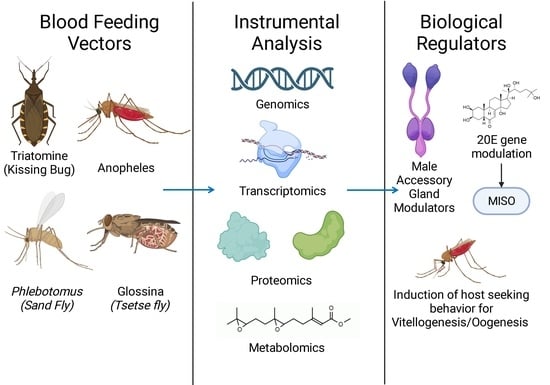
Current Status of Omics Studies Elucidating the Features of Reproductive Biology in Blood-Feeding Insects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Genomic Studies
3. Transcriptomic Studies
4. Proteomic Studies
5. Metabolomic Studies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Vector-Borne Diseases. Key Facts. World Health Organization. March 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 1 September 2023).
- Erguler, K.; Pontiki, I.; Zittis, G.; Proestos, Y.; Christodoulou, V.; Tsirigotakis, N.; Antoniou, M.; Kasap, O.E.; Alten, B.; Lelieveld, J. A climate-driven and field data-assimilated population dynamics model of sand flies. Sci. Rep. 2019, 9, 2469. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288. [Google Scholar] [CrossRef] [PubMed]
- Halani, S.; Tombindo, P.E.; O’Reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.; Longbottom, J.; Gleave, K.; Shearer, F.M.; Sinka, M.E.; Massey, N.C.; Cameron, E.; Bhatt, S.; Gething, P.W.; Hemingway, J.; et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar. J. 2017, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (Also known as American Trypanosomiasis). Key Facts. World Health Organization. April 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 1 September 2023).
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). World Health Organization. May 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 1 September 2023).
- PAHO. “Chagas Disease”. 2022. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 5 September 2023).
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- Elsinga, J.; Gerstenbluth, I.; van der Ploeg, S.; Halabi, Y.; Lourents, N.T.; Burgerhof, J.G.; Van Der Veen, H.T.; Bailey, A.; Grobusch, M.P.; Tami, A. Long-term Chikungunya sequelae in Curaçao: Burden, determinants, and a novel classification tool. J. Infect. Dis. 2017, 216, 573–581. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rowhani-Rahbar, A.; Staples, J.E.; Weaver, M.R.; Halloran, M.E.; Ellis, E.M. Persistent arthralgia associated with Chikungunya virus outbreak, US Virgin Islands, December 2014–February 2016. Emerg. Infect. Dis. 2017, 23, 673–676. [Google Scholar] [CrossRef]
- Moro, M.L.; Grilli, E.; Corvetta, A. For the Study Group ‘Infezioni Da Chikungunya in Emilia-Romagna’. Long-Term Chikungunya Infection Clinical Manifestations after an Outbreak in Italy: A Prognostic Cohort Study. J. Infect. 2012, 65, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Correction: Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- CDC. Fighting the World’s Deadliest Animal. In: Centers for Disease Control and Prevention. 17 August 2023. Available online: https://www.cdc.gov/globalhealth/stories/2019/world-deadliest-animal.html (accessed on 6 September 2023).
- WHO. Malaria. In: World Health Organization. 29 March 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 6 September 2023).
- Gaythorpe, K.A.M.; Hamlet, A.; Jean, K.; Garkauskas Ramos, D.; Cibrelus, L.; Garske, T.; Ferguson, N. The global burden of yellow fever. Elife 2021, 10, e64670. [Google Scholar] [CrossRef]
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M.; Yellow Fever Expert Committee. Yellow fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, T.V.B.; de Alencar Ximenes, R.A.; de Barros Miranda-Filho, D.; Souza, W.V.; Montarroyos, U.R.; de Melo, A.P.L.; Valongueiro, S.; Braga, C.; Brandão Filho, S.P.; Cordeiro, M.T.; et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. Lancet Infect. Dis. 2018, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martínez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; WHO Zika Causality Working Group. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: Systematic review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Ko, A.I.; Baud, D. Zika virus infection—After the pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Bonizzoni, M.; Yan, G. Secondary Malaria Vectors of SubSaharan Africa: Threat to Malaria Elimination on the Continent? In Current Topics in Malaria; IntechOpen: London, UK, 2016; pp. 474–490. [Google Scholar]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef]
- Akeju, A.V.; Olusi, T.A.; Simon-Oke, I.A. Molecular identification and wing variations among malaria vectors in Akure North Local Government Area, Nigeria. Sci. Rep. 2022, 12, 7674. [Google Scholar] [CrossRef]
- Zogo, B.; Koffi, A.A.; Alou, L.P.A.; Fournet, F.; Dahounto, A.; Dabiré, R.K.; Baba-Moussa, L.; Moiroux, N.; Pennetier, C. Identification and characterization of Anopheles spp. breeding habitats in the Korhogo area in northern Côte d’Ivoire: A study prior to a Bti-based larviciding intervention. Parasites Vectors 2019, 12, 146. [Google Scholar] [CrossRef]
- Jobe, N.B.; Huijben, S.; Paaijmans, K.P. Non-target effects of chemical malaria vector control on other biological and mechanical infectious disease vectors. Lancet Planet Health 2023, 7, e706–e717. [Google Scholar] [CrossRef]
- Diallo, D.; Diallo, M. Resting behavior of Aedes aegypti in southeastern Senegal. Parasites Vectors 2020, 13, 356. [Google Scholar] [CrossRef]
- Mohammed, R.; Ahmed, H.; Hassan, S.M.; Abdallah, K.; Enan, M. Breeding and Resting Behaviour of Aedes aegypti in Indoor and Outdoor Environment in Kassala City. Health Sci. J. 2014, 13, 672. [Google Scholar]
- Ferede, G.; Tiruneh, M.; Abate, E.; Kassa, W.J.; Wondimeneh, Y.; Damtie, D.; Tessema, B. Distribution and larval breeding habitats of Aedes mosquito species in residential areas of northwest Ethiopia. Epidemiol. Health 2018, 40, e2018015. [Google Scholar] [CrossRef]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef] [PubMed]
- Haines, L.R.; Lehane, S.M.; Pearson, T.W.; Lehane, M.J. Tsetse EP protein protects the fly midgut from trypanosome establishment. PLoS Pathog. 2010, 6, e1000793. [Google Scholar] [CrossRef]
- Nogueira Brito, N.; Diotaiuti, R.; Celencina Fagundes Gomes, A.; de Cássia Moreira de Souza, R.; Abad-Franch, F. Triatoma costalimai (Hemiptera: Reduviidae) in and Around Houses of Tocantins State, Brazil, 2005–2014. J. Med. Entomol. 2017, 54, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Hamer, S.A.; Lane, S.; Levy, M.Z.; Hamer, G.L. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states, USA. Am. J. Trop. Med. Hyg. 2018, 98, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wormington, J.D.; Gillum, C.; Meyers, A.C.; Hamer, G.L.; Hamer, S.A. Daily activity patterns of movement and refuge use in Triatoma gerstaeckeri and Rhodnius prolixus (Hemiptera: Reduviidae), vectors of the Chagas disease parasite. Acta Trop. 2018, 185, 301–306. [Google Scholar] [CrossRef]
- Klotz, S.A.; Dorn, P.L.; Mosbacher, M.; Schmidt, J.O. Kissing bugs in the United States: Risk for vector-borne disease in humans. Environ. Health Insights 2014, 8, 49–59. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef]
- CDC-Centers for Disease Control, Prevention. CDC—Leishmaniasis—General Information—Frequently Asked Questions (FAQs). 15 August 2010. Available online: https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html (accessed on 6 September 2023).
- CDC. How Can Malaria Cases and Deaths Be Reduced? In: Centers for Disease Control and Prevention. 2018. Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/index.html (accessed on 1 September 2023).
- FDA. First FDA-Approved Vaccine for the Prevention of Dengue Disease in Endemic Regions. In: U.S. Food & Drug Administration. 1 May 2019. Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-dengue-disease-endemic-regions (accessed on 1 September 2023).
- WHO. Immunization, Vaccines and Biologicals: Dengue. In: World Health Organization. April 2018. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/dengue#:~:text=The%20first%20dengue%20vaccine%2C%20Dengvaxia,December%2C%202015%2C%20in%20Mexico (accessed on 1 September 2023).
- CDC. Dengue Vaccination: What Everyone Should Know. In: Centers for Disease Control and Prevention. 2019. Available online: https://www.cdc.gov/vaccines/vpd/dengue/public/index.html (accessed on 1 September 2023).
- CDC. Larval Control and Other Vector Control Interventions. In: Centers for Disease Control and Prevention. 2012. Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/vector_control.html (accessed on 1 September 2023).
- EPA. Success in Mosquito Control: An Integrated Approach. In: United States Environmental Protection Agency. 2019. Available online: https://www.epa.gov/mosquitocontrol/success-mosquito-control-integrated-approach (accessed on 1 September 2023).
- Mabaso, M.L.H.; Sharp, B.; Lengeler, C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop. Med. Int. Health 2004, 9, 846–856. [Google Scholar] [CrossRef]
- WHO. Malaria Vector Control. In: World Health Organization. 2023. Available online: https://www.who.int/teams/global-malaria-programme/prevention/vector-control (accessed on 6 September 2023).
- Hoffmann, E.J.; Miller, J.R. Reduction of mosquito (Diptera: Culicidae) attacks on a human subject by combination of wind and vapor-phase DEET repellent. J. Med. Entomol. 2002, 39, 935–938. [Google Scholar] [CrossRef]
- Chandra, G.; Bhattacharjee, I.; Chatterjee, S.N.; Ghosh, A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008, 127, 13–27. [Google Scholar] [PubMed]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.W.; Kelly, P.H.; Dobson, K.L.; Petrie, W.D.; Dobson, S.L. Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, F.L.; via inundative releases of Wolbachia-infected male mosquitoes. J. Med. Entomol. 2019, 56, 1296–1303. [Google Scholar] [CrossRef]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Scolari, F.; Benoit, J.B.; Michalkova, V.; Aksoy, E.; Takac, P.; Abd-Alla, A.M.M.; Malacrida, A.R.; Aksoy, S.; Attardo, G.M. The Spermatophore in Glossina morsitans morsitans: Insights into Male Contributions to Reproduction. Sci. Rep. 2016, 6, 20334. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J. Insect Physiol. 2006, 52, 679–684. [Google Scholar] [CrossRef]
- Craig, G.B., Jr. Mosquitoes: Female monogamy induced by male accessory gland substance. Science 1967, 156, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Helinski, M.E.H.; Deewatthanawong, P.; Sirot, L.K.; Wolfner, M.F.; Harrington, L.C. Duration and dose-dependency of female sexual receptivity responses to seminal fluid proteins in Aedes albopictus and Ae. aegypti mosquitoes. J. Insect Physiol. 2012, 58, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Gillott, C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- Mueller, J.L.; Page, J.L.; Wolfner, M.F. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics 2007, 175, 777–783. [Google Scholar] [CrossRef]
- Sirot, L.K.; Poulson, R.L.; Caitlin McKenna, M.; Girnary, H.; Wolfner, M.F.; Harrington, L.C. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: Potential tools for control of female feeding and reproduction. Insect Biochem. Mol. Biol. 2008, 38, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Dottorini, T.; Nicolaides, L.; Ranson, H.; Rogers, D.W.; Crisanti, A.; Catteruccia, F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc. Natl. Acad. Sci. USA 2007, 104, 16215–16220. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Waterhouse, R.M.; Abai, M.R.; Aganezov, S.S.; Alekseyev, M.A.; Allen, J.E.; Amon, J.; Arcà, B.; Arensburger, P.; Artemov, G.; et al. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 2015, 347, 1258522. [Google Scholar] [CrossRef]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.J.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007, 316, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.J.; Dudchenko, O.; Kingan, S.B.; Koren, S.; Antoshechkin, I.; Crawford, J.E.; Glassford, W.J.; Herre, M.; Redmond, S.N.; Rose, N.H.; et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 2018, 563, 501–507. [Google Scholar] [CrossRef]
- International Glossina Genome Initiative; Attardo, G.M.; Abila, P.P.; Auma, J.E.; Baumann, A.A.; Benoit, J.B.; Brelsfoard, C.L.; Ribeiro, J.M.; Cotton, J.A.; Pham, D.Q.; et al. Genome sequence of the tsetse fly (Glossina morsitans): Vector of African trypano-somiasis. Science 2014, 344, 380–386. [Google Scholar]
- Mesquita, R.D. Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc. Natl. Acad. Sci. USA 2015, 112, 14936–14941. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, F.; Camargo, L.; Avila, C. Male sexual history influences female fertility and re-mating incidence in the mosquito vector Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2020, 121, 104019. [Google Scholar] [CrossRef]
- Helinski, M.E.H.; Harrington, L.C. Male mating history and body size influence female fecundity and longevity of the dengue vector Aedes aegypti. J. Med. Entomol. 2011, 48, 202–211. [Google Scholar] [CrossRef]
- Gabrieli, P.; Kakani, E.G.; Mitchell, S.N.; Mameli, E.; Want, E.J.; Mariezcurrena Anton, A.; Serrao, A.; Baldini, F.; Catteruccia, F. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2014, 111, 16353–16358. [Google Scholar] [CrossRef]
- Pitts, R.J.; Jason Pitts, R.; Rinker, D.C.; Jones, P.L.; Rokas, A.; Zwiebel, L.J. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genom. 2011, 12, 271. [Google Scholar] [CrossRef]
- Olafson, P.U.; Aksoy, S.; Attardo, G.M.; Buckmeier, G.; Chen, X.; Coates, C.J.; Davis, M.; Dykema, J.; Emrich, S.J.; Friedrich, M.; et al. Publisher Correction: The genome of the stable fly, Stomoxys calcitrans, reveals potential mechanisms underlying reproduction, host interactions, and novel targets for pest control. BMC Biol. 2021, 19, 150. [Google Scholar] [CrossRef]
- Tripet, F.; Touré, Y.T.; Dolo, G.; Lanzaro, G.C. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am. J. Trop. Med. Hyg. 2003, 68, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A. A novel marking/release/recapture method for possible use in determining aspects of tsetse fly behaviour. Int. J. Trop. Insect Sci. 1980, 1, 1–7. [Google Scholar] [CrossRef]
- Gillott, C.; Langley, P.A. The control of receptivity and ovulation in the tsetse fly, Glossina morsitans. Physiol. Entomol. 1981, 6, 269–281. [Google Scholar] [CrossRef]
- Pontes, G.B.; Bohman, B.; Unelius, C.R.; Lorenzo, M.G. Metasternal gland volatiles and sexual communication in the triatomine bug, Rhodnius prolixus. J. Chem. Ecol. 2008, 34, 450–457. [Google Scholar] [CrossRef]
- Gilbert, S.F. Oogenesis; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10008/ (accessed on 1 September 2023).
- Raikhel, A.S.; Kokoza, V.A.; Zhu, J.; Martin, D.; Wang, S.-F.; Li, C.; Sun, G.; Ahmed, A.; Dittmer, N.; Attardo, G. Molecular biology of mosquito vitellogenesis: From basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 2002, 32, 1275–1286. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Park, J.-H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef]
- Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005, 35, 661–675. [Google Scholar] [CrossRef]
- Swevers, L.; Raikhel, A.S.; Sappington, T.W.; Shirk, P.; Iatrou, K. 1.3. Vitellogenesis and Post-Vitellogenic Maturation of the Insect Ovarian Follicle. In Reproduction and Development; Gilbert, L.I., Gill, S., Iatrou, K., Eds.; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Le, B.V.; Nguyen, J.B.; Logarajah, S.; Wang, B.; Marcus, J.; Williams, H.P.; Catteruccia, F.; Baxter, R.H. Characterization of Anopheles gambiae transglutaminase 3 (AgTG3) and its native substrate plugin. J. Biol. Chem. 2013, 288, 4844–4853. [Google Scholar] [CrossRef]
- Rogers, D.W.; Whitten, M.M.A.; Thailayil, J.; Soichot, J.; Levashina, E.A.; Catteruccia, F. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc. Natl. Acad. Sci. USA 2008, 105, 19390–19395. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Kakani, E.G.; South, A.; Howell, P.I.; Waterhouse, R.M.; Catteruccia, F. Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 2015, 347, 985–988. [Google Scholar] [CrossRef]
- Hammond, A. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef]
- Chakraborty, M. Hidden genomic features of an invasive malaria vector, Anopheles stephensi, revealed by a chromo-some-level genome assembly. BMC Biol. 2021, 19, 28. [Google Scholar] [CrossRef]
- Duvall, L.B.; Basrur, N.S.; Molina, H.; McMeniman, C.J.; Vosshall, L.B. A Peptide Signaling System that Rapidly Enforces Paternity in the Aedes aegypti Mosquito. Curr. Biol. 2017, 27, 3734–3742.e5. [Google Scholar] [CrossRef]
- Chen, X.-G. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl. Acad. Sci. USA 2015, 112, E5907–E5915. [Google Scholar] [CrossRef]
- Pagete, D.E. An end-to-end assembly of the Aedes aegypti genome. arXiv. [q-bio.GN]. 2016. Available online: http://arxiv.org/abs/1605.04619 (accessed on 1 September 2023).
- Brown, N.; Nash, R.; Poletti, P.; Guzzetta, G.; Manica, M.; Zardini, A.; Flatken, M.; Vidal, J.; Gueunet, C.; Belikov, E.; et al. Utilising urgent computing to tackle the spread of mosquito-borne diseases. In Proceedings of the 2021 IEEE/ACM HPC for Urgent Decision Making (UrgentHPC), St. Louis, MO, USA, 19 November 2021; IEEE: Piscataway, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Lee, S.G.; Susan, D.; Mccombs, S.H. Sperm Precedence of Irradiated Mediterranean Fruit Fly Males (Diptera: Tephritidae). Proc. Hawaii. Entomol. Soc. 2003, 36, 47–59. [Google Scholar]
- Scolari, F.; Yuval, B.; Gomulski, L.M.; Schetelig, M.F.; Gabrieli, P.; Bassetti, F.; Wimmer, E.A.; Malacrida, A.R.; Gasperi, G. Polyandry in the medfly—Shifts in paternity mediated by sperm stratification and mixing. BMC Genet. 2014, 15 (Suppl. S2), S10. [Google Scholar] [CrossRef]
- Oliva, C.F.; Damiens, D.; Benedict, M.Q. Male reproductive biology of Aedes mosquitoes. Acta Trop. 2014, 132, S12–S19. [Google Scholar] [CrossRef]
- Chiang, R.G.; Chiang, J.A. Reproductive physiology in the blood feeding insect, Rhodnius prolixus, from copulation to the control of egg production. J. Insect Physiol. 2017, 97, 27–37. [Google Scholar] [CrossRef]
- Fuentes-Vicente, J.A.; De, A.E.; Gutiérrez-Cabrera, A.; Flores-Villegas, C.; Lowenberger, G.; Benelli, P.M.; Salazar-Schettino, P.M.; Cordoba-Aguilar, A. What Makes an Effective Chagas Disease Vector? Factors Underlying Trypanosoma Cruzi-Triatomine Interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- Alfonso-Parra, C. Synthesis, depletion and cell-type expression of a protein from the male accessory glands of the dengue vector mosquito Aedes aegypti. J. Insect Physiol. 2014, 70, 117–124. [Google Scholar] [CrossRef]
- Degner, E.C. Proteins, Transcripts, and Genetic Architecture of Seminal Fluid and Sperm in the Mosquito Aedes aegypti. Mol. Cell. Proteom. 2019, 18, S6–S22. [Google Scholar] [CrossRef]
- Hall, A.B.; Basu, S.; Jiang, X.; Qi, Y.; Timoshevskiy, V.A.; Biedler, J.K.; Sharakhova, M.V.; Elahi, R.; Anderson, M.A.; Chen, X.G.; et al. Sex Determination. A male-determining factor in the mosquito Aedes aegypti. Science 2015, 348, 1268–1270. [Google Scholar] [CrossRef]
- Thailayil, J.; Gabrieli, P.; Caputo, B.; Bascuñán, P.; South, A.; Diabate, A.; Dabire, R.; Della Torre, A.; Catteruccia, F. Analysis of natural female post-mating responses of Anopheles gambiae and Anopheles coluzzii unravels similarities and differences in their reproductive ecology. Sci. Rep. 2018, 8, 6594. [Google Scholar] [CrossRef] [PubMed]
- Carmel, I.; Tram, U.; Heifetz, Y. Mating induces developmental changes in the insect female reproductive tract. Curr. Opin. Insect Sci. 2016, 13, 106–113. [Google Scholar] [CrossRef]
- Shaw, W.R.; Teodori, E.; Mitchell, S.N.; Baldini, F.; Gabrieli, P.; Rogers, D.W.; Catteruccia, F. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2014, 111, 5854–5859. [Google Scholar] [CrossRef]
- Baldini, F.; Gabrieli, P.; South, A.; Valim, C.; Mancini, F.; Catteruccia, F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013, 11, e1001695. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Parra, C.; Ahmed-Braimah, Y.H.; Degner, E.C.; Avila, F.W.; Villarreal, S.M.; Pleiss, J.A.; Wolfner, M.F.; Harrington, L.C. Mating-Induced Transcriptome Changes in the Reproductive Tract of Female Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004451. [Google Scholar] [CrossRef] [PubMed]
- Attardo, G.M.; Ribeiro, J.M.C.; Wu, Y.; Berriman, M.; Aksoy, S. Transcriptome analysis of reproductive tissue and intrauterine developmental stages of the tsetse fly (Glossina morsitans morsitans). BMC Genom. 2010, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Procházka, E. Gene expression in reproductive organs of tsetse females—Initial data in an approach to reduce fecundity 06 Biological Sciences 0604 Genetics. BMC Microbiol. 2018, 18, 144. [Google Scholar]
- Leyria, J.; Orchard, I.; Lange, A. Transcriptomic analysis of regulatory pathways involved in female reproductive physiology of Rhodnius prolixus under different nutritional states. Sci. Rep. 2020, 10, 11431. [Google Scholar] [CrossRef]
- Gooding, R.H.; Rolseth, B.M.; Nesbitt, S.A.T. Mapping four loci in Glossina morsitans submorsitans Newstead (Diptera: Glossinidae). Can. Entomol. 1989, 121, 823–824. [Google Scholar] [CrossRef]
- Bruce, D. Further Report on the Tsetse Fly Disease or Nagana in Zululand; Bennett & Davis: Durban, South Africa, 1896. [Google Scholar]
- Curtis, C.F.; Langley, P.A.; Trewern, M.A.; Curtis, C.F. Sterility from crosses between sub-species of the tsetse fly Glossina morsitans. Heredity 1972, 45, 250–268. [Google Scholar]
- Rawlings, P. The genetics of hybrid sterility between subspecies of the complex of Glossina morsitans Westwood (Diptera: Glossinidae). Bull. of Entomol. Res. 1985, 75, 689–699. [Google Scholar] [CrossRef]
- Gooding, R.H. Genetic basis of sterility in hybrids from crosses of Glossina morsitans submorsitans and Glossina morsitans morsitans (Diptera: Glossinidae). Genome 1989, 32, 479–485. [Google Scholar] [CrossRef]
- Mccullough, E.L.; Mcdonough, C.E.; Pitnick, S.; Dorus, S. Quantitative proteomics reveals rapid divergence in the post-mating response of female reproductive tracts among sibling species. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201030. [Google Scholar]
- Lavoipierre, M.M.J. Biting behaviour of mated and unmated females of an African strain of Aedes aegypti. Nature 1958, 181, 1781–1782. [Google Scholar] [CrossRef]
- Fuchs, M.S.; Craig, G.B., Jr.; Despommier, D.D. The protein nature of the substance inducing female monogamy in Aedes aegypti. J. Insect Physiol. 1969, 15, 701–709. [Google Scholar] [CrossRef]
- Houseman, J.G.; Downe, A.E.R. Diptera: Culicidae. Methods of measuring blood meal size and proteinase activity for determining effects of mated state on digestive processes of female Aedes aegypti (L). Can. Entomol. 1986, 118, 241–248. [Google Scholar] [CrossRef]
- Klowden, M.J.; Chambers, G.M. Male accessory gland substances activate egg development in nutritionally stressed Aedes aegypti mosquitoes. J. Insect Physiol. 1991, 37, 721–726. [Google Scholar] [CrossRef]
- Klowden, M.J. Mating and nutritional state affect the reproduction of Aedes albopictus mosquitoes. J. Am. Mosq. Control Assoc. 1993, 9, 169–173. [Google Scholar] [PubMed]
- Fernandez, N.M.; Klowden, M.J. Male accessory gland substances modify the host-seeking behavior of gravid Aedes aegypti mosquitoes. J. Insect Physiol. 1995, 41, 965–970. [Google Scholar] [CrossRef]
- Boes, K.E.; Ribeiro, J.M.C.; Wong, A.; Harrington, L.C.; Wolfner, M.F.; Sirot, L.K. Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus. PLoS Negl. Trop. Dis. 2014, 8, e2946. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, S.M.; Pitcher, S.; Helinski, M.E.H.; Johnson, L.; Wolfner, M.F.; Harrington, L.C. Male contributions during mating increase female survival in the disease vector mosquito Aedes aegypti. J. Insect Physiol. 2018, 108, 1–9. [Google Scholar] [CrossRef]
- Destephano, D.B.; Brady, U.E.; Lovins, R.E. Synthesis of prostaglandin by reproductive tissue of the male house cricket, Acheta domesticus. Prostaglandins 1974, 6, 71–79. [Google Scholar] [CrossRef]
- Loher, W.; Ganjian, I.; Kubo, I.; Stanley-Samuelson, D.; Tobe, S.S. Prostaglandins: Their role in egg-laying of the cricket Teleogryllus commodus. Proc. Natl. Acad. Sci. USA 1981, 78, 7835–7838. [Google Scholar] [CrossRef]
- Pondeville, E.; Maria, A.; Jacques, J.-C.; Bourgouin, C.; Dauphin-Villemant, C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc. Natl. Acad. Sci. USA 2008, 105, 19631–19636. [Google Scholar] [CrossRef]
- Baldini, F.; Gabrieli, P.; Rogers, D.W.; Catteruccia, F. Function and composition of male accessory gland secretions in Anopheles gambiae: A comparison with other insect vectors of infectious diseases. Pathog. Glob. Health 2012, 106, 82–93. [Google Scholar] [CrossRef]
- Sirot, L.K.; Hardstone, M.C.; Helinski, M.E.H.; Ribeiro, J.M.C.; Kimura, M.; Deewatthanawong, P.; Wolfner, M.F.; Harrington, L.C. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. PLoS Negl. Trop. Dis. 2011, 5, e989. [Google Scholar] [CrossRef] [PubMed]
- Mcdonough-Goldstein, C.E. Pronounced Postmating Response in the Drosophila Female Reproductive Tract Fluid Proteome. Mol. Cell. Proteom. 2021, 20, 100156. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.D.; Yi, X.; Maccoss, M.J.; Swanson, W. Proteomics Reveals Novel Drosophila Seminal Fluid Proteins Transferred at Mating. PLoS Biol. 2008, 6, e178. [Google Scholar] [CrossRef]
- Wasbrough, E.R.; Dorus, S.; Hester, S.; Howard-Murkin, J.; Lilley, K.; Wilkin, E.; Polpitiya, A.; Petritis, K.; Karr, T.L. The Drosophila melanogaster sperm proteome-II (DmSP-II). J. Proteom. 2010, 73, 2171–2185. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H. Infection with endosymbiotic Spiroplasma disrupts tsetse (Glossina fuscipes fuscipes) metabolic and reproductive homeostasis. PLoS Pathog. 2021, 17, e1009539. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Rodriguez, E.; Clifton, B.D.; Ranz, J.M. On the genetic basis of the effect of Spiroplasma on the male reproductive fitness of Glossina fuscipes fuscipes. PLoS Pathog. 2022, 18, e1010442. [Google Scholar] [CrossRef]
- Lung, O.; Kuo, L.; Wolfner, M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001, 47, 617–622. [Google Scholar] [CrossRef]
- Leyria, J.; Orchard, I.; Lange, A.B. What happens after a blood meal? A transcriptome analysis of the main tissues involved in egg production in Rhodnius prolixus, an insect vector of Chagas disease. PLoS Negl. Trop. Dis. 2020, 14, e0008516. [Google Scholar] [CrossRef]
- Ouali, R.; Valentim de Brito, K.C.; Salmon, D.; Bousbata, S. High-throughput identification of the Rhodnius prolixus midgut proteome unravels a sophisticated hematophagic machinery. Proteomes 2020, 8, 16. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Heuberger, A.L.; Prenni, J.E. Large Scale Non-targeted Metabolomic Profiling of Serum by Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS). J. Vis. Exp. 2013, 73, e50242. [Google Scholar] [CrossRef]
- Rivera, R.; Garrido, N. Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–285. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Van Eeckhaut, A. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Scolari, F.; Khamis, F.M.; Pérez-Staples, D. Beyond Sperm and Male Accessory Gland Proteins: Exploring Insect Reproductive Metabolomes. Front. Physiol. 2021, 12, 729440. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Martins, G.F.; Serrão, J.E.; Ramalho-Ortigão, J.M.; Pimenta, P.F.P. A comparative study of fat body morphology in five mosquito species. Mem. Inst. Oswaldo Cruz. 2011, 106, 742–747. [Google Scholar] [CrossRef]
- Price, D.P.; Schilkey, F.D.; Ulanov, A.; Hansen, I.A. Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasites Vectors 2015, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.D.; Dagan, S.; Scaraffia, P.Y. Unraveling mosquito metabolism with mass spectrometry-based metabolomics: (Trends in Parasitology, 37:8 p: 747–761; 2021). Trends Parasitol. 2021, 37, 764. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef]
- Pietri, J.E.; Pakpour, N.; Napoli, E.; Song, G.; Pietri, E.; Potts, R.; Cheung, K.W.; Walker, G.; Riehle, M.A.; Starcevich, H.; et al. Two insulin-like peptides differentially regulate malaria parasite infection in the mosquito through effects on intermediary metabolism. Biochem. J. 2016, 473, 3487–3503. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.-J.; Nie, S.; Yang, Q.; Harshman, L.G.; Mao, C.; Williamson, N.A.; Hoffmann, A.A. Lipidomic profiling reveals concerted temporal patterns of functionally related lipids in Aedes aegypti females following blood feeding. Metabolites 2023, 13, 421. [Google Scholar] [CrossRef]
- Rivera-Perez, C.; Nouzova, M.; Lamboglia, I.; Noriega, F.G. Metabolic analysis reveals changes in the mevalonate and juvenile hormone synthesis pathways linked to the mosquito reproductive physiology. Insect Biochem. Mol. Biol. 2014, 51, 1–9. [Google Scholar] [CrossRef]
- Huck, D.T.; Klein, M.S.; Meuti, M.E. Determining the effects of nutrition on the reproductive physiology of male mosquitoes. J. Insect Physiol. 2021, 129, 104191. [Google Scholar] [CrossRef] [PubMed]
- Hagan, R.W.; Didion, E.M.; Rosselot, A.E.; Holmes, C.J.; Siler, S.C.; Rosendale, A.J.; Hendershot, J.M.; Elliot, K.S.; Jennings, E.C.; Nine, G.A.; et al. Dehydration prompts increased activity and blood feeding by mosquitoes. Sci. Rep. 2018, 8, 6804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, Y.; Saha, T.T.; Pei, G.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef] [PubMed]
- Lampe, L.; Jentzsch, M.; Kierszniowska, S.; Levashina, E.A. Metabolic balancing by miR-276 shapes the mosquito reproductive cycle and Plasmodium falciparum development. Nat. Commun. 2019, 10, 5634. [Google Scholar] [CrossRef]
- Ferdous, Z.; Fuchs, S.; Behrends, V.; Trasanidis, N.; Waterhouse, R.M.; Vlachou, D.; Christophides, G.K. Anopheles coluzzii stearoyl-CoA desaturase is essential for adult female survival and reproduction upon blood feeding. PLoS Pathog. 2021, 17, e1009486. [Google Scholar] [CrossRef]
- Bing, X.; Attardo, G.M.; Vigneron, A.; Aksoy, E.; Scolari, F.; Malacrida, A.; Weiss, B.L.; Aksoy, S. Unravelling the relationship between the tsetse fly and its obligate symbiont Wigglesworthia: Transcriptomic and metabolomic landscapes reveal highly integrated physiological networks. Proc. Biol. Sci. 2017, 284, 20170360. [Google Scholar] [CrossRef]
- Scolari, F.; Attardo, G.M.; Aksoy, E.; Weiss, B.; Savini, G.; Takac, P.; Abd-Alla, A.; Parker, A.G.; Aksoy, S.; Malacrida, A.R. Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans. BMC Microbiol. 2018, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.E.; Correa, S.; Rivera-Perez, C.; Nouzova, M.; Noriega, F.G. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J. Insect Physiol. 2014, 64, 40–47. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Ribeiro, J.M.C.; dos Santos, D.V.; Pereira, M.H.; Araújo, R.N.; Gontijo, N.F.; Pessoa, G.C.; Sant’Anna, M.R.; Sorgine, M.H.; Majerowicz, D.; et al. Analysis of the testicle’s transcriptome of the Chagas disease vector Rhodnius prolixus. bioRxiv 2019. [Google Scholar] [CrossRef]
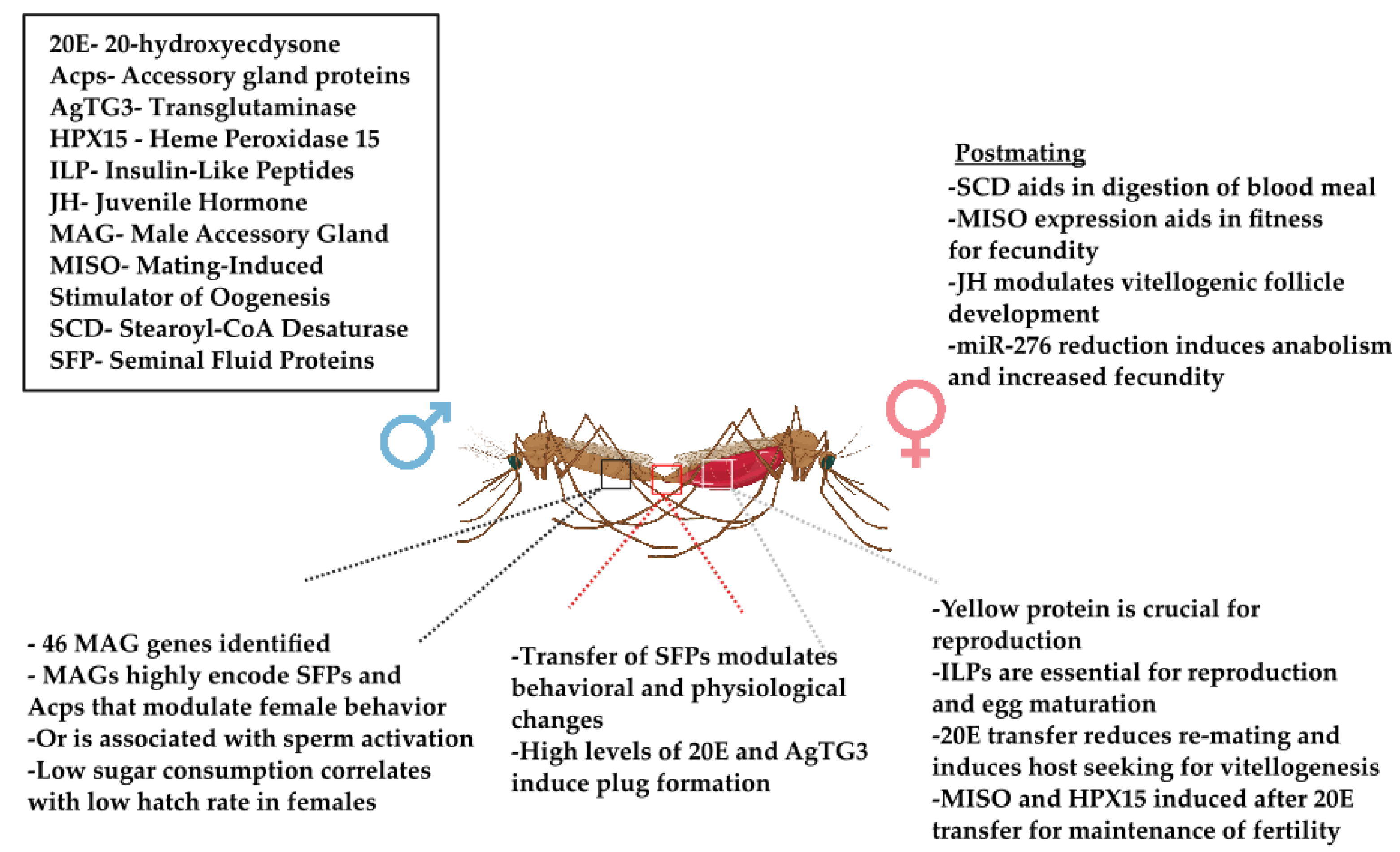
| Omics Analysis | Organism | Target | Location of Target Analysis | Function/Outcome |
|---|---|---|---|---|
| Genomics | Glossina morsitans | Yolk protein (yp2) | Ovaries | Expressed in the ovaries and involved in oogenesis |
| Aedes aegypti | Male reproductive gland protein-encoding genes | Male accessory glands | Multiple genes encoding the respective accessory gland proteins, influence female behavior, physiology, survival and reproduction | |
| Heterochromatin Protein 1 (HP1) | Seminal fluids | Induce female refractoriness to mating | ||
| M-locus gene (Nix) | Chromosome 1 | Male sex locus determination gene | ||
| Anopheles gambiae | Male accessory gland genes (MAGs) | Male accessory glands | Multiple genes that encode for the respective accessory gland proteins | |
| Yellow, -b, -e, -g | Ovaries | Crucial for female reproduction | ||
| Transglutaminase (AgTG3) | Seminal fluids | MAG-specific enzyme involved in formation of mating plug | ||
| Odorant receptor (Or) | Antennae/Palps | Sperm activation | ||
| Anopheles stephensi | Yellow, -b, -e, -g | Ovaries | Consistent with roles in female reproduction | |
| Stomoxys calcitrans | Odorant receptor (Or) | Maxillary palps | Potential role in sperm activation | |
| Transcripts | Anopheles gambiae | Mating induced stimulator of oogenesis (MISO) | Atrium | Induction of oogenesis post blood feeding |
| Heme peroxidase 15 (HPX15) | Sperm storage organs | Involved in activation by sex that are important to preserve the functionality of stored sperm and long-term fertility | ||
| Proteomic | Anopheles gambiae | Accessory gland genes (Acps) | Accessory gland | Control post-mating behavioral changes in females |
| Seminal fluid proteins(SFPs) / Male accessory gland proteins (MAGs) | Male accessory glands | Refractoriness to multiple coapulations | ||
| Aedes aegypti | Seminal fluid proteins(SFPs) / Male acessory gland proteins (MAGs) | Male accessory glands | Refractoriness to multiple copulations and/or ecdysteroidogenesis | |
| Putative male reproductive gland proteins (mRGPs) | Seminal fluids | Induces behavioral and physiological changes in females | ||
| Glossina morsitans morsitans | Seminal fluid proteins(SFPs) / Male acessory gland proteins (MAGs) | Male accessory glands | Modulate female reproductive physiology and behavior, impacting sperm storage/use, ovulation, oviposition, and mating receptivity | |
| Glossina fuscipes | Ortholog of short wing proteins (sw) | Male testes, ovaries | Possible reduction in fitness of the insects after a Spiroplasma infection | |
| Metabolomic | Anopheles gambiae | 20-hydroxyecdysone (20E) | Seminal fluids/ Accessory glands | Formation of mating plug |
| Anopheles coluzzii | Stearoyl-CoA desaturase (SCD) | Midgut lumen | Digestion of the bloodmeal in females, oocyte maturation, maintains cell membrane fluidity | |
| Anopheles stephensi | Insulin-like peptides (ILPs) | Brain | Stimulates egg maturation | |
| Aedes aegypti | Isoprenoid precursors for mevalonate pathway (MVAP) | Corpora allata | Controls synthesis of important metabolites most notably juvenile hormone | |
| Juvenile Hormone (JH) | Corpora allata | Controls nutrients provided to ovaries, impacts the fate of vitellogenic follicle development | ||
| Insulin-like peptides (ILPs) | Brain | Metabolic homeostasis and reproduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Delgadillo, F.M.; Gayathrinathan, S.; Grajeda, B.I.; Roy, S. Current Status of Omics Studies Elucidating the Features of Reproductive Biology in Blood-Feeding Insects. Insects 2023, 14, 802. https://doi.org/10.3390/insects14100802
Kulkarni A, Delgadillo FM, Gayathrinathan S, Grajeda BI, Roy S. Current Status of Omics Studies Elucidating the Features of Reproductive Biology in Blood-Feeding Insects. Insects. 2023; 14(10):802. https://doi.org/10.3390/insects14100802
Chicago/Turabian StyleKulkarni, Aditi, Frida M. Delgadillo, Sharan Gayathrinathan, Brian I. Grajeda, and Sourav Roy. 2023. "Current Status of Omics Studies Elucidating the Features of Reproductive Biology in Blood-Feeding Insects" Insects 14, no. 10: 802. https://doi.org/10.3390/insects14100802
APA StyleKulkarni, A., Delgadillo, F. M., Gayathrinathan, S., Grajeda, B. I., & Roy, S. (2023). Current Status of Omics Studies Elucidating the Features of Reproductive Biology in Blood-Feeding Insects. Insects, 14(10), 802. https://doi.org/10.3390/insects14100802