Vertical Distribution of Fruit Flies (Diptera: Drosophilidae) in Deciduous Forests in the Center of European Russia
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling
2.3. Statistical Analysis
3. Results
3.1. Faunistic Composition
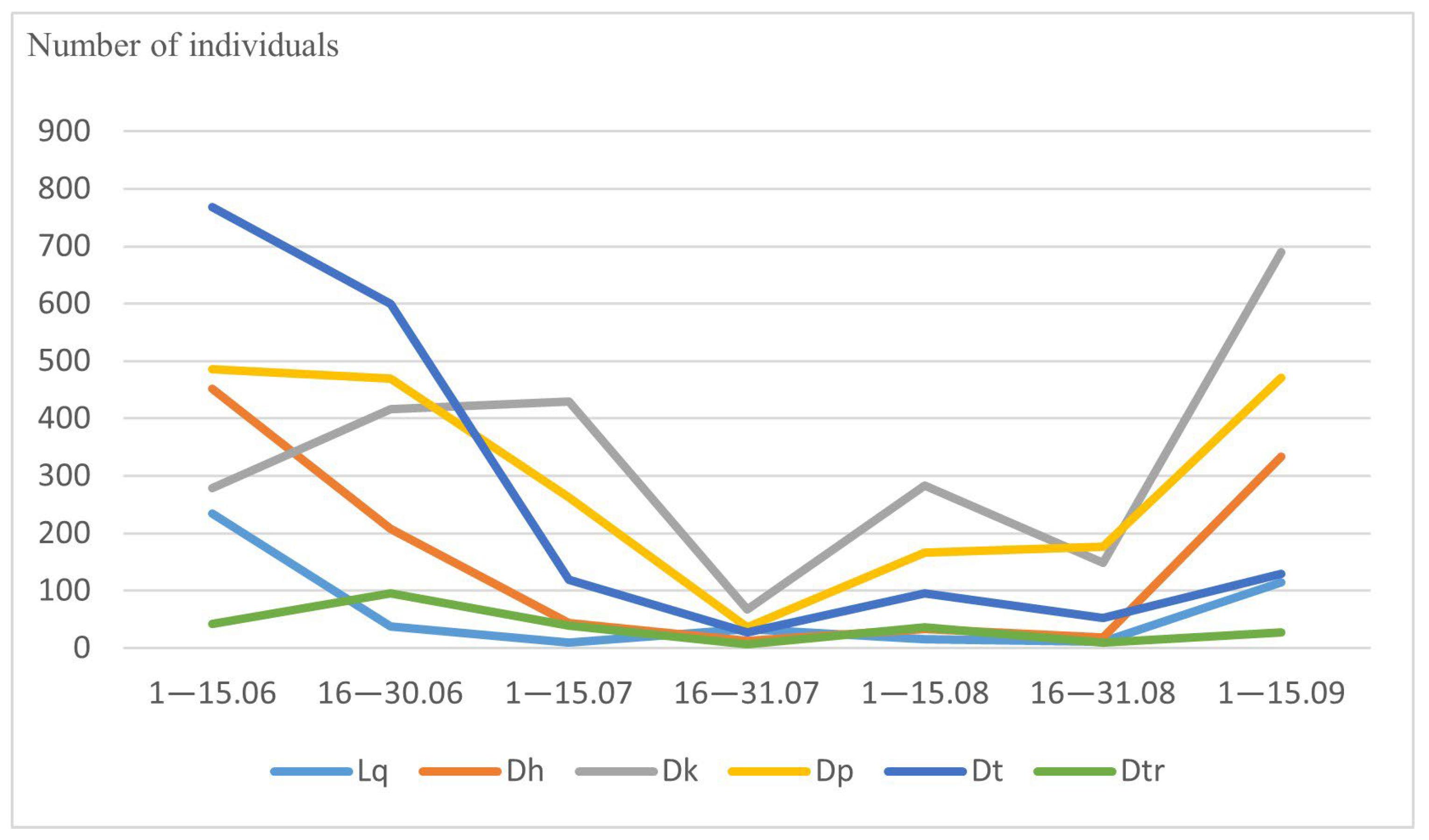
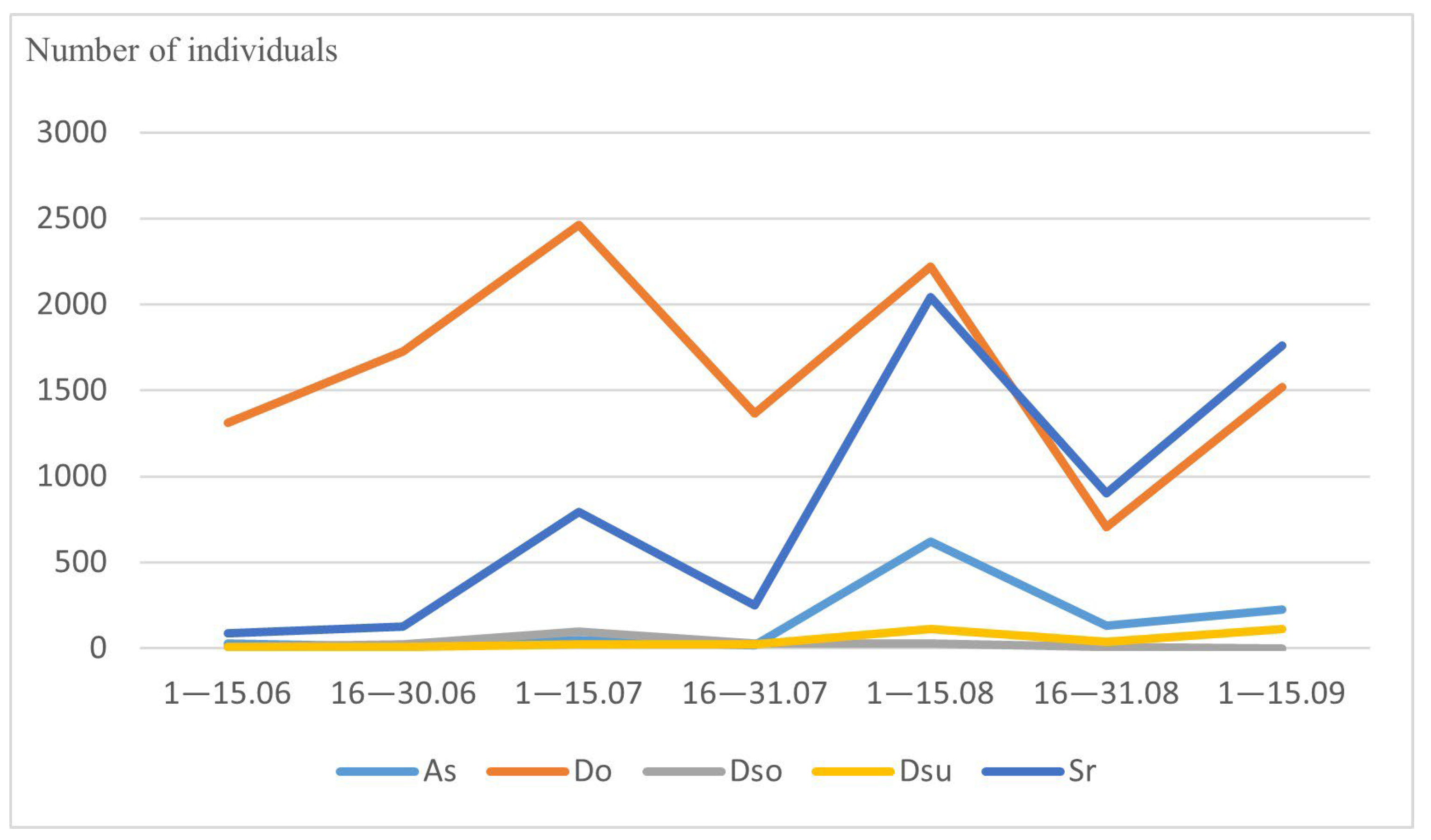
3.2. Abundance and Seasonal Dynamics of Drosophilidae
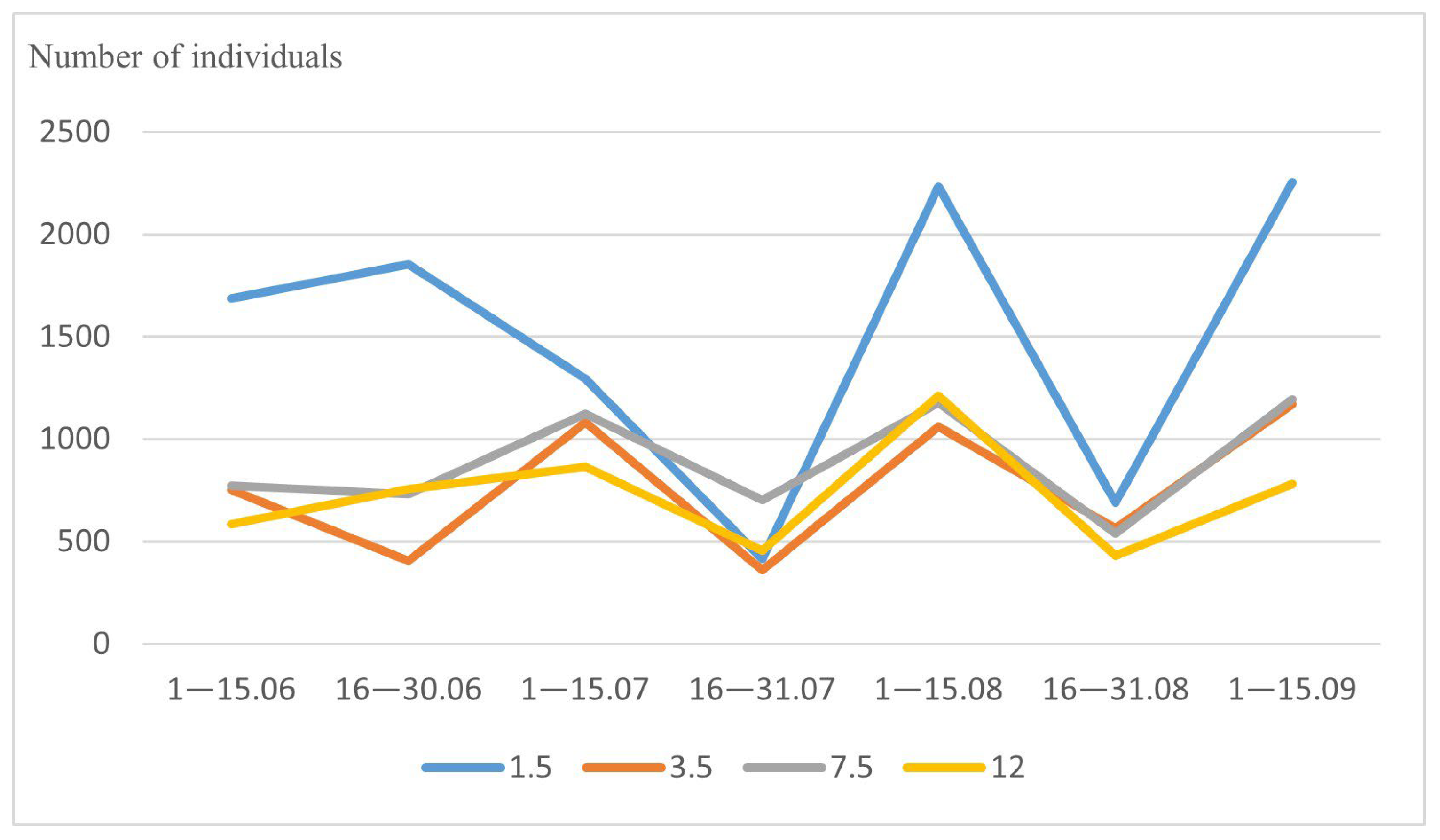
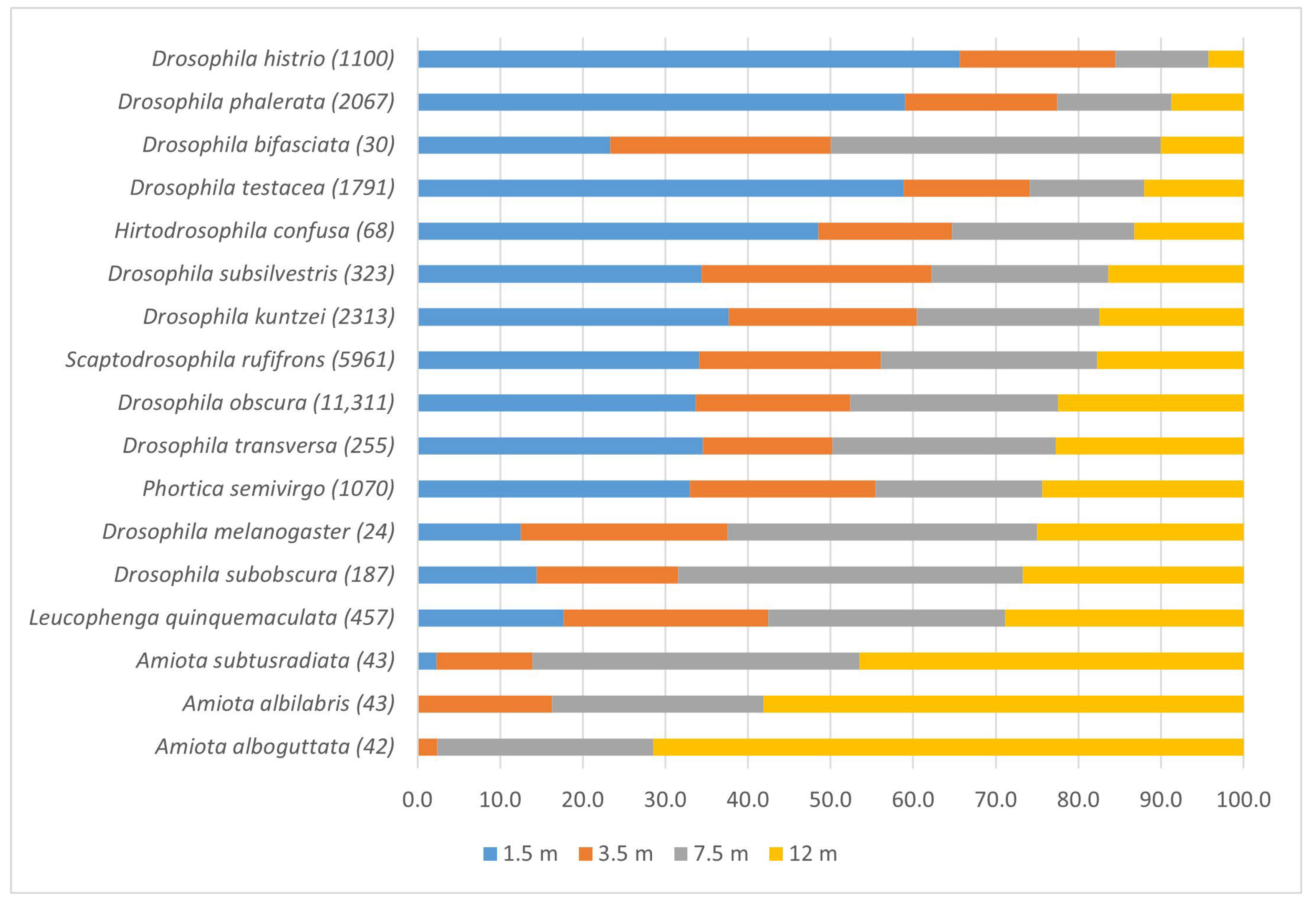
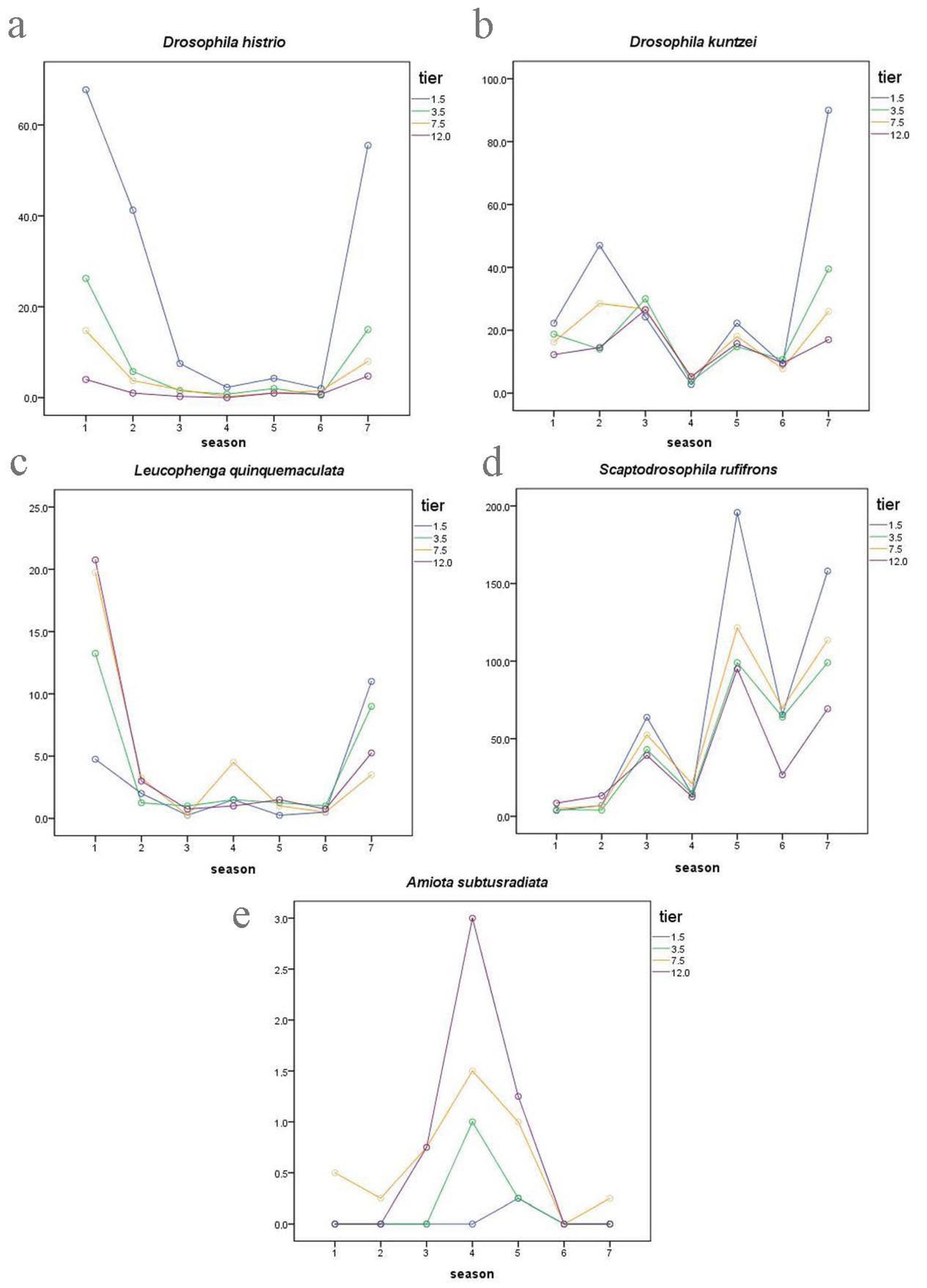
3.3. Vertical Distribution of Drosophilidae
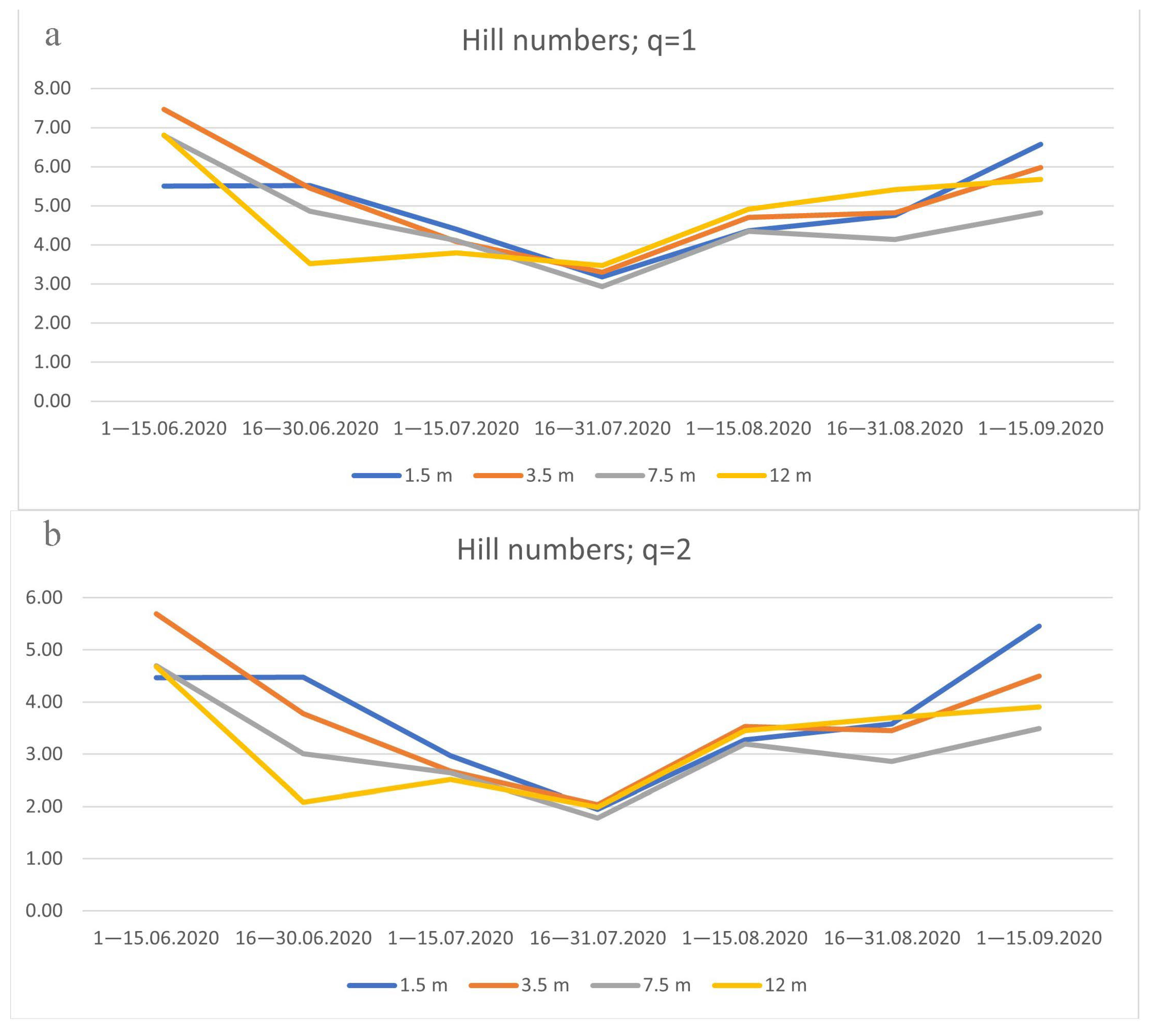
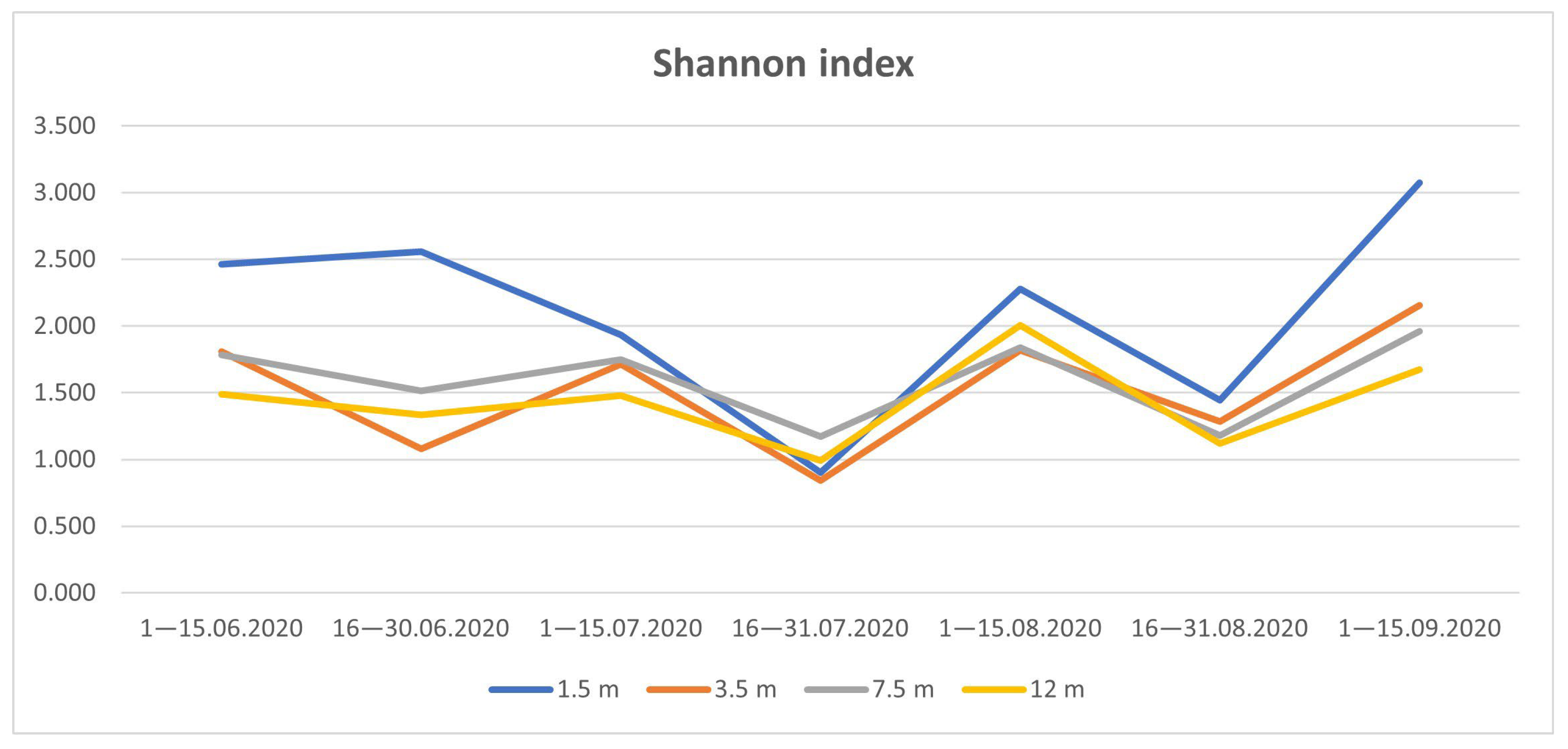
3.4. Species Diversity of Drosophildae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Brygadyrenko, V.V. Modeling the bioclimatic range of Pterostichus melanarius (Coleoptera, Carabidae) in conditions of global climate change. Biosyst. Divers. 2021, 29, 140–150. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Shashkov, M.P. Tree stand assessment before and after windthrow based on open-access biodiversity data and aerial photography. Nat. Conserv. Res. 2022, 7, 52–63. [Google Scholar] [CrossRef]
- Kharitonova, A.O.; Kharitonova, T.I. The effect of landscape pattern on the 2010 wildfire spread in the Mordovia State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 29–41. [Google Scholar] [CrossRef]
- Dedyukhin, S.V. Fauna and biotopic distribution of weevils (Coleoptera: Curculionoidea) of the Zhiguli State Nature Reserve, Russia. Nat. Conserv. Res. 2022, 7, 55–69. [Google Scholar] [CrossRef]
- Weiss, M.; Didham, R.K.; Procházka, J.; Schlaghamerský, J.; Basset, Y.; Odegaard, F.; Tichechkin, A.; Schmidl, J.; Floren, A.; Curletti, G.; et al. Saproxylic beetles in tropical and temperate forests—A standardized comparison of vertical stratification patterns. For. Ecol. Manag. 2019, 444, 50–58. [Google Scholar] [CrossRef]
- Wagle, Y.; Bhattarai, B.P.; Adhikari, J.N. Factors influencing distribution and habitat utilisation of Leptoptilos javanicus in and around Barandabhar corridor forest, Chitwan, Nepal. Nat. Conserv. Res. 2022, 7, 19–26. [Google Scholar] [CrossRef]
- Kirstová, M.; Pyszko, P.; Šipoš, J.; Drozd, P.; Kočárek, P. Vertical distribution of earwigs (Dermaptera: Forficulidae) in a temperate lowland forest, based on sampling with a mobile aerial lift platform. Entomol. Sci. 2017, 20, 57–64. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Soon, V.; Hanula, J.L. Vertical distribution and seasonality of predatory wasps (Hymenoptera: Vespidae) in a temperate deciduous forest. Fla. Entomol. 2011, 94, 1068–1070. [Google Scholar] [CrossRef]
- Parhomenko, O.; Langraf, V.; Petrovičová, K.; Komlyk, V.; Brygadyrenko, V. Morphometric variability of ground beetles Bembidion minimum (Coleoptera, Carabidae): Who should change more, males or females? Nat. Conserv. Res. 2022, 7, 42–69. [Google Scholar] [CrossRef]
- Hirao, T.; Murakami, M.; Kashizaki, A. Importance of the understory stratum to entomofaunal diversity in a temperate deciduous forest. Ecol. Res. 2009, 24, 263–272. [Google Scholar] [CrossRef]
- Bouget, C.; Brin, A.; Brustel, H. Exploring the “last biotic frontier”: Are temperate forest canopies special for saproxylic beetles? For. Ecol. Manag. 2011, 261, 211–220. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification of beetles in deciduous forest communities in the centre of European Russia. Diversity 2021, 13, 508. [Google Scholar] [CrossRef]
- Giovanni, F.; Mei, M.; Cerretti, P. Vertical stratification of selected Hymenoptera in a remnant forest of the Po Plain (Italy, Lombardy) (Hymenoptera: Ampulicidae, Crabronidae, Sphecidae). Fragm. Entomol. 2017, 49, 71–77. [Google Scholar] [CrossRef][Green Version]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodivers. J. Biol. Divers. 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Urban-Mead, K.R.; Muñiz, P.; Gillung, J.; Espinoza, A.; Fordyce, R.; van Dyke, M.; Mc Art, S.H.; Danforth, B.N. Bees in the trees: Diverse spring fauna in temperate forest edge canopies. For. Ecol. Manag. 2021, 482, 118903. [Google Scholar] [CrossRef]
- Sutton, S.L.; Hudson, P.J. The vertical distribution of flying insects in the lowland rain forest of Zaire. Zool. J. Linn. Soc. 1980, 68, 111–123. [Google Scholar] [CrossRef]
- Haack, N.; Borges, P.A.V.; Grimm-Seyfarth, A.; Schlegel, M.; Wirth, C.; Bernhard, D.; Brunk, I.; Henle, K.; Pereira, H.M. Response of common and rare beetle species to tree species and vertical stratification in a floodplain forest. Insects 2022, 13, 161. [Google Scholar] [CrossRef]
- Leksono, A.S.; Takada, K.; Koji, S.; Nakagoshi, N.; Anggraeni, T.; Nakamura, K. Vertical and seasonal distribution of flying beetles in a suburban temperate deciduous forest collected by water pan trap. Insect Sci. 2005, 12, 199–206. [Google Scholar] [CrossRef]
- Charles, E.; Basset, Y. Vertical stratification of leaf-beetle assemblages (Coleoptera: Chrysomelidae) in two forest types in Panama. J. Trop. Ecol. 2005, 21, 329–336. [Google Scholar] [CrossRef]
- Graham, E.E.; Poland, T.M.; Mc Cullough, D.G.; Millar, J.G. A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). J. Econ. Entomol. 2012, 105, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Puker, A.; Correa, C.M.A.; Silva, A.S.; Silva, J.V.O.; Korasaki, V.; Grossi, P.C. Effects of fruit-baited trap height on flower and leaf chafer scarab beetles sampling in Amazon rainforest. Entomol. Sci. 2020, 23, 245–255. [Google Scholar] [CrossRef]
- Maguire, D.Y.; Robert, K.; Brochu, K.; Larrivée, M.; Buddle, C.M.; Wheeler, T.A. Vertical stratification of beetles (Coleoptera) and flies (Diptera) in temperate forest canopies. Environ. Entomol. 2014, 43, 9–17. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Richenbacher, C. Exceptional species diversity of Drosophilidae (Diptera) in a Neotropical forest. Am. Mus. Novit. 2023, 3997, 1–28. [Google Scholar] [CrossRef]
- Yassin, A. Phylogenetic classification of the Drosophilidae Rondani (Diptera): The role of morphology in the postgenomic era. Syst. Entomol. 2013, 38, 349–364. [Google Scholar] [CrossRef]
- Miller, E.M.; Marshall, S.A.; Grimaldi, D.A. A review of the species of Drosophila (Diptera: Drosophilidae) and genera of Drosophilidae of northeastern North America. Can. J. Arthropod Identif. 2017, 31, 1–232. [Google Scholar]
- Gornostaev, N.G. Ecological classification of drosophilid flies (Diptera, Drosophilidae). Entomol. Obozr. 1996, 75, 698–705. (In Russian) [Google Scholar]
- Shorrocks, B.; Charlesworth, P. The distribution and abundance of British fungal-feeding Drosophila. Ecol. Entomol. 1980, 5, 61–78. [Google Scholar] [CrossRef]
- Jones, L.E.; Berkov, A.; Grimaldi, D.A. Saproxylic fly diversity in a Costa Rican forest mosaic. J. Nat. Hist. 2021, 55, 1251–1265. [Google Scholar] [CrossRef]
- Grimaldi, D.; Nguyen, T. Monograph on the spittlebug flies, genus Cladochaeta (Diptera: Drosophilidae: Cladochaetini). Bull. Am. Mus. Nat. Hist. 1999, 241, 1–326. [Google Scholar] [CrossRef]
- Endara, L.; Grimaldi, D.A.; Roy, B.A. Lord of the flies: Pollination of Dracula orchids. Lankesteriana Int. J. Orchid. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Schmitz, H.J.; Valente, V.L. da S. The flower flies and the unknown diversity of Drosophilidae (Diptera): A biodiversity inventory in the Brazilian fauna. Papéis Avulsos Zool. 2019, 59, e20195945. [Google Scholar] [CrossRef]
- Shorrocks, B. The distribution and abundance of woodland species of British Drosophila (Diptera: Drosophilidae). J. Anim. Ecol. 1975, 44, 851–864. [Google Scholar] [CrossRef]
- Lumme, J.; Lakovaara, S.; Muona, O.; Jarvinen, L. Structure of a boreal community of drosophilids (Diptera). Aquila Ser. Zool. 1979, 20, 65–73. [Google Scholar]
- Toda, M.J. Habitat structure of a drosophilid community at Inuvik, NWT, Canada (Diptera: Drosophilidae). Can. Entomol. 1985, 117, 135–137. [Google Scholar] [CrossRef]
- Tidon-Sklorz, R.; Sene, F.M. Vertical and temporal distribution of Drosophila (Diptera, Drosophilidae) species in a wooded area in the State of São Paulo, Brazil. Rev. Bras. Biol. 1992, 52, 311–317. [Google Scholar]
- Roque, F.; da Mata, R.A.; Tidon, R. Temporal and vertical drosophilid (Insecta; Diptera) assemblage fluctuations in a neotropical gallery forest. Biodivers. Conserv. 2013, 22, 657–672. [Google Scholar] [CrossRef]
- Van Klinken, R.D.; Walter, G.H. Subtropical Drosophilids in Australia can be characterized by adult distribution across vegetation type and by height above forest floor. J. Trop. Ecol. 2001, 17, 705–718. [Google Scholar] [CrossRef]
- Toda, M.J. Vertical microdistribution of Drosophilidae (Diptera) within various forests in Hokkaido. I. Natural broad-leaved forest. Jpn. J. Ecol. 1977, 27, 207–214. [Google Scholar]
- Toda, M.J. Guild structure and its comparison between two local drosophilid communities. Physiol. Ecol. Jpn. 1984, 21, 131–172. [Google Scholar]
- Toda, M.J. Vertical microdistribution of Drosophilidae (Diptera) within various forests in Hokkaido. III. The Tomakomai Experiment Forest, Hokkaido University. Res. Bull. Coll. Exp. For. Fac. Agric. Hokkaido Univ. 1987, 44, 611–632. [Google Scholar]
- Toda, M.J. Three-dimensional dispersion of drosophilid flies in a cool temperate forest of northern Japan. Ecol. Res. 1992, 7, 283–295. [Google Scholar] [CrossRef]
- Toda, M.J.; Tanabe, S.; Akutsu, K. Structure and diversity of drosophilid communities in special relation to the three-dimensional structure of forest. Low Temp. Sci. 2011, 69, 101–111. [Google Scholar]
- Beppu, K. Vertical microdistribution of the Drosophilidae (Diptera) within various forests in Hokkaido. II. Streamside in natural broad-leaved forests. Kontyu 1980, 48, 549–557. [Google Scholar]
- Beppu, K. Vertical microdistribution of the Drosophilidae (Diptera) in a beech forest. Kontyu 1984, 52, 58–64. [Google Scholar]
- Beppu, K. Ecological structure of drosophilid assemblage in a subalpine coniferous forest. New Entomol. 1985, 34, 1–10. [Google Scholar]
- Ruchin, A.B.; Khapugin, A.A. Red data book invertebrates in a protected area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvorak, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Silaeva, T.B. The arrangement of threatened plants in Mordovia: The role of biodiversity research centers. Écoscience 2020, 27, 157–164. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Alekseev, S.K.; Khapugin, A.A. Post-fire fauna of carabid beetles (Coleoptera, Carabidae) in forests of the Mordovia State Nature Reserve (Russia). Nat. Conserv. Res. 2019, 4, 11–20. [Google Scholar] [CrossRef]
- Gornostaev, N.G. A key to the drosophilid flies (Diptera, Drosophilidae) from European Russia and neighbouring countries. Entomol. Obozr. 2001, 80, 908–915. (In Russian) [Google Scholar]
- Grimaldi, D.A. A phylogenetic revised classificationof genera in the Drosophilidae (Diptera). Bull. Am. Mus. Nat. Hist. 1990, 197, 1–139. [Google Scholar]
- Tanabe, S. Between-forest variation in vertical stratification of drosophilid populations. Ecol. Entomol. 2002, 27, 720–731. [Google Scholar] [CrossRef]
- Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Kulikov, A.M. Seasonal dynamics of fruit flies (Diptera: Drosophilidae) in forests of the European Russia. Insects 2022, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Basden, E.B. The vertical distribution of Drosophilidae in Scottish woodlands. Drosoph. Inform. Serv. 1953, 27, 84. [Google Scholar]
- Kimura, M.T.; Toda, M.J.; Beppu, K.; Watabe, H. Breeding sites of drosophilid flies in and near Sapporo, northern Japan, with supplementary notes on adult feeding habits. Kontyu 1977, 45, 571–582. [Google Scholar]
- Tanabe, S.; Toda, M.J.; Vinokurova, A.V. Tree shape, forest structure and diversity of drosophilid community: Comparison between boreal and temperate birch forests. Ecol. Res. 2001, 16, 369–385. [Google Scholar] [CrossRef]
- Bächli, G.; Rocha Pité, M.T. Family Drosophilidae. In Catalogue of Palaearctic Diptera: Clusiidae-Chloropidae; Akadémiai Kiadó: Budapest, Hungary, 1984; Volume 10, pp. 186–220. [Google Scholar]
- Nartshuk, E.P. Fruit flies (Diptera: Drosophilidae) of the Russian Arctic. Zoosyst. Ross. 2014, 23, 256–263. [Google Scholar] [CrossRef]
- Beuk, P.L.T. The species of the Drosophila repleta group in Northwestern Europe with special reference to The Netherlands (Diptera: Drosophilidae). Entomol. Ber. Amst. 1993, 53, 96–98. [Google Scholar]
- Toda, M.J.; Sidorenko, V.S.; Watabe, H.; Kholin, S.K.; Vinokurov, N.N. A revision of the Drosophilidae (Diptera) in East Siberia and Russian Far East: Taxonomy and biogeography. Zool. Sci. 1996, 13, 455–477. [Google Scholar] [CrossRef]
- Delbac, L.; Rusch, A.; Binet, D.; Thiéry, D. Seasonal variation of Drosophilidae communities in viticultural landscapes. Basic Appl. Ecol. 2020, 48, 83–91. [Google Scholar] [CrossRef]
- Baspinar, H.; Akşit, T.; Kesici, M.A.; Deutsch, F.; Kiss, B.; Papp, L. Seasonal abundance and diversity of family Drosophilidae, Diptera and records of some other dipterans in fruit orchards in Aydın Province—Türkiye. Türk. Entomol. Derg. 2022, 46, 289–298. [Google Scholar] [CrossRef]
- Zengin, E. Occurrence of invasive species and seasonal dynamics of fruit flies (Diptera: Drosophilidae) species in Uşak province, Turkey. Rev. Soc. Entomol. Argent. 2020, 79, 21–30. [Google Scholar] [CrossRef]


| Subfamily Steganinae | Subfamily Drosophilinae |
|---|---|
1. Amiota (Amiota) albilabris (Roth in Zetterstedt, 1860) | 1. Chymomyza amoena (Loew, 1862) |
2. Amiota (Amiota) alboguttata (Wahlberg, 1839) | 2. Chymomyza caudatula (Oldenberg, 1914) |
3. Amiota (Amiota) rufescens (Oldenberg, 1914) | 3. Chymomyza costata (Zetterstedt, 1838) |
4. Amiota (Amiota) subtusradiata (Duda, 1934) | 4. Chymomyza fuscimana (Zetterstedt, 1838) |
5. Phortica (Phortica) semivirgo (Maca, 1977) | 5. Drosophila (Dorsilopha) busckii (Coquillett, 1901) |
6. Gitona distigma (Meigen, 1830) | 6. Drosophila (Drosophila) funebris (Fabricius, 1787) |
7. Leucophenga maculata (Dufour, 1839) | 7. Drosophila (Drosophila) histrio (Meigen, 1830) |
8. Leucophenga quinquemaculata (Strobl, 1893) | 8. Drosophila (Drosophila) hydei (Sturtevant, 1921) |
9. * Stegana (Steganina) hypoleuca (Meigen, 1830) | 9. Drosophila (Drosophila) immigrans (Sturtevant, 1921) |
10. Drosophila (Drosophila) kuntzei (Duda, 1924) | |
11. * Drosophila (Drosophila) littoralis (Meigen, 1830) | |
12. Drosophila (Drosophila) phalerata (Meigen, 1830) | |
13. Drosophila (Drosophila) testacea (von Roser, 1840) | |
14. Drosophila (Drosophila) transversa (Fallen, 1823) | |
15. Drosophila (Sophophora) bifasciata (Pomini, 1940) | |
16. Drosophila (Sophophora) melanogaster (Meigen, 1830) | |
17. Drosophila (Sophophora) obscura (Fallen, 1823) | |
18. * Drosophila (Sophophora) subobscura (Collin in Gordon, 1936) | |
19. * Drosophila (Sophophora) subsilvestris (Hardy et Kaneshiro, 1968) | |
20. Drosophila (Sophophora) tristis (Fallen, 1823) | |
21. Hirtodrosophila confusa (Staeger, 1844) | |
22. * Hirtodrosophila toyohiokadai (Sidorenko,1990) | |
23. Hirtodrosophila trivittata (Strobl, 1893) | |
24. Scaptodrosophila rufifrons (Loew, 1873) | |
25. * Scaptomyza (Parascaptomyza) pallida (Zetterstedt, 1847) |
| Species | 1–15 June | 16–30 June | 1–15 July | 16–30 July | 1–15 August | 16–30 August | 1–15 September | Total Amount |
|---|---|---|---|---|---|---|---|---|
| Amiota albilabris | 11 | 4 | 2 | 12 | 12 | 0 | 2 | 43 |
| Amiota alboguttata | 5 | 0 | 2 | 18 | 6 | 3 | 8 | 42 |
| Amiota rufescens | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| Phortica semivirgo | 25 | 6 | 48 | 18 | 618 | 132 | 223 | 1070 |
| Amiota subtusradiata | 2 | 1 | 6 | 22 | 11 | 0 | 1 | 43 |
| Gitona distigma | 7 | 0 | 0 | 0 | 0 | 2 | 3 | 12 |
| Leucophenga maculata | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| Leucophenga quinquemaculata | 234 | 38 | 9 | 34 | 16 | 11 | 115 | 457 |
| Stegana hypoleuca | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Chymomyza amoena | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 3 |
| Chymomyza caudatula | 13 | 1 | 0 | 0 | 0 | 0 | 0 | 14 |
| Chymomyza costata | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Chymomyza fuscimana | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Drosophila bifasciata | 6 | 3 | 19 | 2 | 0 | 0 | 0 | 30 |
| Drosophila busckii | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Drosophila funebris | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Drosophila histrio | 451 | 207 | 44 | 13 | 33 | 19 | 333 | 1100 |
| Drosophila hydei | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Drosophila immigrans | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 4 |
| Drosophila kuntzei | 278 | 416 | 430 | 67 | 283 | 149 | 690 | 2313 |
| Drosophila littoralis | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Drosophila melanogaster | 7 | 5 | 3 | 0 | 4 | 4 | 1 | 24 |
| Drosophila obscura | 1313 | 1724 | 2464 | 1368 | 2219 | 705 | 1518 | 11,311 |
| Drosophila phalerata | 485 | 469 | 262 | 37 | 166 | 177 | 471 | 2067 |
| Drosophila subobscura | 8 | 22 | 97 | 29 | 25 | 6 | 0 | 187 |
| Drosophila subsilvestris | 9 | 10 | 21 | 22 | 114 | 36 | 111 | 323 |
| Drosophila testacea | 768 | 600 | 119 | 28 | 95 | 52 | 129 | 1791 |
| Drosophila transversa | 42 | 95 | 39 | 6 | 36 | 10 | 27 | 255 |
| Drosophila tristis | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Hirtodrosophila confusa | 31 | 15 | 3 | 2 | 6 | 5 | 6 | 68 |
| Hirtodrosophila toyohiokadai | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Hirtodrosophila trivittata | 4 | 0 | 1 | 0 | 0 | 0 | 2 | 7 |
| Scaptodrosophila rufifrons | 86 | 124 | 794 | 249 | 2045 | 904 | 1759 | 5961 |
| Scaptomyza pallida | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Number of species | 27 | 22 | 20 | 17 | 18 | 17 | 21 | 34 |
| Total number of individuals | 3794 | 3747 | 4366 | 1928 | 5693 | 2217 | 5406 | 27,151 |
| Species | Stratification in Each Site * | LIP | |||
|---|---|---|---|---|---|
| Wk | χ2 | d.f. | p | ||
| Amiota albilabris | 0.92 | 1.87 | 3 | 0.0021 | 1.87 |
| Amiota alboguttata | 0.91 | 2.66 | 3 | 0.0023 | 2.66 |
| Amiota subtusradiata | 0.89 | 1.64 | 3 | 0.0026 | 1.64 |
| Phortica semivirgo | 0.29 | 1.05 | 3 | 0.3260 | 1.05 |
| Leucophenga quinquemaculata | 0.40 | 1.03 | 3 | 0.1870 | 1.03 |
| Drosophila histrio | 0.93 | 2.23 | 3 | 0.0112 | 2.23 |
| Drosophila kuntzei | 0.85 | 1.12 | 3 | 0.0167 | 1.12 |
| Drosophila phalerata | 0.75 | 1.85 | 3 | 0.0293 | 1.85 |
| Drosophila testacea | 0.70 | 1.82 | 3 | 0.0384 | 1.82 |
| Drosophila transversa | 0.23 | 1.08 | 3 | 0.4402 | 1.08 |
| Drosophila bifasciata | 0.28 | 1.11 | 3 | 0.3394 | 1.11 |
| Drosophila melanogaster | 0.34 | 1.00 | 3 | 0.2561 | 1.00 |
| Drosophila obscura | 0.68 | 1.06 | 3 | 0.0440 | 1.06 |
| Drosophila subobscura | 0.41 | 1.22 | 3 | 0.1755 | 1.22 |
| Drosophila subsilvestris | 0.43 | 1.09 | 3 | 0.1646 | 1.09 |
| Hirtodrosophila confusa | 0.58 | 1.36 | 3 | 0.0735 | 1.36 |
| Scaptodrosophila rufifrons | 0.73 | 1.08 | 3 | 0.0336 | 1.08 |
| Species | Site * | Tier * | ||
|---|---|---|---|---|
| Median Test p | K–W H Test p | Median Test p | K–W H Test p | |
| Amiota albilabris | 0.572 | 0.613 | 0.046 | 0.014 |
| Amiota alboguttata | 0.572 | 0.503 | 0.019 | 0.015 |
| Amiota subtusradiata | 0.859 | 0.673 | 0.005 | 0.016 |
| Phortica semivirgo | 0.425 | 0.420 | 0.031 | 0.193 |
| Leucophenga quinquemaculata | 0.185 | 0.095 | 0.077 | 0.287 |
| Drosophila histrio | 0.572 | 0.907 | 0.019 | 0.004 |
| Drosophila kuntzei | 0.019 | 0.015 | 0.572 | 0.282 |
| Drosophila phalerata | 0.425 | 0.617 | 0.031 | 0.011 |
| Drosophila testacea | 0.261 | 0.533 | 0.112 | 0.022 |
| Drosophila transversa | 0.261 | 0.205 | 0.572 | 0.405 |
| Drosophila bifasciata | 0.362 | 0.273 | 0.785 | 0.373 |
| Drosophila melanogaster | 0.572 | 0.304 | 0.572 | 0.339 |
| Drosophila obscura | 0.572 | 0.522 | 0.019 | 0.051 |
| Drosophila subobscura | 0.859 | 0.639 | 0.077 | 0.124 |
| Drosophila subsilvestris | 0.572 | 0.417 | 0.572 | 0.184 |
| Hirtodrosophila confusa | 0.362 | 0.555 | 0.022 | 0.037 |
| Scaptodrosophila rufifrons | 0.572 | 0.687 | 0.019 | 0.023 |
| Effect | Criteria | Value | F | df1 | df2 | Significance |
|---|---|---|---|---|---|---|
| Free member | Wilkes’ Lambda | 0.072 | 51,844 | 17.000 | 68.000 | 0.000 |
| Hotelling Trace | 12.961 | 51,844 | 17.000 | 68.000 | 0.000 | |
| Season | Wilkes’ Lambda | 0.000 | 3.124 | 357.000 | 956.097 | 0.000 |
| Hotelling Trace | 31.599 | 5.852 | 357.000 | 1124.000 | 0.000 | |
| Tier | Wilkes’ Lambda | 0.204 | 2.817 | 51.000 | 203.253 | 0.000 |
| Hotelling Trace | 2.455 | 3.210 | 51.000 | 200.000 | 0.000 | |
| Site | Wilkes’ Lambda | 0.294 | 2.026 | 51.000 | 203.253 | 0.000 |
| Hotelling Trace | 1.628 | 2.128 | 51.000 | 200.000 | 0.000 | |
| Season * tier | Wilkes’ Lambda | 0.000 | 3.347 | 476.000 | 1044.741 | 0.000 |
| Hotelling Trace | 34.216 | 4.681 | 476.000 | 1107.000 | 0.000 |
| Adjusted Model | Season | Tier | Site | Season * Tier | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | R2 Corrected | d.f. | F | p | d.f. | F | p | d.f. | F | p | d.f. | F | p | d.f. | F | p |
| Phortica semivirgo | 0.817 | 29 | 18.279 | 0.000 | 21 | 13.401 | 0.000 | 3 | 1.698 | 0.174 | 3 | 2.058 | 0.155 | 28 | 12.685 | 0.000 |
| Leucophenga quinquemaculata | 0.757 | 29 | 13.015 | 0.000 | 21 | 8.755 | 0.000 | 3 | 3.437 | 0.021 | 3 | 0.209 | 0.649 | 28 | 8.728 | 0.000 |
| Drosophila histrio | 0.746 | 29 | 12.341 | 0.000 | 21 | 4.539 | 0.000 | 3 | 10.432 | 0.000 | 3 | 0.380 | 0.539 | 28 | 9.580 | 0.000 |
| Drosophila kuntzei | 0.785 | 29 | 15.086 | 0.000 | 21 | 10.591 | 0.000 | 3 | 1.827 | 0.149 | 3 | 18.486 | 0.000 | 28 | 6.723 | 0.000 |
| Drosophila subobscura | 0.65 | 29 | 8.170 | 0.000 | 21 | 3.021 | 0.000 | 3 | 3.345 | 0.023 | 3 | 7.374 | 0.008 | 28 | 5.908 | 0.000 |
| Drosophila phalerata | 0.765 | 29 | 13.575 | 0.000 | 21 | 2.588 | 0.001 | 3 | 14.893 | 0.000 | 3 | 4.167 | 0.044 | 28 | 8.183 | 0.000 |
| Drosophila testacea | 0.787 | 29 | 15.280 | 0.000 | 21 | 3.056 | 0.000 | 3 | 11.045 | 0.000 | 3 | 3.315 | 0.072 | 28 | 11.636 | 0.000 |
| Drosophila transversa | 0.484 | 29 | 4.627 | 0.000 | 21 | 1.947 | 0.017 | 3 | 1.647 | 0.185 | 3 | 1.151 | 0.286 | 28 | 2.320 | 0.002 |
| Drosophila obscura | 0.758 | 29 | 13.067 | 0.000 | 21 | 1.814 | 0.030 | 3 | 4.157 | 0.009 | 3 | 6.414 | 0.013 | 28 | 2.641 | 0.000 |
| Drosophila subsilvestris | 0.679 | 29 | 9.172 | 0.000 | 21 | 6.430 | 0.000 | 3 | 1.730 | 0.167 | 3 | 0.449 | 0.505 | 28 | 4.995 | 0.000 |
| Scaptodrosophila rufifrons | 0.762 | 29 | 13.341 | 0.000 | 21 | 9.793 | 0.000 | 3 | 2.230 | 0.091 | 3 | 0.547 | 0.462 | 28 | 6.530 | 0.000 |
| Amiota albilabris | 0.48 | 29 | 4.571 | 0.000 | 21 | 1.137 | 0.329 | 3 | 7.460 | 0.000 | 3 | 1.840 | 0.179 | 28 | 3.469 | 0.000 |
| Amiota alboguttata | 0.158 | 29 | 1.722 | 0.029 | 21 | 1.322 | 0.185 | 3 | 3.714 | 0.015 | 3 | 0.985 | 0.324 | 28 | 1.379 | 0.133 |
| Amiota subtusradiata | 0.411 | 29 | 3.699 | 0.000 | 21 | 1.672 | 0.052 | 3 | 4.472 | 0.006 | 3 | 0.347 | 0.557 | 28 | 2.870 | 0.000 |
| Drosophila bifasciata | 0.274 | 29 | 2.457 | 0.001 | 21 | 1.058 | 0.408 | 3 | 0.698 | 0.556 | 3 | 1.203 | 0.276 | 28 | 2.096 | 0.005 |
| Drosophila melanogaster | 0.157 | 29 | 1.721 | 0.029 | 21 | 0.589 | 0.916 | 3 | 1.303 | 0.279 | 3 | 0.074 | 0.787 | 28 | 1.019 | 0.456 |
| Hirtodrosophila confusa | 0.532 | 29 | 5.398 | 0.000 | 21 | 3.884 | 0.000 | 3 | 7.858 | 0.000 | 3 | 1.712 | 0.194 | 28 | 3.447 | 0.000 |
| Factor | Criteria | N | N * | ||||
|---|---|---|---|---|---|---|---|
| d.f. | p | d.f. | p | ||||
| site | χ2 | 33.799 | 30 | 0.289 | 38.952 | 27 | 0.043 |
| c | 0.481 | 0.280 | 0.508 | 0.064 | |||
| tier | χ2 | 27.975 | 30 | 0.572 | 38.075 | 27 | 0.050 |
| c | 0.447 | 0.598 | 0.504 | 0.054 | |||
| season | χ2 | 79.034 | 60 | 0.037 | 75.823 | 54 | 0.027 |
| c | 0.643 | 0.037 | 0.635 | 0.017 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornostaev, N.G.; Ruchin, A.B.; Esin, M.N.; Lazebny, O.E.; Kulikov, A.M. Vertical Distribution of Fruit Flies (Diptera: Drosophilidae) in Deciduous Forests in the Center of European Russia. Insects 2023, 14, 822. https://doi.org/10.3390/insects14100822
Gornostaev NG, Ruchin AB, Esin MN, Lazebny OE, Kulikov AM. Vertical Distribution of Fruit Flies (Diptera: Drosophilidae) in Deciduous Forests in the Center of European Russia. Insects. 2023; 14(10):822. https://doi.org/10.3390/insects14100822
Chicago/Turabian StyleGornostaev, Nikolai G., Alexander B. Ruchin, Mikhail N. Esin, Oleg E. Lazebny, and Alex M. Kulikov. 2023. "Vertical Distribution of Fruit Flies (Diptera: Drosophilidae) in Deciduous Forests in the Center of European Russia" Insects 14, no. 10: 822. https://doi.org/10.3390/insects14100822
APA StyleGornostaev, N. G., Ruchin, A. B., Esin, M. N., Lazebny, O. E., & Kulikov, A. M. (2023). Vertical Distribution of Fruit Flies (Diptera: Drosophilidae) in Deciduous Forests in the Center of European Russia. Insects, 14(10), 822. https://doi.org/10.3390/insects14100822








