The Susceptibility of Bemisia tabaci Mediterranean (MED) Species to Attack by a Parasitoid Wasp Changes between Two Whitefly Strains with Different Facultative Endosymbiotic Bacteria
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Insects
2.2. Response of Eretmocerus mundus Oviposition Rate to Host Density
2.3. Effect of Bemisia tabaci Strain on Parasitism by Eretmocerus mundus
3. Results
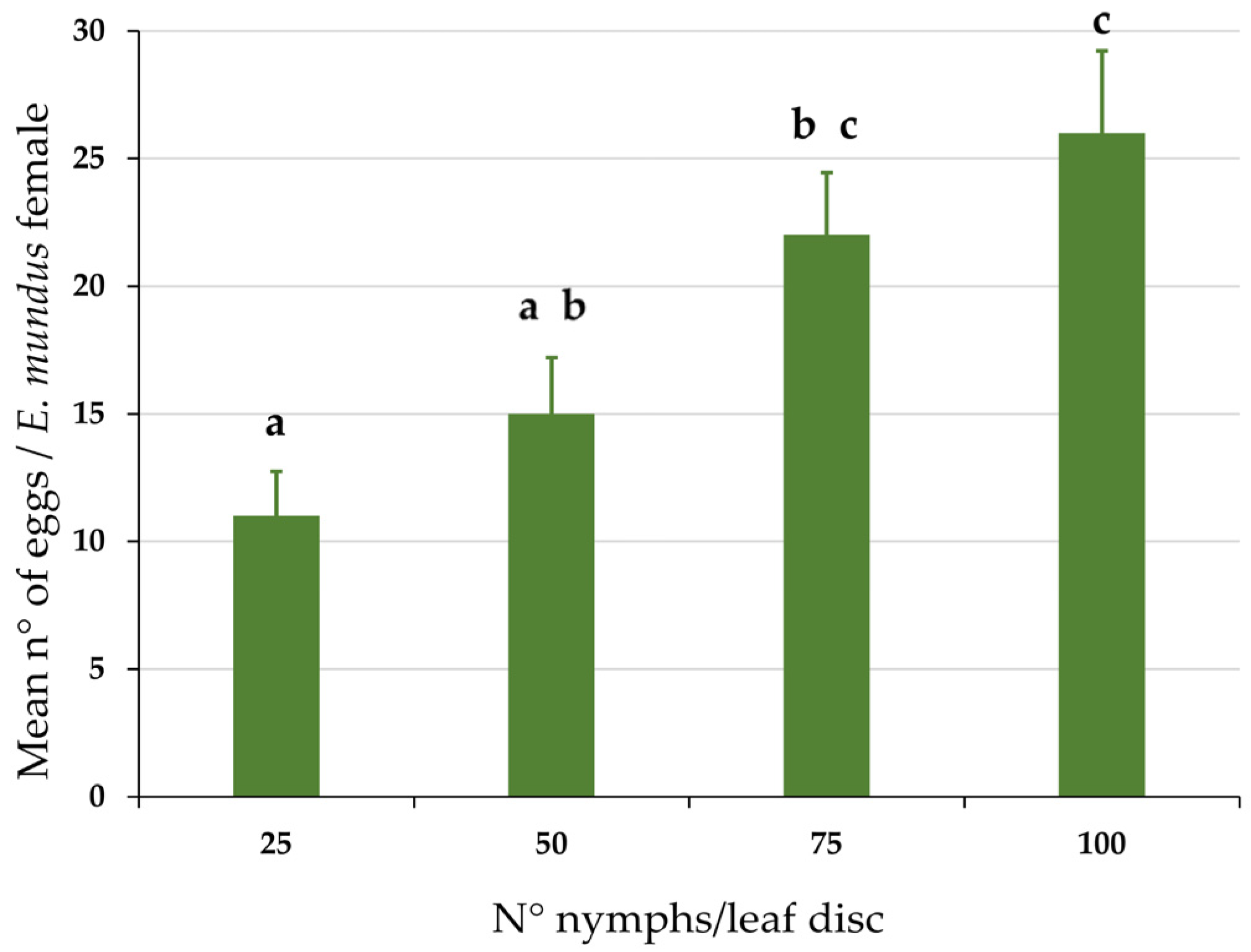
3.1. Response of Eretmocerus mundus Oviposition Rate to Host Density
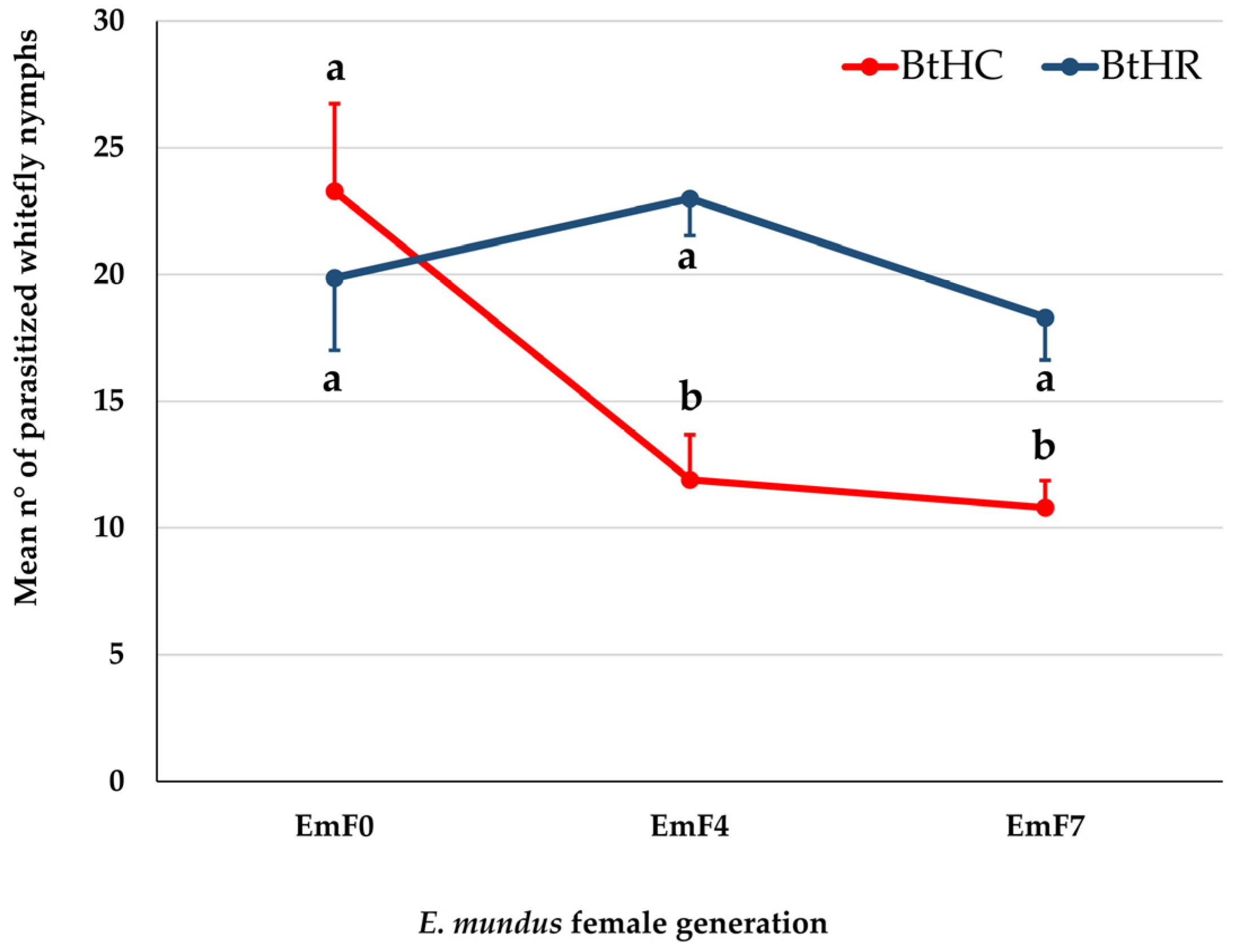
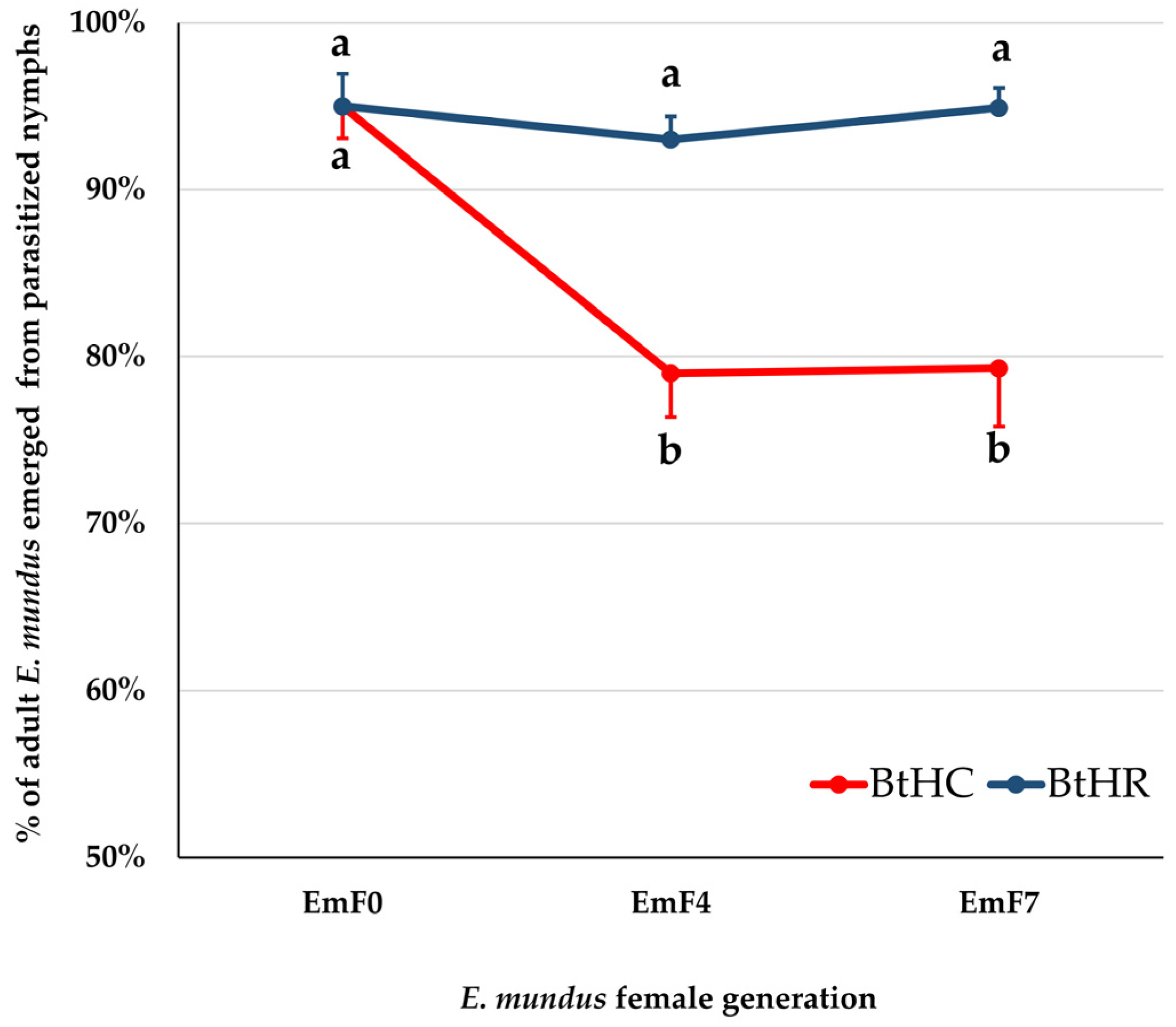
3.2. Effect of Bemisia tabaci Strain on Parasitism by Eretmocerus mundus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.R. Plant Viruses Transmitted by Whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- N’cho, A.-J.; Seka, K.; Assiri, K.P.; Simiand, C.; Otron, D.H.; Ochou, G.; Konan, K.A.J.; Kouadio, M.-F.; Fondio, L.; Atta Diallo, H.; et al. Genetic Diversity of Whitefly Species of the Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) Species Complex, Associated with Vegetable Crops in Côte d’Ivoire. PLoS ONE 2022, 17, e0276993. [Google Scholar] [CrossRef] [PubMed]
- Kanakala, S.; Ghanim, M. Global Genetic Diversity and Geographical Distribution of Bemisia tabaci and Its Bacterial Endosymbionts. PLoS ONE 2019, 14, e0213946. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.; Barro, P. Refined Global Analysis of Bemisia Tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial Cytochrome Oxidase 1 to Identify Species Level Genetic Boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Parrella, G.; Scassillo, L.; Giorgini, M. Evidence for a New Genetic Variant in the Bemisia tabaci Species Complex and the Prevalence of the Biotype Q in Southern Italy. J. Pest Sci. 2012, 85, 227–238. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Lee, G.-S.; Lee, S.; Akimoto, S. Taxonomic Status of the Bemisia tabaci Complex (Hemiptera: Aleyrodidae) and Reassessment of the Number of Its Constituent Species. PLoS ONE 2013, 8, e63817. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B.; Moran, N.A. Endosymbiotic Bacteria as a Source of Carotenoids in Whiteflies. Biol. Lett. 2012, 8, 986–989. [Google Scholar] [CrossRef]
- Ren, F.-R.; Sun, X.; Wang, T.-Y.; Yan, J.-Y.; Yao, Y.-L.; Li, C.-Q.; Luan, J.-B. Pantothenate Mediates the Coordination of Whitefly and Symbiont Fitness. ISME J. 2021, 15, 1655–1667. [Google Scholar] [CrossRef]
- Chiel, E.; Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Katzir, N.; Inbar, M.; Ghanim, M. Biotype-Dependent Secondary Symbiont Communities in Sympatric Populations of Bemisia tabaci. Bull. Entomol. Res. 2007, 97, 407–413. [Google Scholar] [CrossRef]
- Zchori-Fein, E.; Brown, J.K. Diversity of Prokaryotes Associated with Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Ann. Entomol. Soc. Am. 2002, 95, 711–718. [Google Scholar] [CrossRef]
- Bing, X.-L.; Ruan, Y.-M.; Rao, Q.; Wang, X.-W.; Liu, S.-S. Diversity of Secondary Endosymbionts among Different Putative Species of the Whitefly Bemisia tabaci: Distribution of Endosymbionts in Bemisia tabaci. Insect Sci. 2013, 20, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Feldhaar, H. Bacterial Symbionts as Mediators of Ecologically Important Traits of Insect Hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Moran, N.A.; Mc Cutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Giorgini, M.; Strand, M.R.; Pennacchio, F. Arthropod Endosymbiosis and Evolution. In Arthropod Biology and Evolution; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 441–477. [Google Scholar] [CrossRef]
- White, J.A. Caught in the Act: Rapid, Symbiont-Driven Evolution: Endosymbiont Infection Is a Mechanism Generating Rapid Evolution in Some Arthropods—But How Widespread Is the Phenomenon? Bioessays 2011, 33, 823–829. [Google Scholar] [CrossRef]
- Russell, J.A.; Moran, N.A. Costs and Benefits of Symbiont Infection in Aphids: Variation among Symbionts and across Temperatures. Proc. R. Soc. B 2006, 273, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Łukasik, P.; O’Connor, M.P.; Lee, A.; Mayo, G.; Drott, M.T.; Doll, S.; Tuttle, R.; Disciullo, R.A.; Messina, A.; et al. Patterns, Causes and Consequences of Defensive Microbiome Dynamics across Multiple Scales. Mol. Ecol. 2015, 24, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, Z.; Niu, H.; Guo, H. Win by Quantity: A Striking Rickettsia-Bias Symbiont Community Revealed by Seasonal Tracking in the Whitefly Bemisia tabaci. Microb. Ecol. 2021, 81, 523–534. [Google Scholar] [CrossRef]
- Andreason, S.A.; Shelby, E.A.; Moss, J.B.; Moore, P.J.; Moore, A.J.; Simmons, A.M. Whitefly Endosymbionts: Biology, Evolution, and Plant Virus Interactions. Insects 2020, 11, 775. [Google Scholar] [CrossRef]
- Milenovic, M.; Ghanim, M.; Hoffmann, L.; Rapisarda, C. Whitefly Endosymbionts: IPM Opportunity or Tilting at Windmills? J. Pest Sci. 2022, 95, 543–566. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid Spread of a Bacterial Symbiont in an Invasive Whitefly Is Driven by Fitness Benefits and Female Bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.-W.; Luan, J.-B.; Liu, Y.-Q.; Douglas, A.E.; Liu, S.-S. The Inherited Bacterial Symbiont Hamiltonella Influences the Sex Ratio of an Insect Host. Proc. R. Soc. B 2019, 286, 20191677. [Google Scholar] [CrossRef] [PubMed]
- Bockoven, A.A.; Bondy, E.C.; Flores, M.J.; Kelly, S.E.; Ravenscraft, A.M.; Hunter, M.S. What Goes Up Might Come Down: The Spectacular Spread of an Endosymbiont Is Followed by Its Decline a Decade Later. Microb. Ecol. 2020, 79, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Peng, J.; Chen, X.; Guo, C.; Sang, W.; Wang, X.; Ahmed, M.Z.; Xu, Y.; Qiu, B. Antagonistic Interaction between Male-killing and Cytoplasmic Incompatibility Induced by Cardinium and Wolbachia in the Whitefly, Bemisia tabaci. Insect Sci. 2021, 28, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Baiming, L.; Hongran, L.; Tianbo, D.; Yunli, T.; Dong, C. Effect of Cardinium Infection on the Probing Behavior of Bemisia tabaci (Hemiptera: Aleyrodidae) MED. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Rollat-Farnier, P.-A.; Zhu, D.-T.; Santos-Garcia, D.; Silva, F.J.; Moya, A.; Latorre, A.; Klein, C.C.; Vavre, F.; Sagot, M.-F.; et al. Genome Reduction and Potential Metabolic Complementation of the Dual Endosymbionts in the Whitefly Bemisia tabaci. BMC Genom. 2015, 16, 226. [Google Scholar] [CrossRef] [PubMed]
- Rollat-Farnier, P.-A.; Santos-Garcia, D.; Rao, Q.; Sagot, M.-F.; Silva, F.J.; Henri, H.; Zchori-Fein, E.; Latorre, A.; Moya, A.; Barbe, V.; et al. Two Host Clades, Two Bacterial Arsenals: Evolution through Gene Losses in Facultative Endosymbionts. Genome Biol. Evol. 2015, 7, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia Influences Thermotolerance in the Whitefly Bemisia tabaci B Biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Yang, K.; Yuan, M.; Liu, Y.; Guo, C.; Liu, T.; Zhang, Y.; Chu, D. First Evidence for Thermal Tolerance Benefits of the Bacterial Symbiont Cardinium in an Invasive Whitefly, Bemisia tabaci. Pest Manag. Sci. 2021, 77, 5021–5031. [Google Scholar] [CrossRef]
- Hendry, T.A.; Hunter, M.S.; Baltrus, D.A. The Facultative Symbiont Rickettsia Protects an Invasive Whitefly against Entomopathogenic Pseudomonas syringae Strains. Appl. Environ. Microbiol. 2014, 80, 7161–7168. [Google Scholar] [CrossRef]
- Shi, P.-Q.; Chen, X.-Y.; Chen, X.-S.; Lv, N.; Liu, Y.; Qiu, B.-L. Rickettsia Increases Its Infection and Spread in Whitefly Populations by Manipulating the Defense Patterns of the Host Plant. FEMS Microbiol. Ecol. 2021, 97, fiab032. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.; Zchori-Fein, E.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F.; et al. The Transmission Efficiency of Tomato Yellow Leaf Curl Virus by the Whitefly Bemisia tabaci Is Correlated with the Presence of a Specific Symbiotic Bacterium Species. J. Virol. 2010, 84, 9310–9317. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhao, J.; Wang, H.; Liu, Y.; Liu, S. Impact of a Novel Rickettsia Symbiont on the Life History and Virus Transmission Capacity of Its Host Whitefly (Bemisia tabaci). Insect Sci. 2021, 28, 377–391. [Google Scholar] [CrossRef]
- Gueguen, G.; Vavre, F.; Gnankine, O.; Peterschmitt, M.; Charif, D.; Chiel, E.; Gottlieb, Y.; Ghanim, M.; Zchori-Fein, E.; Fleury, F. Endosymbiont Metacommunities, MtDNA Diversity and the Evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) Species Complex. Mol. Ecol. 2010, 19, 4365–4376. [Google Scholar] [CrossRef]
- Gnankiné, O.; Mouton, L.; Henri, H.; Terraz, G.; Houndeté, T.; Martin, T.; Vavre, F.; Fleury, F. Distribution of Bemisia tabaci (Homoptera: Aleyrodidae) Biotypes and Their Associated Symbiotic Bacteria on Host Plants in West Africa: Distribution of Bemisia tabaci Biotypes and Their Associated Symbiotic. Insect Conserv. Divers. 2013, 6, 411–421. [Google Scholar] [CrossRef]
- Parrella, G.; Nappo, A.G.; Manco, E.; Greco, B.; Giorgini, M. Invasion of the Q2 Mitochondrial Variant of Mediterranean Bemisia tabaci in Southern Italy: Possible Role of Bacterial Endosymbionts: Invasion of the Q2 Mitochondrial Variant of MED Bemisia tabaci in Southern Italy. Pest Manag. Sci. 2014, 70, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Li, X.; Ge, D.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Chu, D.; Liu, B.; Xu, B.; et al. Factors Affecting Population Dynamics of Maternally Transmitted Endosymbionts in Bemisia tabaci. PLoS ONE 2012, 7, e30760. [Google Scholar] [CrossRef]
- Brumin, M.; Lebedev, G.; Kontsedalov, S.; Ghanim, M. Levels of the Endosymbiont Rickettsia in the Whitefly Bemisia tabaci Are Influenced by the Expression of Vitellogenin. Insect Mol. Biol. 2020, 29, 241–255. [Google Scholar] [CrossRef]
- Gould, J.; Hoelmer, K.; Goolsby, J. Classical Biological Control of Bemisia tabaci in the United States—A Review of Interagency Research and Implementation; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Arnó, J.; Gabarra, R.; Liu, T.-X.; Simmons, A.M.; Gerling, D. Natural Enemies of Bemisia tabaci: Predators and Parasitoids. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 385–421. [Google Scholar] [CrossRef]
- Moreno-Ripoll, R.; Gabarra, R.; Symondson, W.O.C.; King, R.A.; Agustí, N. Do the Interactions among Natural Enemies Compromise the Biological Control of the Whitefly Bemisia tabaci? J. Pest Sci. 2014, 87, 133–141. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Hoelmer, K.A.; Gould, J.R. Biological control of silverleaf whitefly in the United States. In Contributions of Classical Biological Control to the U.S. Food Security, Forestry, and Biodiversity; Van Driesche, R.G., Winston, R.L., Perring, T.M., Lopez, V.M., Eds.; FHAAST-2019-05; USDA Forest Service: Morgantown, WV, USA, 2022; pp. 59–72. Available online: https://bugwoodcloud.org/resource/files/23194.pdf (accessed on 30 June 2022).
- Naranjo, S.E. Conservation and Evaluation of Natural Enemies in IPM Systems for Bemisia tabaci. Crop Prot. 2001, 20, 835–852. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C. The Contribution of Conservation Biological Control to Integrated Control of Bemisia tabaci in Cotton. Biol. Control 2009, 51, 458–470. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative Bacterial Symbionts in Aphids Confer Resistance to Parasitic Wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Smith, A.H.; Russell, J.A. Defensive Symbiosis in the Real World—Advancing Ecological Studies of Heritable, Protective Bacteria in Aphids and Beyond. Funct. Ecol. 2014, 28, 341–355. [Google Scholar] [CrossRef]
- Vorburger, C.; Perlman, S.J. The Role of Defensive Symbionts in Host-Parasite Coevolution: Symbiont-Mediated Coevolution. Biol. Rev. 2018, 93, 1747–1764. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, A.R.; Hutcheson, K.A.; Limentani, E.C.; Godfray, H.C.J. Costs of counter defenses to host resistance in a parasitoid of Drosophila. Evolution 2001, 55, 1815–1821. [Google Scholar]
- Dupas, S.; Carton, Y.; Poirie, M. Genetic dimension of the coevolution of virulence-resistance in Drosophila parasitoid wasp relationships. Heredity 2003, 90, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Rouchet, R.; Vorburger, C. Strong specificity in the interaction between parasitoids and symbiont-protected hosts. J. Evol. Biol. 2012, 25, 2369–2375. [Google Scholar] [CrossRef]
- Arnó, J.; Matas, M.; Martí, M.; Arino, J.; Roig, J.; Gabarra, R. Coexistence between Trialeurodes vaporariorum and Bemisia tabaci and impact of natural enemies in tomato crops under Mediterranean conditions. IOBC/WPRS Bull. 2005, 28, 1–4. [Google Scholar]
- Nannini, M.; Manca, L.; Giorgini, M. Natural parasitism of Bemisia tabaci and Trialeurodes vaporariorum in a horticultural area of Sardinia, Italy. IOBC/WPRS Bull. 2006, 29, 59–64. [Google Scholar]
- Urbaneja, A.; Sánchez, E.; Stansly, P.A. Life History of Eretmocerus mundus, a Parasitoidof Bemisia tabaci, on Tomato and Sweet Pepper. BioControl 2007, 52, 25. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Shishehbor, P.; Kocheili, F. Parasitism of Cotton Whitefly, Bemisia tabaci on Cucumber by Eretmocerus mundus: Bionomics in Relation to Temperature. Crop Prot. 2009, 28, 963–967. [Google Scholar] [CrossRef]
- López, S.N.; Andorno, A.V. Evaluation of the Local Population of Eretmocerus mundus (Hymenoptera: Aphelinidae) for Biological Control of Bemisia tabaci Biotype B (Hemiptera: Aleyrodidae) in Greenhouse Peppers in Argentina. Biol. Control 2009, 50, 317–323. [Google Scholar] [CrossRef]
- Otim, M.; Kyamanywa, S.; Ecaat, S.; Legg, J.; Gerling, D. The Incidence of Bemisia tabaci (Homoptera: Aleyrodidae) And Its Parasitoids On Cassava And Associated Plants In Uganda. Isr. J. Entomol. 2018, 48, 157–176. [Google Scholar] [CrossRef]
- Keser, B.; Karaca, M.M.; Karut, K. Reproductive Performance and Functional Response of Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae) Obtained from Cold-Stored Red-Eyed Pupae. Egypt J. Biol. Pest Control 2022, 32, 72. [Google Scholar] [CrossRef]
- Heinz, K.H.; Parrella, M.P. Host location and utilization by selected parasitoids of Bemisia argentifolii (Homoptera: Aleyrodidae): Implications for augmentative biological control. Environ. Entomol. 1998, 27, 773–784. [Google Scholar] [CrossRef]
- De Barro, P.J.; Hart, P.J.; Morton, R. The Biology of Two Eretmocerus spp. (Haldeman) and Three Encarsia spp. Forster and Their Potential as Biological Control Agents of Bemisia tabaci Biotype B in Australia. Entomol. Exp. Appl. 2000, 94, 93–102. [Google Scholar] [CrossRef]
- Kirk, A.A.; Lacey, L.A.; Brown, J.K.; Ciomperlik, M.A.; Goolsby, J.A.; Vacek, D.C.; Wendel, L.E.; Napompeth, B. Variation in the Bemisia tabaci s. 1. Species Complex (Hemiptera: Aleyrodidae) and Its Natural Enemies Leading to Successful Biological Control of Bemisia Biotype B in the USA. Bull. Entomol. Res. 2000, 90, 317–327. [Google Scholar] [CrossRef]
- Gerling, D.; Tremblay, E.; Orion, T. Initial stages of the vital capsule formation in the Eretmocerus-Bemisia tabaci association. Redia 1991, 74, 411–415. [Google Scholar]
- Gerling, D.; Orion, T.; Yaakov, D. Eretmocerus penetration and immature development: A novel approach to overcome host immunity. Arch. Insect Biochem. Physiol. 1990, 13, 247–253. [Google Scholar] [CrossRef]
- Gelman, D.B.; Gerling, D.; Blackburn, M.A. Host-Parasitoid Interactions Relating to Penetration of the Whitefly, Bemisia tabaci, by the Parasitoid Wasp, Eretmocerus mundus. J. Insect Sci. 2005, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Mahadav, A.; Gerling, D.; Gottlieb, Y.; Czosnek, H.; Ghanim, M. Parasitization by the Wasp Eretmocerus mundus Induces Transcription of Genes Related to Immune Response and Symbiotic Bacteria Proliferation in the Whitefly Bemisia tabaci. BMC Genom. 2008, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Tsagkarakou, A.; Mouton, L.; Kristoffersen, J.B.; Dokianakis, E.; Grispou, M.; Bourtzis, K. Population Genetic Structure and Secondary Endosymbionts of Q Bemisia tabaci (Hemiptera: Aleyrodidae) from Greece. Bull. Entomol. Res. 2012, 102, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Lahav, T.; Freilich, S. Variations in the Identity and Complexity of Endosymbiont Combinations in Whitefly Hosts. Front. Microbiol. 2014, 5, 310. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.-W.; Liu, S.-S. The Costs and Benefits of Two Secondary Symbionts in a Whitefly Host Shape Their Differential Prevalence in the Field. Front. Microbiol. 2021, 12, 739521. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A.; Degnan, P.H.; Santos, S.R.; Dunbar, H.E.; Ochman, H. The Players in a Mutualistic Symbiosis: Insects, Bacteria, Viruses, and Virulence Genes. Proc. Natl. Acad. Sci. USA 2005, 102, 16919–16926. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Wang, S.; Su, Y.-L.; Bing, X.-L.; Liu, S.-S.; Wang, X.-W. Draft Genome Sequence of “Candidatus Hamiltonella Defensa”, an Endosymbiont of the Whitefly Bemisia tabaci. J. Bacteriol. 2012, 194, 3558. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Harwood, J.D.; Liu, T.; Chu, D. Novel Proteome and Acetylome of Bemisia tabaci Q in Response to Cardinium Infection. BMC Genom. 2018, 19, 523. [Google Scholar] [CrossRef]
- Fang, Y.-W.; Liu, L.-Y.; Zhang, H.-L.; Jiang, D.-F.; Chu, D. Competitive Ability and Fitness Differences between Two Introduced Populations of the Invasive Whitefly Bemisia tabaci Q in China. PLoS ONE 2014, 9, e100423. [Google Scholar] [CrossRef]
- Santos-Garcia, D.; Rollat-Farnier, P.-A.; Beitia, F.; Zchori-Fein, E.; Vavre, F.; Mouton, L.; Moya, A.; Latorre, A.; Silva, F.J. The Genome of Cardinium CBtQ1 Provides Insights into Genome Reduction, Symbiont Motility, and Its Settlement in Bemisia tabaci. Genome Biol. Evol. 2014, 6, 1013–1030. [Google Scholar] [CrossRef]
- Tsagkarakou, A.; Tsigenopoulos, C.S.; Gorman, K.; Lagnel, J.; Bedford, I.D. Biotype Status and Genetic Polymorphism of the Whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) in Greece: Mitochondrial DNA and Microsatellites. Bull. Entomol. Res. 2007, 97, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in Resistance to Parasitism in Aphids Is Due to Symbionts Not Host Genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, Y.; Ghanim, M.; Gueguen, G.; Kontsedalov, S.; Vavre, F.; Fleury, F.; Zchori-Fein, E. Inherited Intracellular Ecosystem: Symbiotic Bacteria Share Bacteriocytes in Whiteflies. FASEB J. 2008, 22, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Vorburger, C.; Gehrer, L.; Rodriguez, P. A Strain of the Bacterial Symbiont Regiella insecticola Protects Aphids against Parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Sieber, R.; Zimmermann, Y.-S.; Vorburger, C. Development, Specificity and Sublethal Effects of Symbiont-Conferred Resistance to Parasitoids in Aphids. Funct. Ecol. 2012, 26, 207–215. [Google Scholar] [CrossRef]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma Bacteria Enhance Survival of Drosophila hydei Attacked by the Parasitic Wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.; Winter, L.; Winter, C.; Higareda-Alvear, V.M.; Martinez-Romero, E.; Xie, J. Independent Origins of Resistance or Susceptibility of Parasitic Wasps to a Defensive Symbiont. Ecol. Evol. 2016, 6, 2679–2687. [Google Scholar] [CrossRef]
- Paredes, J.C.; Herren, J.K.; Schüpfer, F.; Lemaitre, B. The Role of Lipid Competition for Endosymbiont-Mediated Protection against Parasitoid Wasps in Drosophila. mBio 2016, 7, e01006-16. [Google Scholar] [CrossRef]
- Sochard, C.; Bellec, L.; Simon, J.-C.; Outreman, Y. Influence of “Protective” Symbionts throughout the Different Steps of an Aphid–Parasitoid Interaction. Curr. Zool. 2021, 67, 441–453. [Google Scholar] [CrossRef]
- Łukasik, P.; Dawid, M.A.; Ferrari, J.; Godfray, H.C.J. The Diversity and Fitness Effects of Infection with Facultative Endosymbionts in the Grain Aphid, Sitobion Avenae. Oecologia 2013, 173, 985–996. [Google Scholar] [CrossRef]
- Attia, S.; Renoz, F.; Pons, I.; Louâpre, P.; Foray, V.; Piedra, J.-M.; Sanané, I.; Le Goff, G.; Lognay, G.; Hance, T. The Aphid Facultative Symbiont Serratia Symbiotica Influences the Foraging Behaviors and the Life-History Traits of the Parasitoid Aphidius ervi. Entomologia 2022, 42, 21–33. [Google Scholar] [CrossRef]
- Hertaeg, C.; Risse, M.; Vorburger, C.; De Moraes, C.M.; Mescher, M.C. Aphids Harbouring Different Endosymbionts Exhibit Differences in Cuticular Hydrocarbon Profiles That Can Be Recognized by Ant Mutualists. Sci. Rep. 2021, 11, 19559. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Noge, K.; Huang, E.M.; Campos, J.M.; Becerra, J.X.; Hunter, M.S. Parasitic Wasp Responses to Symbiont-Based Defense in Aphids. BMC Biol. 2012, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Mandour, N.S.; Ren, S.X.; Qiu, B.L. Effect of Bemisia tabaci honeydew and its carbohydrates on search time and parasitization of Encarsia bimaculata. J. Appl. Entomol. 2007, 131, 645–651. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Pirk, C.W.W.; Deletre, E. Chemical Cues From Honeydew and Cuticular Extracts of Trialeurodes vaporariorum Serve as Kairomones for The Parasitoid Encarsia formosa. J. Chem. Ecol. 2022, 48, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, A.R.; Van Alphen, J.J.; Godfray, H.C. The Coevolution of Host Resistance and Parasitoid Virulence. Parasitology 1998, 116 (Suppl. S1), S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Dion, E.; Zélé, F.; Simon, J.-C.; Outreman, Y. Rapid Evolution of Parasitoids When Faced with the Symbiont-Mediated Resistance of Their Hosts: Symbiont Effects on Host-Parasitoid Evolution. J. Evol. Biol. 2011, 24, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Rouchet, R.; Vorburger, C. Experimental Evolution of Parasitoid Infectivity on Symbiont-Protected Hosts Leads to the Emergence of Genotype Specificity. Evolution 2014, 68, 1607–1616. [Google Scholar] [CrossRef]
- Dennis, A.B.; Patel, V.; Oliver, K.M.; Vorburger, C. Parasitoid Gene Expression Changes after Adaptation to Symbiont-Protected Hosts. Evolution 2017, 71, 2599–2617. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Godfray, H.C.J. Evolution of host resistance and parasitoid counter-resistance. Adv. Parasitol. 2009, 70, 257–280. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorgini, M.; Formisano, G.; García-García, R.; Bernat-Ponce, S.; Beitia, F. The Susceptibility of Bemisia tabaci Mediterranean (MED) Species to Attack by a Parasitoid Wasp Changes between Two Whitefly Strains with Different Facultative Endosymbiotic Bacteria. Insects 2023, 14, 808. https://doi.org/10.3390/insects14100808
Giorgini M, Formisano G, García-García R, Bernat-Ponce S, Beitia F. The Susceptibility of Bemisia tabaci Mediterranean (MED) Species to Attack by a Parasitoid Wasp Changes between Two Whitefly Strains with Different Facultative Endosymbiotic Bacteria. Insects. 2023; 14(10):808. https://doi.org/10.3390/insects14100808
Chicago/Turabian StyleGiorgini, Massimo, Giorgio Formisano, Rosalía García-García, Saúl Bernat-Ponce, and Francisco Beitia. 2023. "The Susceptibility of Bemisia tabaci Mediterranean (MED) Species to Attack by a Parasitoid Wasp Changes between Two Whitefly Strains with Different Facultative Endosymbiotic Bacteria" Insects 14, no. 10: 808. https://doi.org/10.3390/insects14100808
APA StyleGiorgini, M., Formisano, G., García-García, R., Bernat-Ponce, S., & Beitia, F. (2023). The Susceptibility of Bemisia tabaci Mediterranean (MED) Species to Attack by a Parasitoid Wasp Changes between Two Whitefly Strains with Different Facultative Endosymbiotic Bacteria. Insects, 14(10), 808. https://doi.org/10.3390/insects14100808








