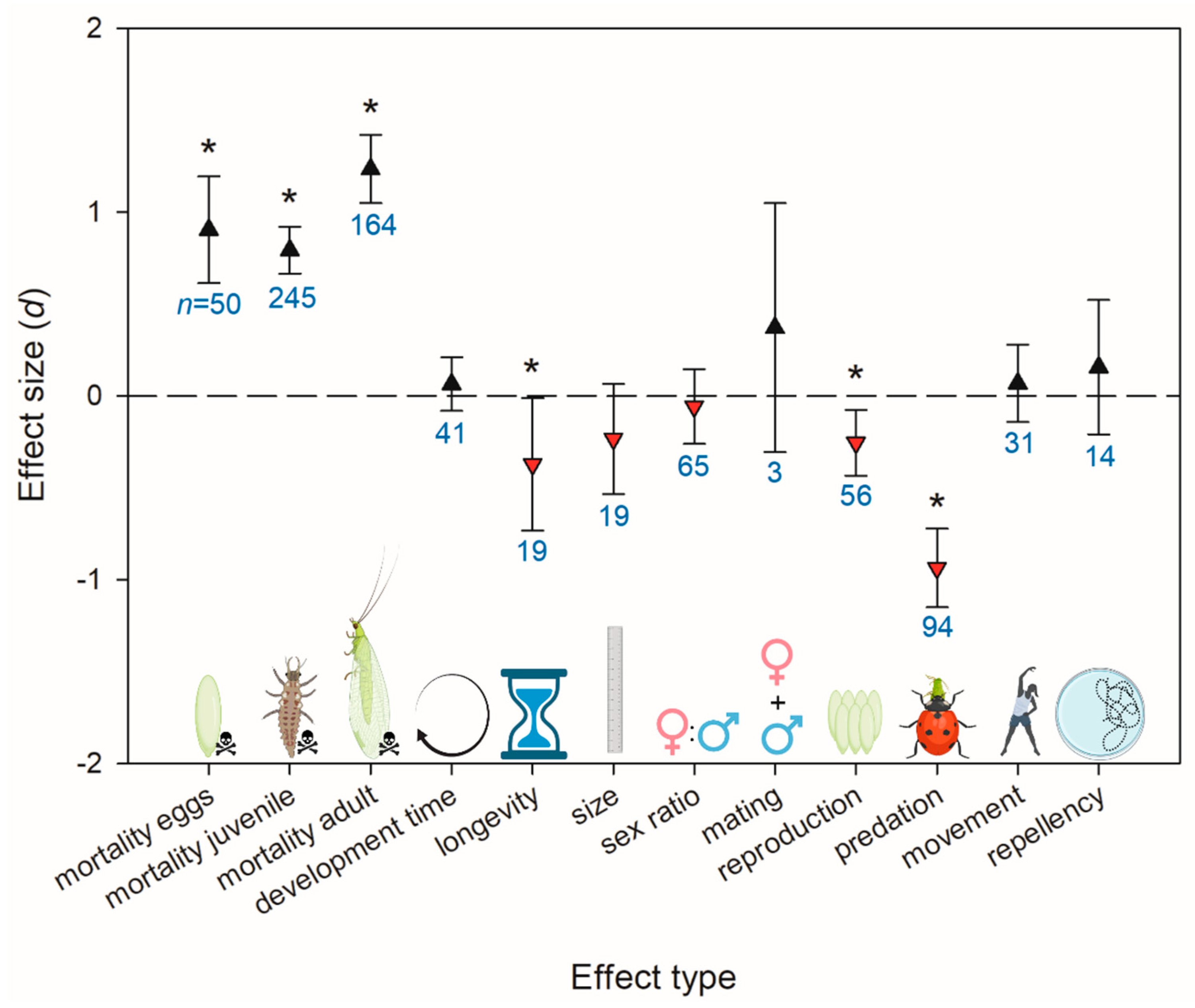
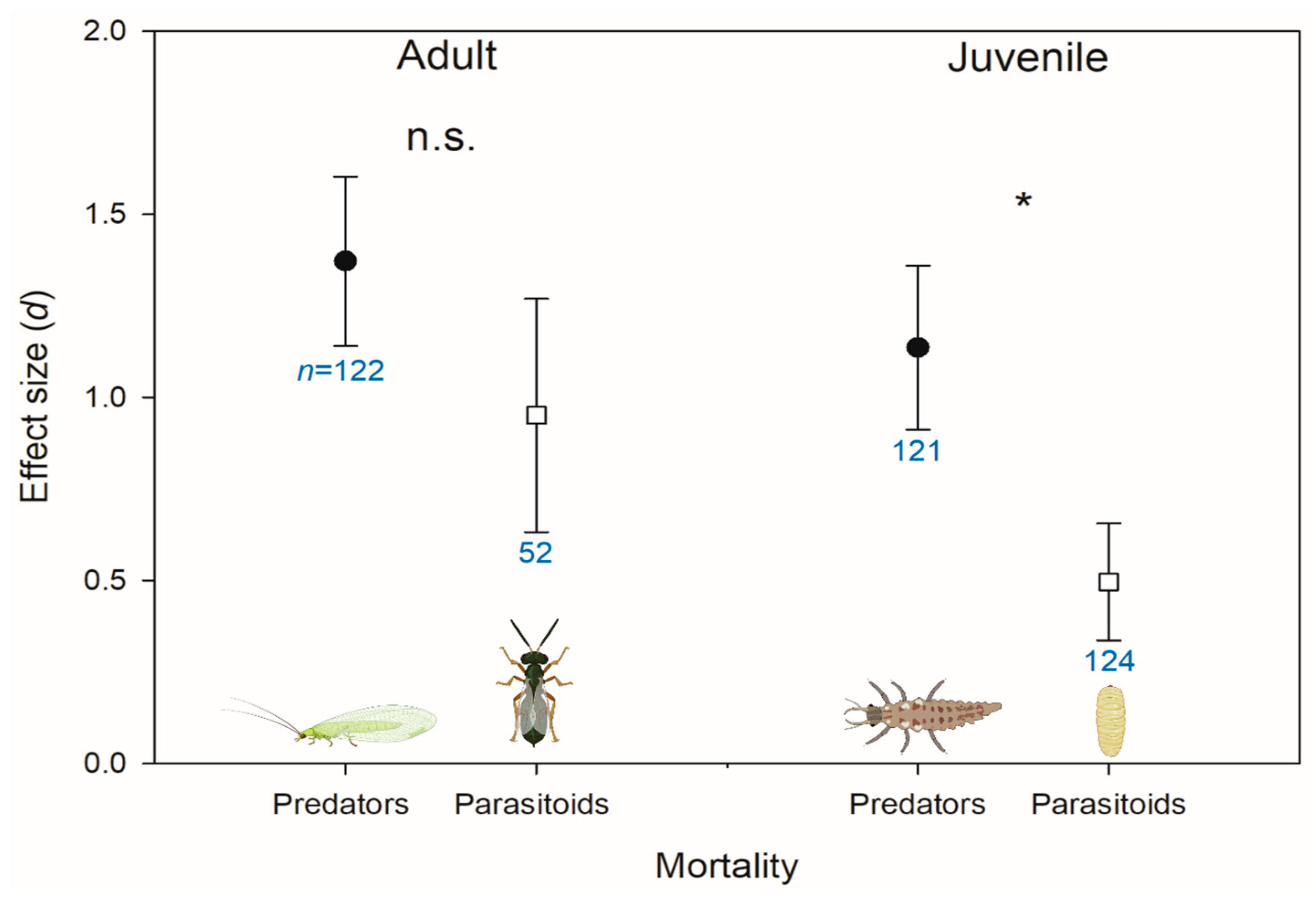
3.2. Herbicide Effects on Natural Enemies
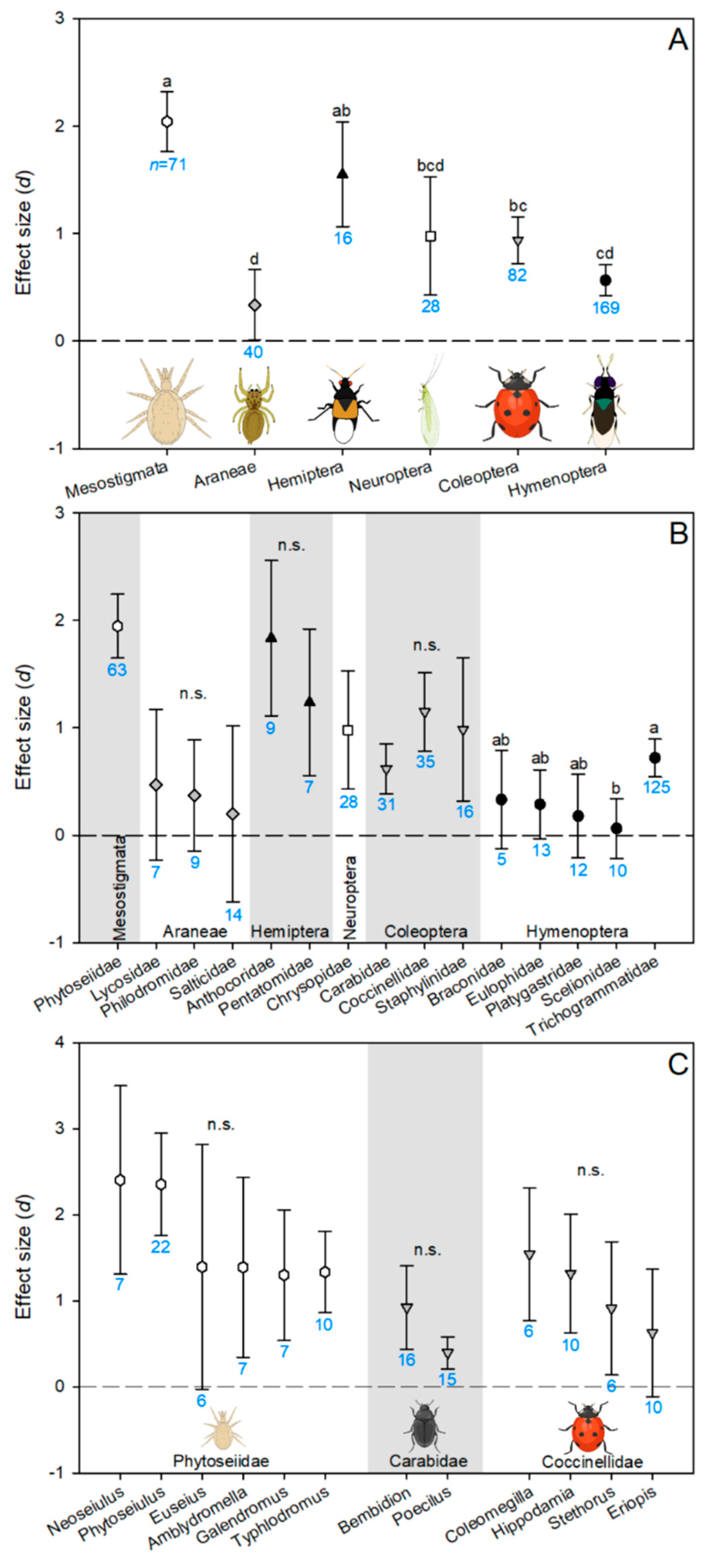
3.3. Taxonomic Variation in Herbicide Effects
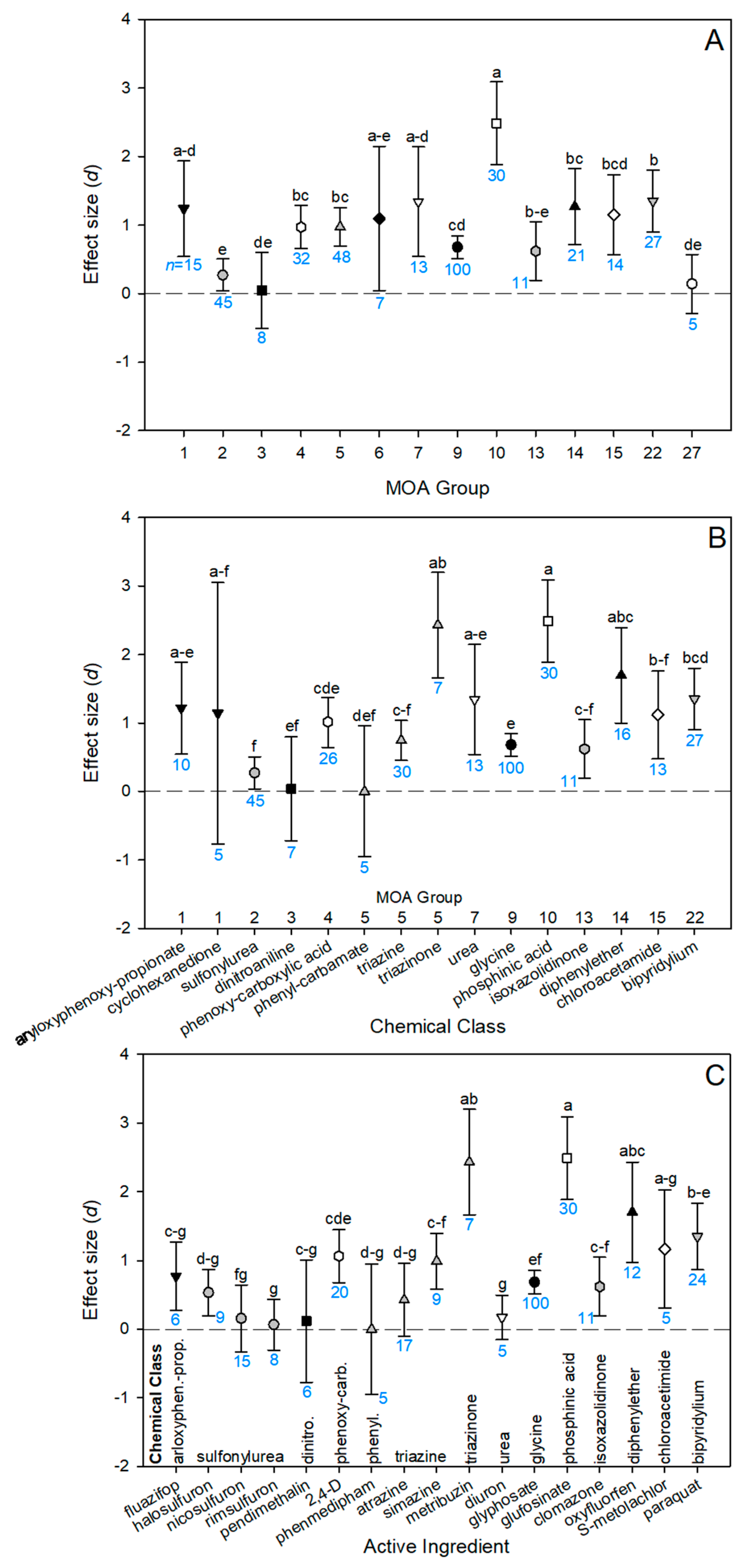
3.4. Variation between Herbicides
3.5. Glyphosate Alternatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theiling, K.M.; Croft, B.A. Pesticide side-effects on arthropod natural enemies: A database summary. Agric. Ecosyst. Environ. 1988, 21, 191–218. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Croft, B.A. Natural enemies and pesticides: An overview. In Arthropod Biological Control Agents and Pesticides; John Wiley & Sons, Inc.: New York, NY, USA, 1990; pp. 3–15. [Google Scholar]
- Pimentel, D. Ecological Effects of Pesticides on Non-Target Species; Executive Office of the President, Office of Science and Technology: Washington, DC, USA, 1971. [Google Scholar]
- Brittain, C.; Potts, S.G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Blacquiere, T.; Field, L.M.; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B 2014, 281, 20140558. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; Dicks, L.V.; Hokkanen, H.M.; Stenberg, J.A. Delivering integrated pest and pollinator management (IPPM). Trends Plant Sci. 2020, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.A.; Beers, E.H. Ecological selectivity: Modifying chemical control practices to preserve natural enemies. In Biological Control in Agricultural IPM Systems; Hoy, M.A., Herzog, D.C., Eds.; Academic Press: New York, NY, USA, 1985; pp. 103–122. [Google Scholar]
- Torres, J.B.; Bueno, A.d.F. Conservation biological control using selective insecticides—A valuable tool for IPM. Biol. Control 2018, 126, 53–64. [Google Scholar] [CrossRef]
- Doutt, R.L. Ecological considerations in chemical control: Implications to non-target invertebrates. Bull. Entomol. Soc. Am. 1964, 10, 83–88. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- FAO. License: CC BY-NC-SA 2.0 IGO. Available online: https://www.fao.org/faostat/ (accessed on 15 February 2022).
- Schmidt-Jeffris, R.A.; Beers, E.H.; Sater, C. Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest. Manag. Sci. 2021, 77, 4848–4862. [Google Scholar] [CrossRef]
- Croft, B.A. Pesticide effects on arthropod natural enemies: A database summary. In Arthropod Biological Control Agents and Pesticides; John Wiley & Sons, Inc.: New York, NY, USA, 1990; pp. 17–46. [Google Scholar]
- Fountain, M.T.; Medd, N. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica 2015, 43, 657–667. [Google Scholar] [CrossRef]
- Pekar, S. Spiders (Araneae) in the pesticide world: An ecotoxicological review. Pest Manag. Sci. 2012, 68, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Ken, C.F.; Ashworth, M.B. Farming without glyphosate? Plants 2020, 9, 96. [Google Scholar] [CrossRef]
- Pleasants, J.M.; Oberhauser, K.S. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Conserv. Diver. 2013, 6, 135–144. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.-L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef]
- Abraham, J.; Benhotons, G.S.; Krampah, I.; Tagba, J.; Amissah, C.; Abraham, J.D. Commercially formulated glyphosate can kill non-target pollinator bees under laboratory conditions. Entomol. Exp. Appl. 2018, 166, 695–702. [Google Scholar] [CrossRef]
- Battisti, L.; Potrich, M.; Sampaio, A.R.; de Castilhos Ghisi, N.; Costa-Maia, F.M.; Abati, R.; Dos Reis Martinez, C.B.; Sofia, S.H. Is glyphosate toxic to bees? A meta-analytical review. Sci. Total Environ. 2021, 767, 145397. [Google Scholar] [CrossRef]
- Stenoien, C.; Nail, K.R.; Zalucki, J.M.; Parry, H.; Oberhauser, K.S.; Zalucki, M.P. Monarchs in decline: A collateral landscape-level effect of modern agriculture. Insect Sci. 2018, 25, 528–541. [Google Scholar] [CrossRef]
- Beckie, H.J. Herbicide-resistant weed management: Focus on glyphosate. Pest Manag. Sci. 2011, 67, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar]
- Jansen, J.-P. Pest Select Database: A new tool to use selective pesticides for IPM. Comm. Appl. Biol. Sci. 2013, 78, 115–119. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer 4.2. Available online: https://automeris.io/WebPlotDigitizer (accessed on 1 January 2019).
- Croft, B.A. Factors affecting susceptibility. In Arthropod Biological Control Agents and Pesticides; John Wiley & Sons, Inc.: New York, NY, USA, 1990; pp. 71–100. [Google Scholar]
- Leccia, F.; Kysilková, K.; Kolářová, M.; Hamouzová, K.; Líznarová, E.; Korenko, S. Disruption of the chemical communication of the European agrobiont ground-dwelling spider Pardosa agrestis by pesticides. J. Appl. Entomol. 2016, 140, 609–616. [Google Scholar] [CrossRef]
- Michalková, V.; Pekár, S. How glyphosate altered the behaviour of agrobiont spiders (Araneae: Lycosidae) and beetles (Coleoptera: Carabidae). Biol. Control 2009, 51, 444–449. [Google Scholar] [CrossRef]
- Godfrey, J.A.; Rypstra, A.L. Impact of an atrazine-based herbicide on an agrobiont wolf spider. Chemosphere 2018, 201, 459–465. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J.; Poisot, T. Open MEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Converting among effect sizes. In Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: West Sussex, UK, 2009; pp. 45–49. [Google Scholar]
- Schmidt-Jeffris, R.A.; Moretti, E.A.; Bergeron, P.E.; Zilnik, G. Nontarget impacts of herbicides on spiders in orchards. J. Econ. Entomol. 2022, 115, 65–73. [Google Scholar] [CrossRef]
- Bergeron, P.; Schmidt-Jeffris, R. Herbicides harm key orchard predatory mites. Insects 2023, 14, 480. [Google Scholar] [CrossRef]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Neonicotinoid insecticides negatively affect performance measures of non-target terrestrial arthropods: A meta-analysis. Ecol. Appl. 2018, 28, 1232–1244. [Google Scholar] [CrossRef]
- Naranjo, S.E. Effects of GE crops on non-target organisms. Plant Biotechnol. Exp. Future Prospect. 2021, 127–144. [Google Scholar]
- Jervis, M. Functional and evolutionary aspects of mouthpart structure in parasitoid wasps. Biol. J. Linn. Soc. 1998, 63, 461–496. [Google Scholar] [CrossRef]
- Overton, K.; Hoffmann, A.A.; Reynolds, O.L.; Umina, P.A. Toxicity of insecticides and miticides to natural enemies in Australian grains: A review. Insects 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Pekar, S.; Haddad, C.R. Can agrobiont spiders (Araneae) avoid a surface with pesticide residues? Pest Manag. Sci. 2005, 61, 1179–1185. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A. Nontarget pesticide impacts on pest natural enemies: Progress and gaps in current knowledge. Curr. Opin. Insect Sci. 2023, 58, 101056. [Google Scholar] [CrossRef]
- Gerson, U.; Smiley, R.L.; Ochoa, R. The effect of agricultural chemicals on acarine biocontrol agents. In Mites (Acari) for Pest Control; Blackwell Science: Oxford, UK, 2003; pp. 367–383. [Google Scholar]
- Carmo, E.L.; Bueno, A.F.; Bueno, R.C.O.F. Pesticide selectivity for the insect egg parasitoid Telenomus remus. BioControl 2010, 55, 455–464. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.A.; Cutulle, M.A. Non-target effects of herbicides on Tetranychus urticae and its predator, Phytoseiulus persimilis: Implications for biological control. Pest Manag. Sci. 2019, 75, 3226–3234. [Google Scholar] [CrossRef]
- Mullin, C.A. Effects of ‘inactive’ ingredients on bees. Curr. Opin. Insect Sci. 2015, 10, 194–200. [Google Scholar] [CrossRef]
- Niedobová, J.; Skalský, M.; Ouředníčková, J.; Michalko, R. Glyphosate based formulation with tank mixing adjuvants alter predatory behaviour of the ground dwelling lycosid spider Pardosa sp. IOBC/WPRS Bull. 2019, 143, 20–24. [Google Scholar]
- Niedobová, J.; Skalsky, M.; Ouředníčková, J.; Michalko, R.; Bartošková, A. Synergistic effects of glyphosate formulation herbicide and tank-mixing adjuvants on Pardosa spiders. Environ. Pollut. 2019, 249, 338–344. [Google Scholar] [CrossRef]
- Rakes, M.; Pasini, R.A.; Morais, M.C.; Araujo, M.B.; de Bastos Pazini, J.; Seidel, E.J.; Bernardi, D.; Grutzmacher, A.D. Pesticide selectivity to the parasitoid Trichogramma pretiosum: A pattern 10-year database and its implications for Integrated Pest Management. Ecotoxicol. Environ. Saf. 2021, 208, 111504. [Google Scholar] [CrossRef] [PubMed]
- Kniss, A.R. Long-term trends in the intensity and relative toxicity of herbicide use. Nat. Commun. 2017, 8, 14865. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.A. The Bipyridinum Herbicides; Academic Press: London, UK, 1980. [Google Scholar]
- Jansen, J.-P.; Hautier, L.; Mabon, N.; Schiffers, B. Pesticides selectivity list to beneficial arthropods in four field vegetable crops. IOBC/WPRS Bull. 2008, 35, 66–77. [Google Scholar]
- Hassan, S.A.; Hafes, B.; Degrande, P.E.; Herai, K. The side-effects of pesticides on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae), acute dose-response and persistence tests. J. Appl. Entomol. 1998, 122, 569–573. [Google Scholar] [CrossRef]
- Freydier, L.; Lundgren, J.G. Unintended effects of the herbicides 2,4-D and dicamba on lady beetles. Ecotoxicology 2016, 25, 1270–1277. [Google Scholar] [CrossRef]
- Metzger, J.A.; Pfeiffer, D.G. Topical toxicity of pesticides used in Virginia vineyards to the predatory mite, Neoseiulus fallacis (Garman). J. Entomol. Sci. 2002, 37, 329–337. [Google Scholar] [CrossRef]
- Pfeiffer, D.G. Effects of field applications of paraquat on densities of Panonychus ulmi (Koch) and Neoseiulus fallacis (Garman). J. Agric. Entomol. 1986, 3, 322–325. [Google Scholar]
- House, G.J.; Stinner, B.R. Arthropods in no-tillage soybean agroecosystems: Community composition and ecosystem interactions. Environ. Manag. 1983, 7, 23–28. [Google Scholar] [CrossRef]
- Rowen, E.; Regan, K.; Barbercheck, M.; Tooker, J.F. Is tillage beneficial or detrimental for invertebrate pest management? A meta-analysis. Agric. Ecosyst. Environ. 2020, 294, 106849. [Google Scholar] [CrossRef]
- House, G.J.; Brust, G.E. Ecology of low-input, no-tillage agroecosystems. Agric. Ecosyst. Environ. 1989, 27, 331–345. [Google Scholar] [CrossRef]
- Dassou, A.G.; Tixier, P. Response of pest control by generalist predators to local-scale plant diversity: A meta-analysis. Ecol. Evol. 2016, 6, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Croft, B.A. Ecological influences. In Arthropod Biological Control Agents and Pesticides; John Wiley & Sons: New York, NY, USA, 1990; pp. 185–218. [Google Scholar]
- Bàrberi, P.; Burgio, G.; Dinelli, G.; Moonen, A.C.; Otto, S.; Vazzana, C.; Zanin, G. Functional biodiversity in the agricultural landscape: Relationships between weeds and arthropod fauna. Weed Res. 2010, 50, 388–401. [Google Scholar] [CrossRef]
- Norris, R.F.; Kogan, M. Ecology of interactions between weeds and arthropods. Annu. Rev. Entomol. 2005, 50, 479–503. [Google Scholar] [CrossRef]
- Adams, J.B. Effects of spraying 2,4-D amine on coccinellid larvae. Can. J. Zool. 1960, 38, 285–288. [Google Scholar] [CrossRef]
- Addison, P.J.; Barker, G.M. Effect of various pesticides on the non-target species Microctonus hyperodae, a biological control agent of Listronotus bonariensis. Entomol. Exp. Appl. 2006, 119, 71–79. [Google Scholar] [CrossRef]
- Ahn, Y.-J.; Kim, Y.-J.; Yoo, J.-K. Toxicity of the herbicide glufosinate-ammonium to predatory insects and mites of Tetranychus urticae (Acari: Tetranychidae) under laboratory conditions. J. Econ. Entomol. 2001, 94, 157–161. [Google Scholar] [CrossRef]
- Alhewairini, S.S.; Al-Azzazy, M.M. Evaluation of the side effects of oxamyl and hymexazol on five species of soil-dwelling predatory mites. Pak. J. Agri. Sci. 2019, 56, 531–536. [Google Scholar]
- Bastos, C.S.; de Almeida, R.P.; Suinaga, F.A. Selectivity of pesticides used on cotton (Gossypium hirsutum) to Trichogramma pretiosum reared on two laboratory-reared hosts. Pest Manag. Sci. 2006, 62, 91–98. [Google Scholar] [CrossRef]
- Behrend, J.E.; Rypstra, A.L. Contact with a glyphosate-based herbicide has long-term effects on the activity and foraging of an agrobiont wolf spider. Chemosphere 2018, 194, 714–721. [Google Scholar] [CrossRef]
- Benamú, M.A.; Schneider, M.I.; Sanchez, N.E. Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 2010, 78, 871–876. [Google Scholar] [CrossRef]
- Bernard, M.B.; Cole, P.; Kobelt, A.; Horne, P.A.; Altmann, J.; Wratten, S.D.; Yen, A.L. Reducing the impact of pesticides on biological control in Australian vineyards: Pesticide mortality and fecundity effects on an indicator species, the predatory mite Euseius victoriensis (Acari: Phytoseiidae). J. Econ. Entomol. 2010, 103, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Boller, E.F.; Janser, E.; Potter, C. Evaluation of side-effects of vineyard herbicides on Tetranychus urticae and its predator Typhlodromus pyri under laboratory and semi-field conditions. J. Plant Dis. Protect. 1984, 91, 561–568. [Google Scholar]
- Bueno, A.F.; Bueno, R.C.O.F.; Parra, J.R.P.; Vieira, S.S. Effects of pesticides used in soybean crops to the egg parasitoid Trichogramma pretiosum. Ciênc. Rural 2008, 38, 1495–1503. [Google Scholar] [CrossRef]
- Carmo, E.L.; Bueno, A.F.; Bueno, R.C.O.F.; Viera, S.S.; Gobii, A.L.; Vasco, R.F. Selectivity of different pesticides used in soybean to the eggs parasitoid Telenomus remus. Ciênc. Rural 2009, 39, 2293–2300. [Google Scholar] [CrossRef]
- Carmo, E.L.; Bueno, A.F.; Bueno, R.C.O.F.; Viera, S.S.; Goulart, M.M.P.; Carneiro, T.R. Selectivity of pesticides used in soybean crops to Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae) pupae. Arq. Inst. Biol. 2010, 77, 282–290. [Google Scholar] [CrossRef]
- Castilhos, R.V.; Grützmacher, A.D.; Nava, D.E.; Zotti, M.J.; Siqueira, P.R.B.; Spagnol, D. Selectivity of pesticides used in peach orchards on the larval stage of the predator Chrysoperla externa (Hagen). Semin. Cienc. Agrar. 2013, 34, 3585–3596. [Google Scholar] [CrossRef]
- Castilhos, R.V.; Grützmacher, A.D.; Siqueira, P.R.B.; de Moraes, Í.L.; Gauer, C.J. Selectivity of pesticides used in peach orchards on eggs and pupae of the predator Chrysoperla externa. Ciênc. Rural 2014, 44, 1921–1928. [Google Scholar] [CrossRef][Green Version]
- Cruz, R.A.; Zanuncio, J.C.; Lacerda, M.C.; Wilcken, C.F.; Fernandes, F.L.; Tavares, W.S.; Soares, M.A.; Sediyama, C.S. Side-effects of pesticides on the generalist endoparasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae). Sci. Rep. 2017, 7, 10064. [Google Scholar] [CrossRef]
- Czarnecka, M.; Parma, P.; Kulec-Ploszczyca, E. Total effects of selected plant protection products applied to different natural substrates on the predatory mite Typhlodromus pyri Sch. IOBC/WPRS Bull. 2014, 103, 51–60. [Google Scholar]
- Evans, S.C.; Shaw, E.M.; Rypstra, A.L. Exposure to a glyphosate-based herbicide affects agrobiont predatory arthropod behaviour and long-term survival. Ecotoxicology 2010, 19, 1249–1257. [Google Scholar] [CrossRef]
- Giolo, F.P.; Grützmacher, A.D.; Procópio, S.O.; Manzoni, C.G.; Lima, C.A.B.; Nörnberg, S.D. Side-effects of glyphosate formulations on Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Planta Daninha 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Giolo, F.P.; Grützmacher, A.D.; Manzoni, C.G.; Härter, W.R.; Castilhos, R.V.; Müller, C. Toxicity of pesticides used in peach production on the egg parasitoids Trichogramma atopovirilia Oatman & Platner, 1983 (Hymenoptera: Trichogrammatidae). Ciênc. Rural 2007, 37, 308–314. [Google Scholar]
- Giolo, F.P.; Grützmacher, A.D.; Manzoni, C.G.; Nörnberg, S.D.; Härter, W.d.R.; Castilhos, R.V. Selectivity of pesticides used in peach production to immature stages of Trichogramma atopovirilia Oatman & Platner, 1983 (Hymenoptera: Trichogrammatidae). Ciênc. Rural 2008, 38, 1220–1226. [Google Scholar]
- Hassan, S.A.; Klingauf, F.; Shahin, F. Role of Chrysopa carnea as an aphid predator on sugar beet and the effect of pesticides. Z. Angew. Entomol. 1985, 100, 163–174. [Google Scholar] [CrossRef]
- Haughton, A.J.; Bell, J.R.; Wilcox, A.; Boatman, N.D. The effect of the herbicide glyphosate on non-target spiders: Part I. Direct effects on Lepthyphantes tenuis under laboratory conditions. Pest Manag. Sci. 2001, 57, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Helsen, H.; Booij, K. Effects of amitrole (3-amino-1,2,4-triazole) on the common earwig Forficula auricularia L. (Dermaptera: Forficulidae). IOBC/WPRS Bull. 2013, 91, 143–146. [Google Scholar]
- Kegel, B. Laboratory experiments on the side effects of selected herbicides and insecticides on the larvae of three sympatric Poecilus-species (Col., Carabidae). J. Appl. Entomol. 1989, 108, 144–155. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, H.; Ruberson, J.R. Lethal and behavioral effects of selected novel pesticides on adults of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2015, 71, 1640–1648. [Google Scholar] [CrossRef]
- Khan, M.A.; Ruberson, J.R. Lethal effects of selected novel pesticides on immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2017, 73, 2465–2472. [Google Scholar] [CrossRef]
- Khan, M.A. Lethal and parasitism effects of selected novel pesticides on adult Trichogramma chilonis (Hymenoptera: Trichogrammatidae). J. Plant Dis. Protect. 2020, 127, 81–90. [Google Scholar] [CrossRef]
- Korenko, S.; Niedobová, J.; Kolářová, M.; Hamouzová, K.; Kysilková, K.; Michalko, R. The effect of eight common herbicides on the predatory activity of the agrobiont spider Pardosa agrestis. BioControl 2016, 61, 507–517. [Google Scholar] [CrossRef]
- Lacava, M.; Garcia, L.F.; Viera, C.; Michalko, R. The pest-specific effects of glyphosate on functional response of a wolf spider. Chemosphere 2021, 262, 127785. [Google Scholar] [CrossRef] [PubMed]
- Lacoume, S.; Bressac, C.; Chevrier, C. Male hypofertility induced by Paraquat consumption in the non-target parasitoid Anisopteromalus calandrae (Hymenoptera: Pteromalidae). Biol. Control 2009, 49, 214–218. [Google Scholar] [CrossRef]
- Leite, G.L.D.; Paulo, P.D.; Zanuncio, J.C.; Alvarenga, A.C.; Soares, M.A.; Tavares, W.S.; Tuffi-Santos, L.D.; Spínola-Filho, P.R.C. Effects of atrazine-based herbicide on emergence and sex ratio of Trichogrammatidae (Hymenoptera). Fla. Entomol. 2015, 98, 899–902. [Google Scholar] [CrossRef]
- Leite, G.L.; de Paulo, P.D.; Zanuncio, J.C.; Tavares, W.S.; Alvarenga, A.C.; Dourado, L.R.; Bispo, E.P.; Soares, M.A. Herbicide toxicity, selectivity and hormesis of nicosulfuron on 10 Trichogrammatidae (Hymenoptera) species parasitizing Anagasta ( = Ephestia) kuehniella (Lepidoptera: Pyralidae) eggs. J. Environ. Sci. Health B 2017, 52, 70–76. [Google Scholar] [CrossRef]
- Leite, G.L.D.; de Paulo, P.D.; Zanuncio, J.C.; Tavares, W.S.; Alvarenga, A.C.; Dourado, L.R.; Bispo, E.P.R.; Soares, M.A. Nicosulfuron plus atrazine herbicides and Trichogrammatidae (Hymenoptera) in no-choice test: Selectivity and hormesis. Bull. Environ. Contam. Toxicol. 2017, 99, 589–594. [Google Scholar] [CrossRef]
- Leite, G.L.D.; Paulo, P.D.; Tuffi-Santos, L.D.; Alvarenga, A.C.; Soares, M.A.; Dourado, L.R.; Bispo, E.P.R. Efficacy of Trichogrammatidae species (Hymenoptera) submitted to the herbicide glyphosate. Planta Daninha 2019, 37, e019216439. [Google Scholar] [CrossRef]
- Maia, J.B.; Carvalho, G.A.; Medina, P.; Garzón, A.; Gontijo, P.C.; Viñuela, E. Lethal and sublethal effects of pesticides on Chrysoperla carnea larvae (Neuroptera: Chrysopidae) and the influence of rainfastness in their degradation pattern over time. Ecotoxicology 2016, 25, 845–855. [Google Scholar] [CrossRef]
- Mandal, S.K.; Debnath, M.; Panja, S. Effect of some herbicides on egg parasitism and development of Trichogramma chilonis Ishii (Trichogrammatidae: Hymenoptera). J. Crop Weed 2006, 2, 26–28. [Google Scholar]
- Mansour, F.; Nentwig, W. Effects of agrochemical residues on four spider taxa: Laboratory methods for pesticide tests with web-building spiders. Phytoparasitica 1988, 16, 317–326. [Google Scholar] [CrossRef]
- Manzoni, C.G.; Grützmacher, A.D.; Giolo, F.P.; Härter, W.; Müller, C. Side effects of pesticides used in integrated production of apple in adults of Trichogramma pretiosum. Pesq. Agropec. Bras. 2006, 41, 1461–1467. [Google Scholar] [CrossRef]
- Menezes, C.W.G.; Santos, J.B.; Assis Júnior, S.L.; Fonseca, A.J.; França, A.C.; Soares, M.A.; Fernandes, A.F. Selectivity of atrazin and nicosulfurom to Podisus nigrispinus (Heteroptera: Pentatomidae). Planta Daninha 2012, 30, 327–334. [Google Scholar] [CrossRef]
- Menezes, C.W.G.; Soares, M.A.; Santos, J.B.; Assis Júnior, S.L.; Fonseca, A.J.; Zanuncio, J.C. Reproductive and toxicological impacts of herbicides used in Eucalyptus culture in Brazil on the parasitoid Palmistichus elaeisis (Hymenoptera: Eulophidae). Weed Res. 2012, 52, 520–525. [Google Scholar] [CrossRef]
- Menezes, C.W.G.; Soares, M.A.; Fonseca, A.J.; Santos, J.B.; Camilo, S.S.; Zanuncio, J.C. Palmistichus elaeisis (Hymenoptera: Eulophidae) as an indicator of toxicity of herbicides registered for corn in Brazil. Chil. J. Agric. Res. 2014, 74, 361–365. [Google Scholar] [CrossRef]
- Michaud, J.P.; Vargas, G. Relative toxicity of three wheat herbicides to two species of Coccinellidae. Insect Sci. 2010, 17, 434–438. [Google Scholar] [CrossRef]
- Mirande, L.; Haramboure, M.; Smagghe, G.; Pineda, S.; Schneider, M.I. Side-effects of glyphosate on the life parameters of Eriopis connexa (Coleoptera: Coccinellidae) in Argentina. Comm. Appl. Biol. Sci. 2010, 75, 367–372. [Google Scholar]
- Niedobová, J.; Krištofová, L.; Michalko, R.; Hula, V.; Kýnický, J.; Brtnický, M. Effects of glufosinate-ammonium herbicide and pod sealant on spider Pardosa agrestis. J. Appl. Entomol. 2019, 143, 196–203. [Google Scholar] [CrossRef]
- Nörnberg, S.D.; Grützmacher, A.D.; Giolo, F.P.; Júnior, G.J.E.; Lima, C.A.B.; Grützmacher, D.D. Selectivity of glyphosate formulations applied on immature stages of Trichogramma pretiosum. Planta Daninha 2008, 26, 611–617. [Google Scholar] [CrossRef]
- Oliveira, H.N.; Antigo, M.R.; Carvalho, G.A.; Glaeser, D.F. Effect of selectivity of herbicides and plant growth regulators used in sugarcane crops on immature stages of Trichogramma galloi (Hymenoptera: Trichogrammatidae). Planta Daninha 2014, 32, 125–131. [Google Scholar] [CrossRef]
- Oliver, J.B.; Reding, M.E.; Moyseenko, J.J.; Klein, M.G.; Mannion, C.M.; Bishop, B. Survival of adult Tiphia vernalis (Hymenoptera: Tiphiidae) after insecticide, fungicide, and herbicide exposure in laboratory bioassays. J. Econ. Entomol. 2006, 99, 288–294. [Google Scholar] [CrossRef]
- Oomen, P.A.; Romeijn, G.; Wiegers, G.L. Side-effects of 100 pesticides on the predatory mite Phytoseiulus persimilis, collected and evaluated according to the EPPO guideline. EPPO Bull. 1991, 21, 701–712. [Google Scholar] [CrossRef]
- Overmeer, W.P.J.; Van Zon, A.Q. A standarized method for testing the side effects of pesticides on the predacious mite, Amblyseius potentillae (Acarina: Phytoseiidae). Entomophaga 1982, 27, 357–364. [Google Scholar] [CrossRef]
- Pasini, R.A.; Pazini, J.B.; Grützmacher, A.D.; Rakes, M.; Armas, F.S. Comparative selectivity of herbicides used in wheat crop on the predators Chrysoperla externa and Eriopis connexa. Planta Daninha 2018, 36, e018179968. [Google Scholar] [CrossRef]
- Pekár, S. Susceptibility of the spider Theridion impressum to 17 pesticides. J. Pest Sci. 2002, 75, 51–55. [Google Scholar] [CrossRef]
- Pekár, S.; Beneš, J. Aged pesticide residues are detrimental to agrobiont spiders (Araneae). J. Appl. Entomol. 2008, 132, 614–622. [Google Scholar] [CrossRef]
- Pontes, J.P.; Leite, G.L.D.; Bispo, E.P.R.; Tavares, W.S.; Menezes, C.W.G.; Wilcken, C.F.; Zanuncio, J.C. A glyphosate-based herbicide in a free-choice test on parasitism, emergence, and female-biased sex ratio of 10 Trichogrammatidae. J. Plant Dis. Protect. 2020, 127, 73–79. [Google Scholar] [CrossRef]
- Rittman, S.; Wrinn, K.M.; Evans, S.C.; Webb, A.W.; Rypstra, A.L. Glyphosate-based herbicide has contrasting effects on prey capture by two co-occurring wolf spider species. J. Chem. Ecol. 2013, 39, 1247–1253. [Google Scholar] [CrossRef]
- Samsøe-Petersen, L. Laboratory method for testing side-effects of pesticides on the rove beetle Aleochara bilineata—Adults. Entomophaga 1987, 32, 73–81. [Google Scholar] [CrossRef]
- Santos, T.P.; Menezes, C.W.G.; Batista, C.H.; Brito, E.S.G.; Tavares, W.S.; Zanuncio, J.C. Selectivity of registered pesticides for the corn crop on immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Ciênc. Agrotecnol. 2019, 43, e020719. [Google Scholar] [CrossRef]
- Saska, P.; Skuhrovec, J.; Lukas, J.; Vlach, M.; Chi, H.; Tuan, S.J.; Honek, A. Treating prey with glyphosate does not alter the demographic parameters and predation of the Harmonia axyridis (Coleoptera: Coccinellidae). J. Econ. Entomol. 2017, 110, 392–399. [Google Scholar]
- Schneider, M.I.; Sanchez, N.; Pineda, S.; Chi, H.; Ronco, A. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): Ecological approach. Chemosphere 2009, 76, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Stecca, C.S.; Bueno, A.F.; Pasini, A.; Silva, D.M.; Andrade, K.; Filho, D.M.Z. Side-effects of glyphosate to the parasitoid Telenomus remus Nixon (Hymenoptera: Platygastridae). Neotrop. Entomol. 2016, 45, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Stefanello Júnior, G.J.; Grützmacher, A.D.; Grützmacher, D.D.; Lima, C.A.B.; Dalmozo, D.O.; Paschoal, M.D.F. Selectivity of herbicides registered on corn to Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Planta Daninha 2008, 26, 343–351. [Google Scholar] [CrossRef]
- Stefanello Júnior, G.J.; Grutzmacher, A.D.; Pasini, R.A.; Bonez, C.; Moreira, D.C.; Spagnol, D. Selectivity of herbicides registered for corn at the immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Planta Daninha 2011, 29, 1069–1077. [Google Scholar] [CrossRef]
- Tahir, H.M.; Butt, A.; Khan, S.Y.; Ahmad, K.R.; Arshad, M.; Nawaz, S. Effects of acetochlor (herbicide) on the survival and avoidance behaviour of spiders. Afr. J. Biotechnol. 2011, 10, 6265–6268. [Google Scholar]
- Tahir, H.M.; Noor, T.; Bhatti, M.F.; Bano, M.; Butt, A.; Alam, I.; Arshad, M.; Mukhtar, M.K.; Khan, S.Y.; Ahmed, K.R.; et al. Acetochlor application at field-rate compromises the locomotion of the jumping spider Plexippus paykulli (Araneae: Salticidae). Afr. J. Agric. Res. 2012, 7, 3329–3333. [Google Scholar]
- Tanigoshi, L.K.; Congdon, B.D. Laboratory toxicity of commonly-used pesticides in California citriculture to Euseius hibisci (Chant) (Acarina: Phytoseiidae). J. Econ. Entomol. 1983, 76, 247–250. [Google Scholar] [CrossRef]
- Valente, E.C.N.; Broglio, S.M.F.; Dias-Pini, N.S.; Micheletti, L.B.; Lima, A.S.T.; Barbosa, T. Selectivity of pesticides to egg parasitoid in sugarcane. Sugar Tech 2018, 20, 765–769. [Google Scholar] [CrossRef]
- Walters, P.J. Susceptibility of three Stethorus spp. (Coleoptera: Coccinellidae) to selected chemicals used in N.S.W. apple orchards. J. Aust. Entomol. Soc. 1976, 15, 49–52. [Google Scholar] [CrossRef]
- Wrinn, K.M.; Evans, S.C.; Rypstra, A.L. Predator cues and an herbicide affect activity and emigration in an agrobiont wolf spider. Chemosphere 2012, 87, 390–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilnik, G.; Bergeron, P.E.; Chuang, A.; Diepenbrock, L.; Hanel, A.; Middleton, E.; Moretti, E.; Schmidt-Jeffris, R. Meta-Analysis of Herbicide Non-Target Effects on Pest Natural Enemies. Insects 2023, 14, 787. https://doi.org/10.3390/insects14100787
Zilnik G, Bergeron PE, Chuang A, Diepenbrock L, Hanel A, Middleton E, Moretti E, Schmidt-Jeffris R. Meta-Analysis of Herbicide Non-Target Effects on Pest Natural Enemies. Insects. 2023; 14(10):787. https://doi.org/10.3390/insects14100787
Chicago/Turabian StyleZilnik, Gabriel, Paul E. Bergeron, Angela Chuang, Lauren Diepenbrock, Aldo Hanel, Eric Middleton, Erica Moretti, and Rebecca Schmidt-Jeffris. 2023. "Meta-Analysis of Herbicide Non-Target Effects on Pest Natural Enemies" Insects 14, no. 10: 787. https://doi.org/10.3390/insects14100787
APA StyleZilnik, G., Bergeron, P. E., Chuang, A., Diepenbrock, L., Hanel, A., Middleton, E., Moretti, E., & Schmidt-Jeffris, R. (2023). Meta-Analysis of Herbicide Non-Target Effects on Pest Natural Enemies. Insects, 14(10), 787. https://doi.org/10.3390/insects14100787






