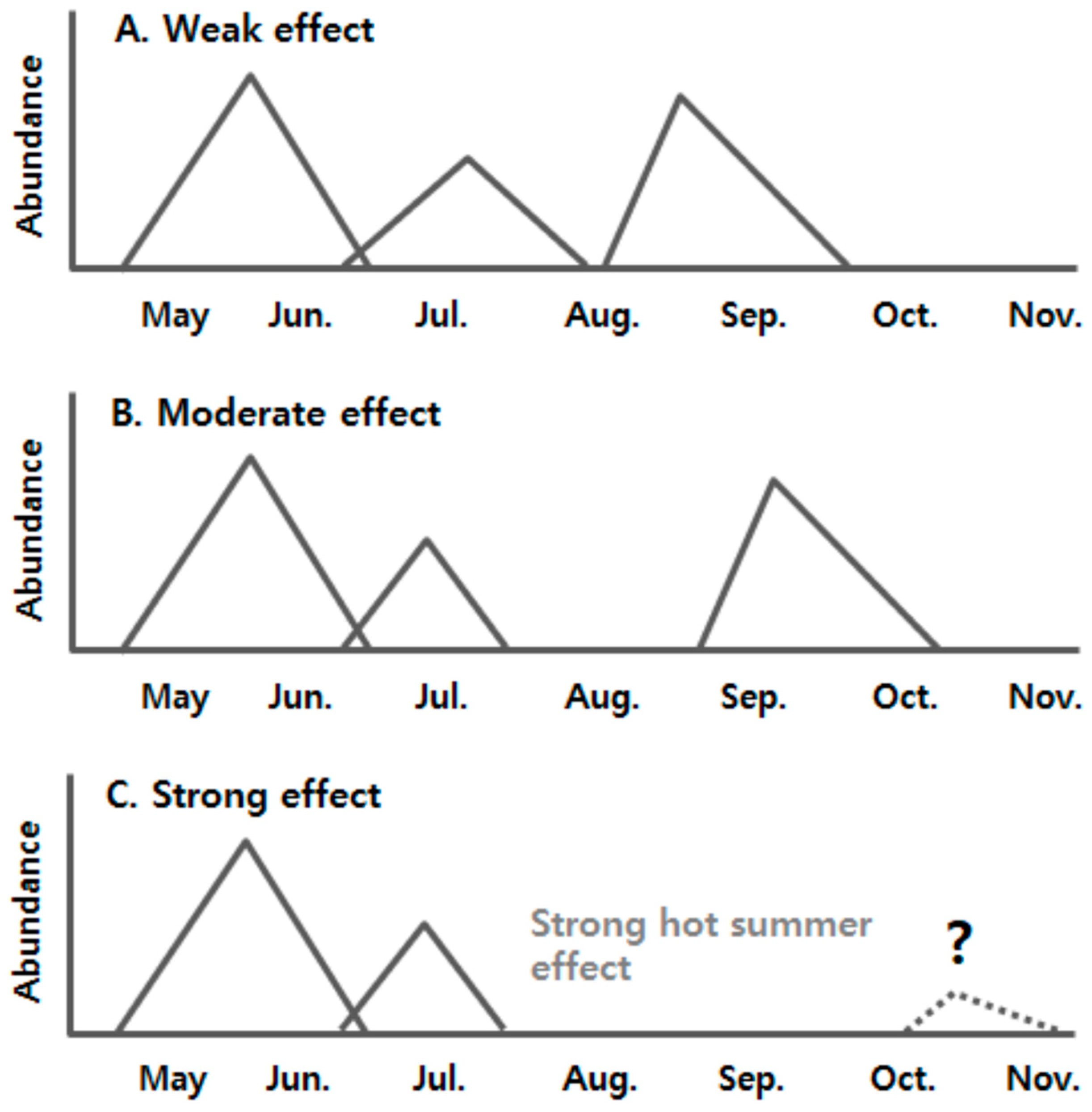
A New Perspective to Understand the Late Season Abundance of Delia antiqua (Diptera: Anthomyiidae): A Modeling Approach for the Hot Summer Effect
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
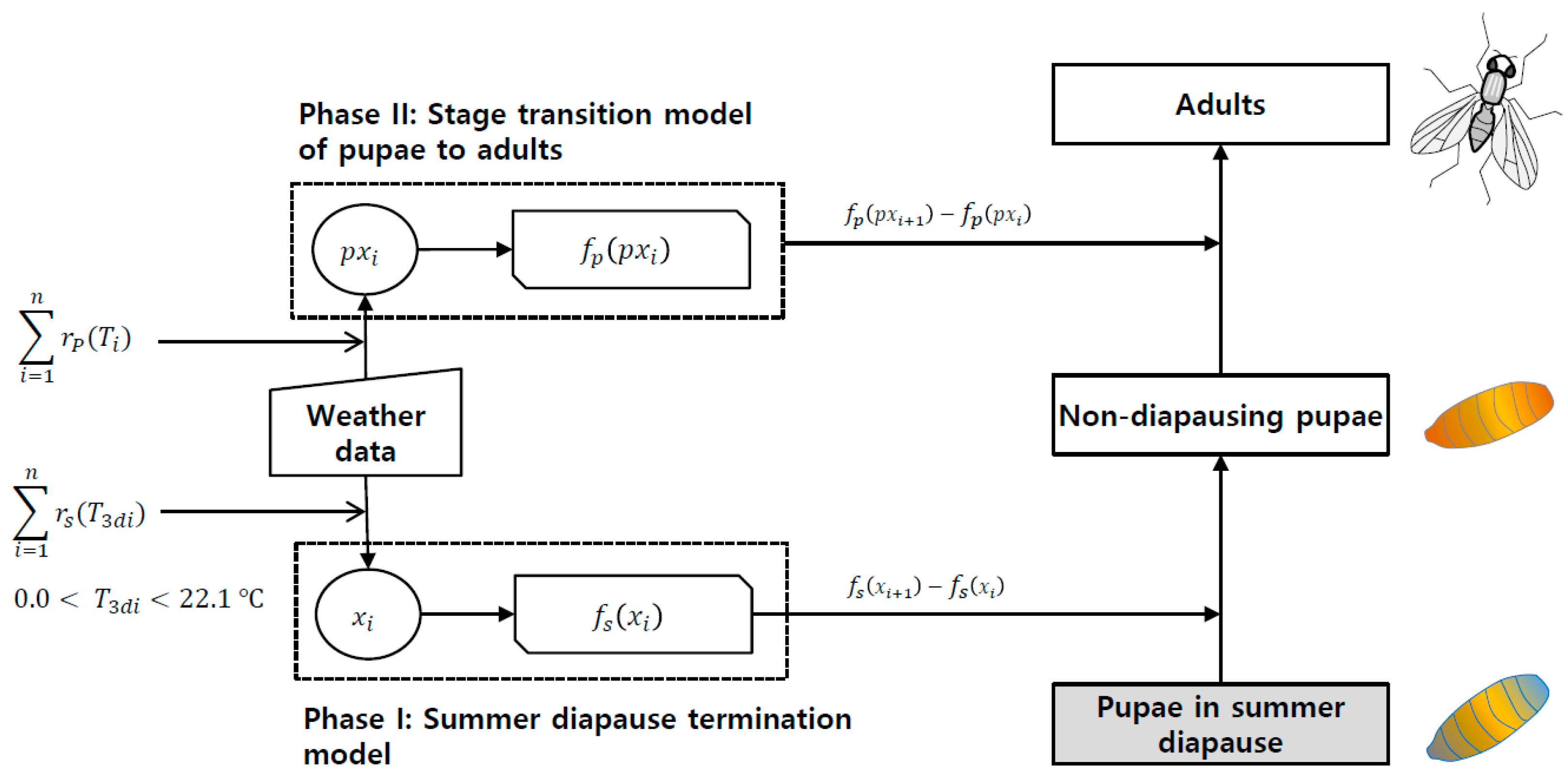
2.1. The Summer Diapause Termination and Post-Diapause Development
2.1.1. Model for the Summer Diapause Termination (SDT), Phase I
2.1.2. Stage Transition of Non-Diapausing Pupae, Phase II
2.2. Estimation of Thermal Constant (Degree Days) for Stage Development
2.3. Model Simulation and Field Comparison
2.3.1. Model Overview and Simulation
2.3.2. Field Data Collection
2.3.3. Meteorological Data
2.3.4. Model Comparison with Field Data
3. Results
3.1. The Summer Diapause Termination and Post-Diapause Development
3.1.1. Model for the Summer Diapause Termination (SDT)
3.1.2. Stage Transition of Non-Diapausing Pupae
3.2. Estimation of Degree Days for Generation Time
3.3. Model Simulation and Comparison with Field Data
4. Discussion
4.1. Model Parameters and Simulation
4.2. Comparison of Model Outputs with Field Data and Its Application
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch, S. Ecological considerations in the management of Delia pest species in vegetable crops. Ann. Rev. Entomol. 1989, 34, 117–137. [Google Scholar]
- Collier, R.; Mazzi, D.; Schjøll, A.F.; Schorpp, Q.; Thöming, G.; Johansen, T.J.; Meadow, R.; Meyling, N.V.; Cortesero, A.-M.; Vogler, U.; et al. The potential for decision support tools to improve the management of root-feeding fly eests of vegetables in Western Europe. Insects 2020, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.P.; Petzoldt, C.H.; Frodsham, A.C. Integrated Pest Management for Onions; Cornell Cooperative Extension: New York, NY, USA, 1996; p. 78. [Google Scholar]
- Larraín, P.S. Population variation, and damage of Delia antiqua (Meigen) and Delia platura (Meigen) (Diptera: Anthomyiidae) on onions seedlings (Allium cepa L.) in north-central area of Chile. Agric. Tec. 1994, 54, 60–64, (In Spanish with English abstract). [Google Scholar]
- Smith, E.; Nault, B. Onion Maggot: Delia antiqua Meigen; Cornell University: Ithaca, NY, USA, 2014. [Google Scholar]
- Ishikawa, Y.; Tsukada, S.; Matsumoto, Y. Effect of temperature and photoperiod on the larval development and diapause induction in the onion fly, Hylemya antiqua Meigen (Diptera: Anthomyiidae). Appl. Entomol. Zool. 1987, 22, 610–616. [Google Scholar] [CrossRef]
- Eckenrode, C.J.; Vea, E.V.; Stone, K.W. Population trends of onion maggots correlated with air thermal unit accumulations. Environ. Entomol. 1975, 4, 785–789. [Google Scholar] [CrossRef]
- Perron, J.; Lafrance, J. Notes on the life-history of the onion maggot, Hylemya antiqua (Meig.) (Diptera: Anthomyiidae) reared in field cages. Can. Entomol. 1961, 93, 101–106. [Google Scholar] [CrossRef]
- Liu, H.J.; McEwen, F.L.; Ritcey, G. Forecasting events in the life cycle of the onion maggot, Hylemya antiqua (Diptera: Anthomyiidae): Application to control schemes. Environ. Entomol. 1982, 11, 751–755. [Google Scholar] [CrossRef]
- Otto, M.; Hommes, M. Development of a simulation model for the population dynamics of the onion fly Delia antiqua in Germany. OEPP/EPPO Bull. 2000, 30, 115–119. [Google Scholar] [CrossRef]
- Grichanov, I.Y.; Ovsyannikova, E.I. Delia antiqua (Meigen), Onion fly. AgroAtlas, Russia. Available online: http://www.agroatlas.ru/en/content/pests/Delia_antiqua/index.html (accessed on 6 January 2023).
- Workman, R.B., Jr. Biology of the Onion Maggot, Hylemya antiqua (Meigen) under Field and Greenhouse Conditions. PhD Thesis, Oregon State University, Corvallis, OR, USA, 1958. [Google Scholar]
- Nault, B.A.; Straub, R.W.; Taylor, A.G. Performance of novel insecticide seed treatments for managing onion maggot (Diptera: Anthomyiidae) in onion fields. Crop Prot. 2006, 25, 58–65. [Google Scholar] [CrossRef]
- Walters, T.W.; Eckenrode, C.J. Integrated management of the onion maggot (Diptera: Anthomyiidae). J. Econ. Entomol. 1996, 89, 1582–1586. [Google Scholar] [CrossRef]
- Moretti, E.; Nault, B.A. Onion maggot control in onion, 2019. Arthropod Manag. Tests. 2020, 45, 1. [Google Scholar] [CrossRef]
- Sapáková, E.; Šefrová, H.; Hasíková, L.; Hřivna, L. Harmfulness of Delia antiqua (Diptera: Anthomyiidae) in garlic in Southeast and Central Moravia. Acta Univ. Agric. Silvic. Mendel. Brunensi. 2016, 64, 871–876. [Google Scholar] [CrossRef]
- Park, C.G.; Hyun, J.S.; Shin, W.K. Relationship between pupation depth, pupation type, thermal unit accumulation and the emergence overwintered pupae of onion fly, Delia antique Meigen (Diptera: Anthomyiidae). Res. Rept. RDA (Plant prot.) 1987, 29, 130–137, (In Korean with English abstract). [Google Scholar]
- Werling, B.P. Onion Maggot Delia antique (Diptera: Anthomyiidae), Adult Activity and Oviposition in New York Onion Field: Implications for Management. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2004. [Google Scholar]
- Thöming, G.; Schjøll, A.F.; Johansen, T.J. Developing tools for monitoring and forecasting of onion fly Delia antiqua in Norway. IOBC/WPRS Bull. 2019, 142, 42–49. [Google Scholar]
- Ning, S.; Wei, J.; Feng, J. Predicting the current potential and future worldwide distribution of the onion maggot, Delia antiqua using maximum entropy ecological niche modeling. PLoS ONE 2017, 12, e0171190. [Google Scholar] [CrossRef]
- Moretti, E. Factors Influencing Damage from Delia antiqua in Onion and Activity of Spinosad Seed Treatments Used in Their Control. Master’s Thesis, Cornell University, Ithaca, NY, USA, 2020. [Google Scholar]
- Miles, M. Studies of British Anthomyiid Flies. IX. Biology of the onion fly, Delia antiqua (Mg.). Bull. Entomol. Res. 1958, 49, 405–414. [Google Scholar] [CrossRef]
- Keller, J.E.; Miller, J.R. Onion fly oviposition as influenced by soil temperature. Entomol. Exp. Appl. 1990, 54, 37–42. [Google Scholar] [CrossRef]
- Robinson, A.S.; Zurlini, G. The response of two stains of Hylemya antiqua (Diptera: Anthomyiidae) to a constant and an alternative temperature regime. Can. Entomol. 1970, 111, 1207–1217. [Google Scholar] [CrossRef]
- Park, C.G. Ecological Studies on the Summer and Winter Diapause of the Onion Maggot, Delia antiqua (Meigen) (Diptera: Anthomyiidae). Ph.D. Dissertation, Seoul National University, Suwon, Republic of Korea, 1989. (In Korean with English abstract). [Google Scholar]
- Ishikawa, Y.; Yamashita, T.; Nomura, M. Characteristics of summer diapause in the onion maggot, Delia antiqua (Diptera: Anthomyiidae). J. Insect Physiol. 2000, 46, 161–167. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.; Sharpe, P.J.H.; Schoolfield, R.M.; Coulson, R.N. Modeling insect development rates: A literature review and application of a biophysical model. Ann. Entomol. Soc. Am. 1984, 77, 208–225. [Google Scholar] [CrossRef]
- Jandel Scientific. TableCurve 2D: Automated Curve Fitting and Equation Discovery, version 5.01; Jandel Scientific: San Rafael, CA, USA, 2002. [Google Scholar]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.L.; Craigen, R. Improved rate model of temperature-dependent development by arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, D.-S. Temperature-dependent development of Ascotis selenaria (Lepidoptera: Geometridae) and its stage emergence models with field validation. Crop Prot. 2014, 66, 72–79. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.-A.; Kim, G.-H.; Kim, S.-B.; Kim, D.-S. Temperature-dependent development of Agrotis ipsilon (Lepidoptera: Noctuidae) and its stage transition models. Phytoparasitica 2023, 51, 199–214. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Damos, P.T.; Savopoulou-Soultani, M. Temperature dependent bionomics and modeling of Anarsia lineatella (Lepidoptera: Gelechiidae) in the laboratory. J. Econ. Entomol. 2008, 101, 1557–1567. [Google Scholar] [CrossRef]
- Wagner, T.L.; Wu, H.; Sharpe, P.J.H.; Coulson, R.N. Modeling distributions of insect development time: A literature review and application of the Weibull function. Ann. Entomol. Soc. Am. 1984, 77, 475–487. [Google Scholar] [CrossRef]
- Weibull, W. A Statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar] [CrossRef]
- Cockfield, S.D.; Butkewich, S.L.; Samoil, K.S.; Mahr, D.L. Forecasting fright activity of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. J. Econ. Entomol. 1994, 87, 193–196. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-H.; Yiem, M.S. Temperature-dependent development of Carposina sasakii (Lepidoptera: Carposinidae), and its stage emergence models. Environ. Entomol. 2002, 30, 298–305. [Google Scholar] [CrossRef]
- Curry, G.L.; Feldman, R.M.; Sharped, P.J.H. Foundation of stochastic development. J. Theor. Biol. 1978, 74, 397–410. [Google Scholar] [CrossRef]
- Curry, G.L.; Feldman, R.M.; Smith, K.C. A stochastic model of a temperature-dependent population. J. Theor. Pop. Biol. 1978, 13, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.L.; Wu, H.; Feldman, R.M.; Sharpe, P.J.H.; Coulson, R.N. Multiple-cohort approach for simulating development of insect populations under variable temperatures. Ann. Entomol. Soc. Am. 1985, 78, 691–704. [Google Scholar] [CrossRef]
- WebPlotDigitizer. Web Based Plot Digitizer, Version 4.5.archived on 15 August 2021. Available online: https://apps.automeris.io/wpd/ (accessed on 10 October 2022).
- Kim, S.B.; Kim, D.-S. A tentative evaluation for population establishment of Bactrocera dorsalis (Diptera:Tephritidae) by its population modeling: Considering the temporal distribution of host plants in a selected area in Jeju, Korea. J. Asia-Pac. Entomol. 2018, 21, 451–465. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.-S. A new direction to understand the life cycle of the Japanese pine sawyer considering the selection strategy of instar pathways. Sci. Rep. 2020, 10, 16952. [Google Scholar] [CrossRef]
- Lin, S.; Hudson, A.C.; Richards, A.G. An analysis of threshold temperatures for the development of Oncopeltus and Tribolium eggs. Physiol. Zool. 1954, 27, 287–310. [Google Scholar] [CrossRef]
- Curry, G.L.; Feldman, R.M. Mathematical Foundations of Population Dynamics; Texas A&M University Press: College Station, TX, USA, 1987; p. 246. [Google Scholar]
- Kim, D.-S.; Lee, J.-H. A population model for the peach fruit moth, Carposin sasakii Matsumura (Lepidoptera: Carposinidae), in a Korean orchard system. Ecol. Model. 2010, 221, 268–280. [Google Scholar] [CrossRef]
- Shaffer, P.L.; Gold, H.J. A simulation model of population dynamics of the codling moth, Cydia pomonella. Ecol. Model. 1985, 30, 247–274. [Google Scholar]
- Willett, D.S.; Filgueiras, C.C.; Nyrop, J.P.; Nault, B.A. Field monitoring of onion maggot (Delia antiqua) fly through improved trapping. J. Appl. Entomol. 2020, 144, 382–387. [Google Scholar] [CrossRef]
- Savage, J.; Fortier, A.-M.; Fournier, F.; Bellavance, V. Identification of Delia pest species (Diptera: Anthomyiidae) in cultivated crucifers and other vegetable crops in Canada. Can. J. Arthropod Identif. 2016, 29, 1–40. [Google Scholar] [CrossRef]
- Choi, C.S. A study on the life cycle and control of onion maggot. In Annual Research Report for 1980; Jeonnam Agricultural Research and Extension Services (RDA): Naju, Republic of Korea, 1981; pp. 561–564. (In Korean) [Google Scholar]
- Park, C.G.; Cho, D.S.; Shin, W.G. A study on the ecology of onion maggot in the southern region in Korea. In Final Research Report for 1984 to 1987; Gyeongnam Agricultural Research and Extension Services (RDA): Jinju, Republic of Korea, 1988; pp. 485–495. (In Korean) [Google Scholar]
- Choi, K.S.; Ahn, J.J.; Hwang, S.-Y.; Samyoa, A.C. Development of population model of foreign fruit fly and prediction of their settlement to cope with climate change. In The Final Report of International Cooperative Research Project; Project No. PJ012075 (1 March 2016–31 December 2018); NIHHS; RDA: Cheonju, Republic of Korea, 2019; (In Korean with English abstract). [Google Scholar]
- Park, C.G.; Hyun, J.S.; Cho, D.J.; Choi, K.M. Seasonal occurrence and summer diapause of the onion maggot, Delia antiqua (Meigen) (Diptera: Anthomyiidae). Korean J. Appl. Entomol. 1990, 29, 230–237, (In Korean with English abstract). [Google Scholar]
- Kim, H.S.; Kim, S.H.; Choi, G.M. Ecological investigation of major vegetable pests. In Annual Research Report of 1982; Agricultural Science Institute, RDA: Suwon, Republic of Korea, 1982; Entomology 6; pp. 413–443. (In Korean) [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; W.H. Freeman and Co.: San Francisco, CA, USA, 1981; p. 859. [Google Scholar]
- Collier, R.H.; Finch, S. Accumulated temperatures for predicting the time of emergence in the spring of the cabbage root fly, Delia radicum L. (Diptere: Anthomyiidae). Bull. Entomol. Res. 1985, 75, 395–404. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Kim, D.-S.; Cho, M.R.; Chang, Y.D.; Yiem, M.S. Temperature-dependent Development of Pseudococcus comstocki (Homoptera: Pseudococcidae) and its stage transition models. Korean J. Appl. Entomol. 2003, 42, 43–51, (In Korean with English abstract). [Google Scholar]
- Choi, S.; Heo, J.; Kim, S.; Jwa, M.; Shin, S.; Kim, D.-S. Estimation of the source adult population for Agrotis ipsilon (Lepidoptera: Noctuidae) appearing in early spring in Korea: An approach with phenology modeling. Korean J. Agric. For. Meteorol. 2023, 25, 37–47. [Google Scholar]
- Jeffers, J.N.R. Outline Studies in Ecology, Modeling; Chapman and Hill: London, UK; New York, NY, USA, 1982; p. 80. [Google Scholar]
- Gomes, L.R.P.; Zawadneak, M.A.C.; da Costa-Ribeiro, M.C.V.; Jarek, T.M.; de Carvalho, C.J.B. Integrating morphology and DNA barcodes for identification of Delia sanctijacobi (Diptera: Anthomyiidae): New host and new records in South America. Arthropod Sys. Phylogeny 2022, 80, 511–522. [Google Scholar] [CrossRef]
- Sauer, C. Possible impacts of climate change on carrot fly’s population dynamics in Switzerland. In Proceedings of the Working Group, Arenenberg, Switzerland, 2–6 October 2017. [Google Scholar]
- Villeneuve, F.; Latour, F. A few biological specificities of the carrot root fly (Psila rosae Fabre) for a more accurate forecasting in Carrot (Daucus carota L.) production. In Proceedings of the International Symposium on Carrot and Other Apiaceae, Angers, France, 17–19 September 2014. [Google Scholar]
- Ramakers, P.M.J. Diapause induction in the onion fly, Hylemya antiqua (Meigen), under the influence of photoperiod and temperature. P.h.D. Dissertation, Department of Entomology, Wageningen University and Research, Wageningen, The Netherlands, 1973; p. 37, (In Dutch with English abstract). [Google Scholar]
- Loosjes, M. Ecology and Genetic Control of the Onion Flv, Delia antiqua (Meigen). PhD Thesis, University of Leiden (NL), Leiden, The Netherlands, 1976. [Google Scholar]
- La Tanzubil, P.B.; Terbobri, P.; Ansoba, E. Incidence and control of onion fly, Delia antiqua, at some irrigation sites in the Upper East Region of Ghana. Ghana J. Agric. Sci. 1999, 32, 205–211. [Google Scholar] [CrossRef]
- Lee, G.H.; Choi, M.Y. An experiment to improve the control system for Delia flies. In Final Research Report for 1986 to 1988; Honam Agricultural Experiment Station (RDA): Cheonju, Republic of Korea, 1999; pp. 698–701. (In Korean) [Google Scholar]
- Nault, B.A.; Zhao, J.-Z.; Straub, R.W.; Nyrop, J.P.; Hessney, M.L. Onion maggot (Diptera: Anthomyiidae) resistance to chlorpyrifos in New York onion fields. J. Econ. Entomol. 2006, 99, 1375–1380. [Google Scholar] [CrossRef]
- Wilson, R.G.; Orloff, S.B.; Taylor, A.G. Evaluation of insecticides and application methods to protect onions from onion maggot, Delia antiqua, and seedcorn maggot, Delia platura, damage. Crop Prot. 2015, 67, 102–108. [Google Scholar] [CrossRef]
- Salgado, L.D.; Nault, B.A. Evaluating foliar insecticide applications and seed treatments for onion maggot control in onion, 2022. Arthropod Manag. Tests 2023, 48, tsad009. [Google Scholar] [CrossRef]
- Spencer, J.L.; Miller, J.R. Lifetime ovipositional patterns of mated and virgin onion flies, Delia antiqua (Diptera: Anthomyiidae). J. Insect Physiol. 2002, 48, 171–180. [Google Scholar]
- Ning, S.; Zhang, W.; Sun, Y.; Feng, J. 2017. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef]
- Snyder, R.L. DegDay.xls, Version 1.01. University of California, Department of Land, Air and Water Resources, Atmospheric Science, Davis, California, USA. Archived on 10 April 2005. Available online: http://biomet.ucdavis.edu/DegreeDays/DegDay.htm (accessed on 13 May 2021).




| Model | Parameter | Estimated | SE | R2 |
|---|---|---|---|---|
| Termination of summer diapause | 0.16978 | 0.013258 | 0.97 | |
| 22.13802 | 0.268383 | |||
| 5.67016 | 0.395566 | |||
| Distribution of summer diapause completion | 1.15460 | 0.036266 | 0.97 | |
| 1.71717 | 0.119902 |
| Model | Parameter | Estimated | SE | R2 |
|---|---|---|---|---|
| Development rate | 0.151932200 | 0.019070840 | 0.94 | |
| 37.88922166 | 3.322196970 | |||
| 6.572996371 | 0.822062604 | |||
| Distribution of development time | 1.121146325 | 0.010050286 | 0.99 | |
| 3.155020615 | 0.121914537 |
| Percent Emergence | 1986 | 1987 | ||||
|---|---|---|---|---|---|---|
| Observed | Predicted | Difference a | Observed | Predicted | Difference | |
| 10 | 256.0 | 267.0 | 11.0 | 261.0 | 265.0 | 4.0 |
| 15 | 260.0 | 268.0 | 8.0 | 261.5 | 266.5 | 5.0 |
| 25 | 263.5 | 270.0 | 6.5 | 263.5 | 268.5 | 5.0 |
| 50 | 266.5 | 273.5 | 7.0 | 267.5 | 274.5 | 7.0 |
| 75 | 269.5 | 278.5 | 9.0 | 269.5 | 279.0 | 9.5 |
| 90 | 275.0 | 282.0 | 7.0 | 279.0 | 282.0 | 3.0 |
| 95 | 279.0 | 284.0 | 5.0 | 284.0 | 284.0 | 0.0 |
| Mean | 7.64 *b | 4.79 ns | ||||
| SE | 0.7296 | 1.331 | ||||
| t-value | 3.501 | 0.1891 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.K.; Kim, S.; Park, C.G.; Kim, D.-S. A New Perspective to Understand the Late Season Abundance of Delia antiqua (Diptera: Anthomyiidae): A Modeling Approach for the Hot Summer Effect. Insects 2023, 14, 816. https://doi.org/10.3390/insects14100816
Shin YK, Kim S, Park CG, Kim D-S. A New Perspective to Understand the Late Season Abundance of Delia antiqua (Diptera: Anthomyiidae): A Modeling Approach for the Hot Summer Effect. Insects. 2023; 14(10):816. https://doi.org/10.3390/insects14100816
Chicago/Turabian StyleShin, Yong Kyun, Subin Kim, Chung Gyoo Park, and Dong-Soon Kim. 2023. "A New Perspective to Understand the Late Season Abundance of Delia antiqua (Diptera: Anthomyiidae): A Modeling Approach for the Hot Summer Effect" Insects 14, no. 10: 816. https://doi.org/10.3390/insects14100816
APA StyleShin, Y. K., Kim, S., Park, C. G., & Kim, D.-S. (2023). A New Perspective to Understand the Late Season Abundance of Delia antiqua (Diptera: Anthomyiidae): A Modeling Approach for the Hot Summer Effect. Insects, 14(10), 816. https://doi.org/10.3390/insects14100816






