A New Species of Hemilauxania Hennig (Lauxaniidae) from the Lower Eocene Oise Amber—The Oldest Record of Schizophora (Diptera)? †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Amber Specimen
2.3. Techniques of Investigation
2.4. Morphological Terminology
- A1—first branch of anal vein (=CuA+CuP)
- A2—second branch of anal vein (=A1)
- ac—acrostichal (setulae)
- bm—basal medial cell
- C—costa
- ce—cercus
- Cs2, Cs3, Cs4—2nd, 3rd, 4th costal sector
- CuA1—cubitus (=M4)
- cup—posterior cubital cell (=cua)
- cx1, cx2, cx3—fore, mid, hind coxa
- dc—dorsocentral setae
- dm—discal medial cell
- dm-cu—discal medial-cubital (=dm-m, posterior, tp) cross-vein
- f1, f2, f3—fore, mid, hind femur
- frt—frontal triangle
- h—humeral cross-vein
- hu—humeral (=postpronotal) (seta)
- ia—posterior intra-alar
- M—media (=M1)
- mspl—mesopleural (=anepisternal) (seta)
- npl—notopleural (seta)
- oc—ocellar (seta)
- ori—lower fronto-orbital (seta)
- ors—upper fronto-orbital (seta)
- pa—postalar (seta)
- ppl—propleural (=proepisternal) (seta)
- pra—pre-alar (=anterior supra-alar)(seta)
- prs—presutural (=presutural intra-alar) (seta)
- pvt—postvertical (=poc, postocellar) (seta)
- R1—1st branch of radius
- R2+3—2nd branch of radius
- R4+5—3rd branch of radius
- r-m—radial-medial (=anterior, ta) cross-vein
- S1–S10—abdominal sterna
- sa—supraalar (seta)
- sc—scutellar (seta)
- Sc—subcosta
- sctl—scutellum
- stpl—sternopleural (=katepisternal) (seta)
- T1–T10—abdominal terga
- t1, t2, t3—fore, mid, hind tibia
- vte—outer vertical (seta)
- vti—inner vertical (seta)
3. Results
3.1. Systematic Palaeontology
- Class Insecta Linnaeus, 1758.
- Order Diptera Linnaeus, 1758
- Cyclorrhapha Brauer, 1863
- Schizophora Becher, 1882
- Acalyptratae Macquart, 1835
- Superfamily Lauxanioidea Macquart, 1835
- Family Lauxaniidae Macquart, 1835
- Genus Hemilauxania Hennig, 1965
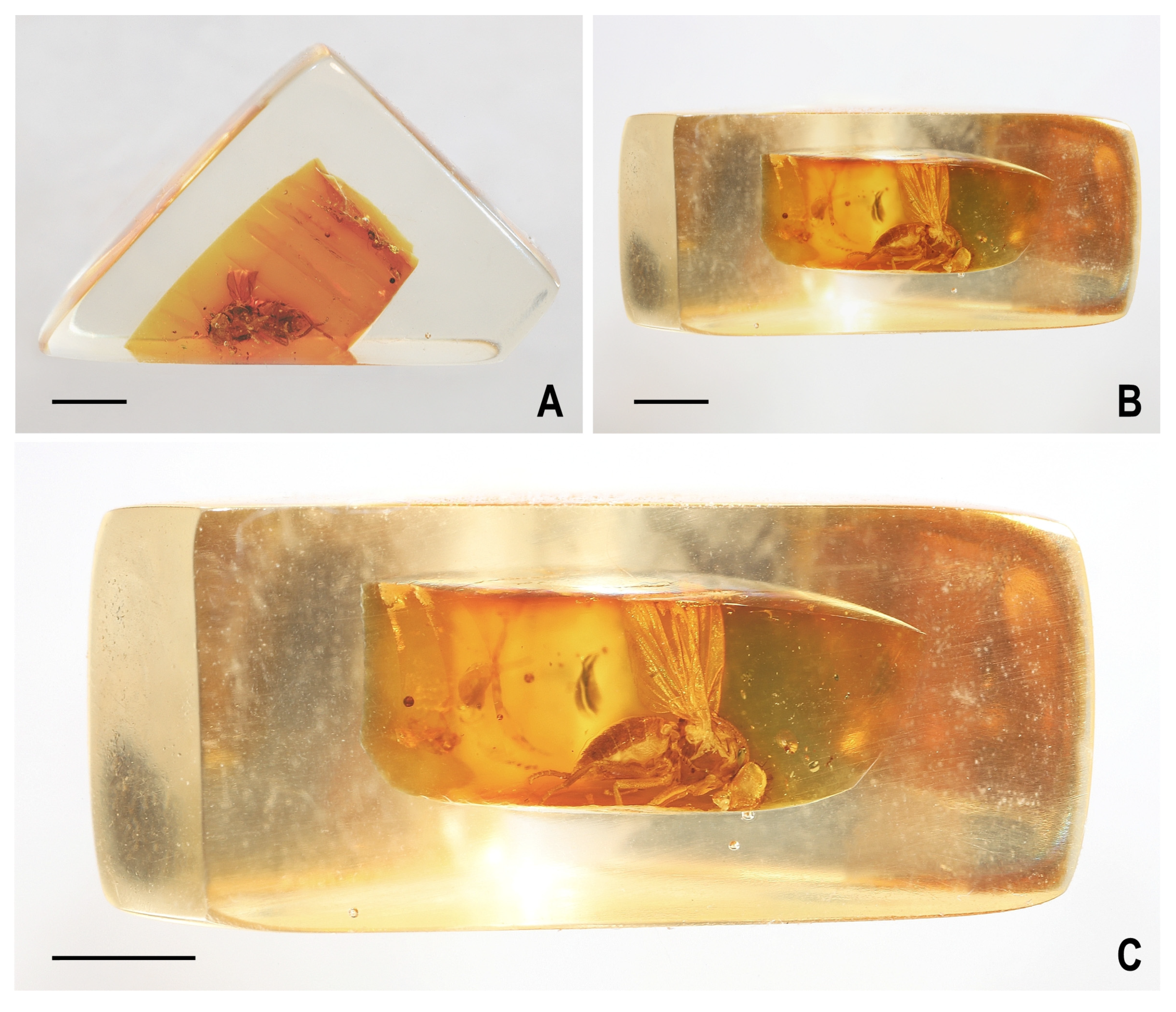
3.2. Hemilauxania parvula sp. nov. (Figure 1, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8)

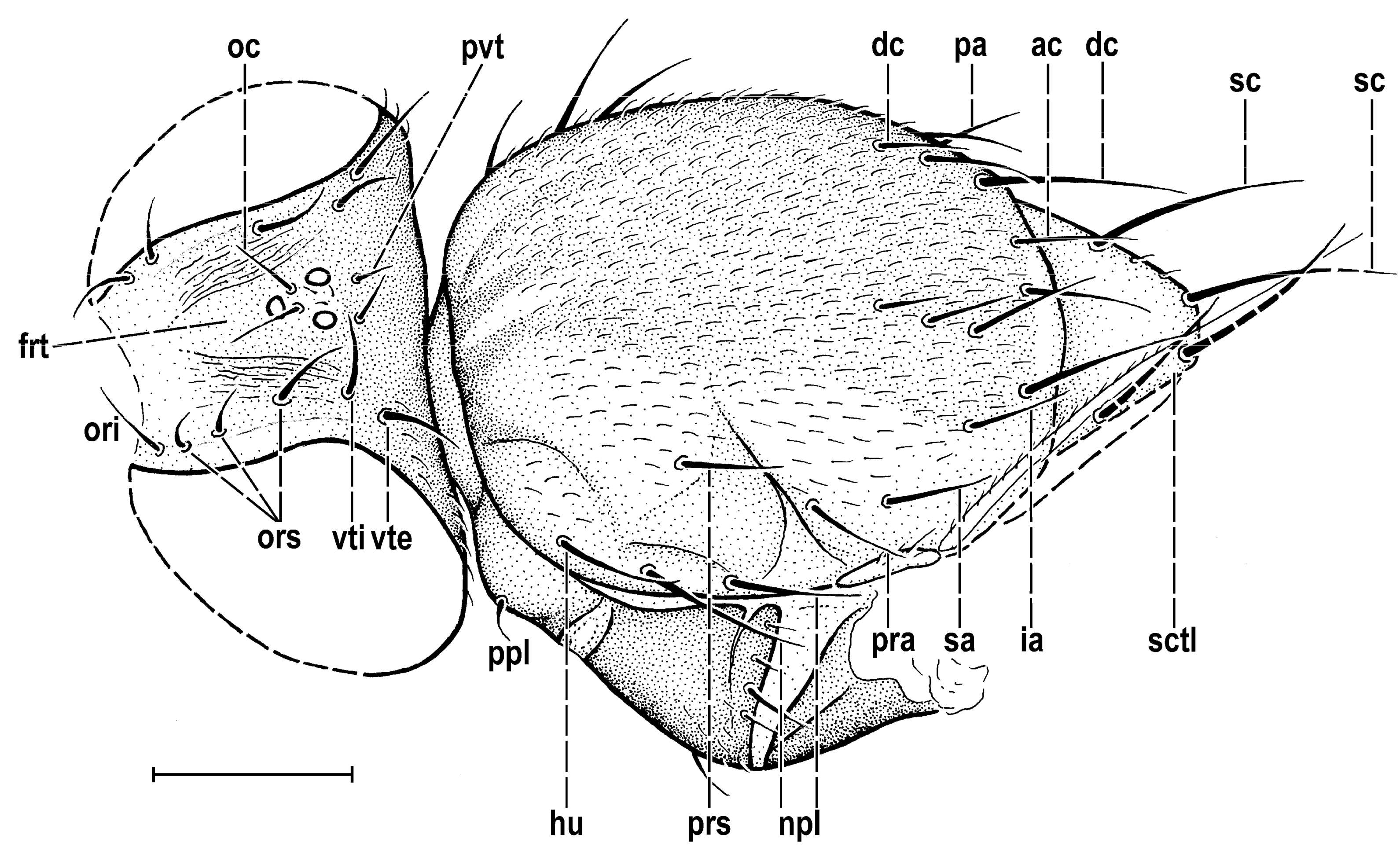

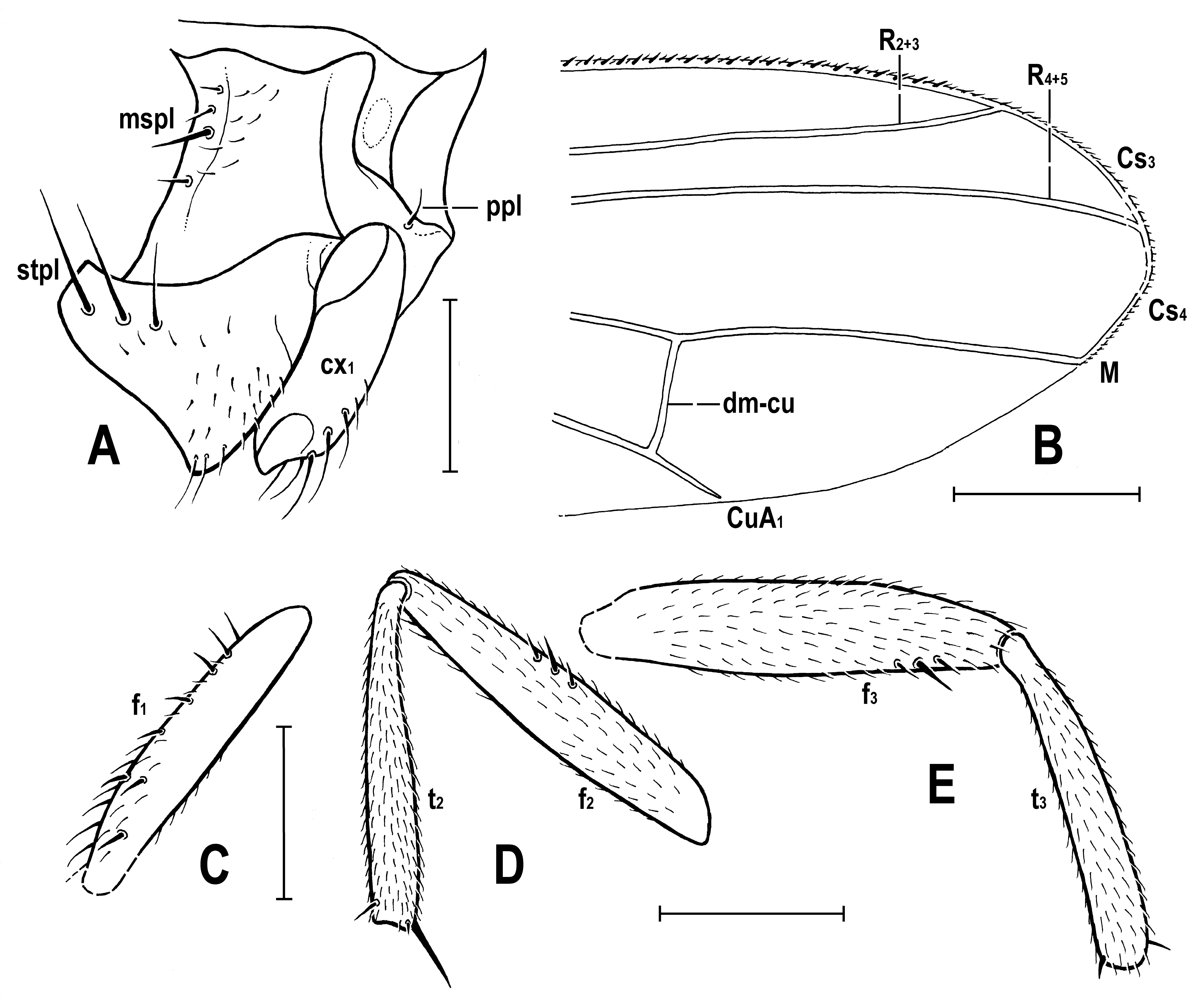

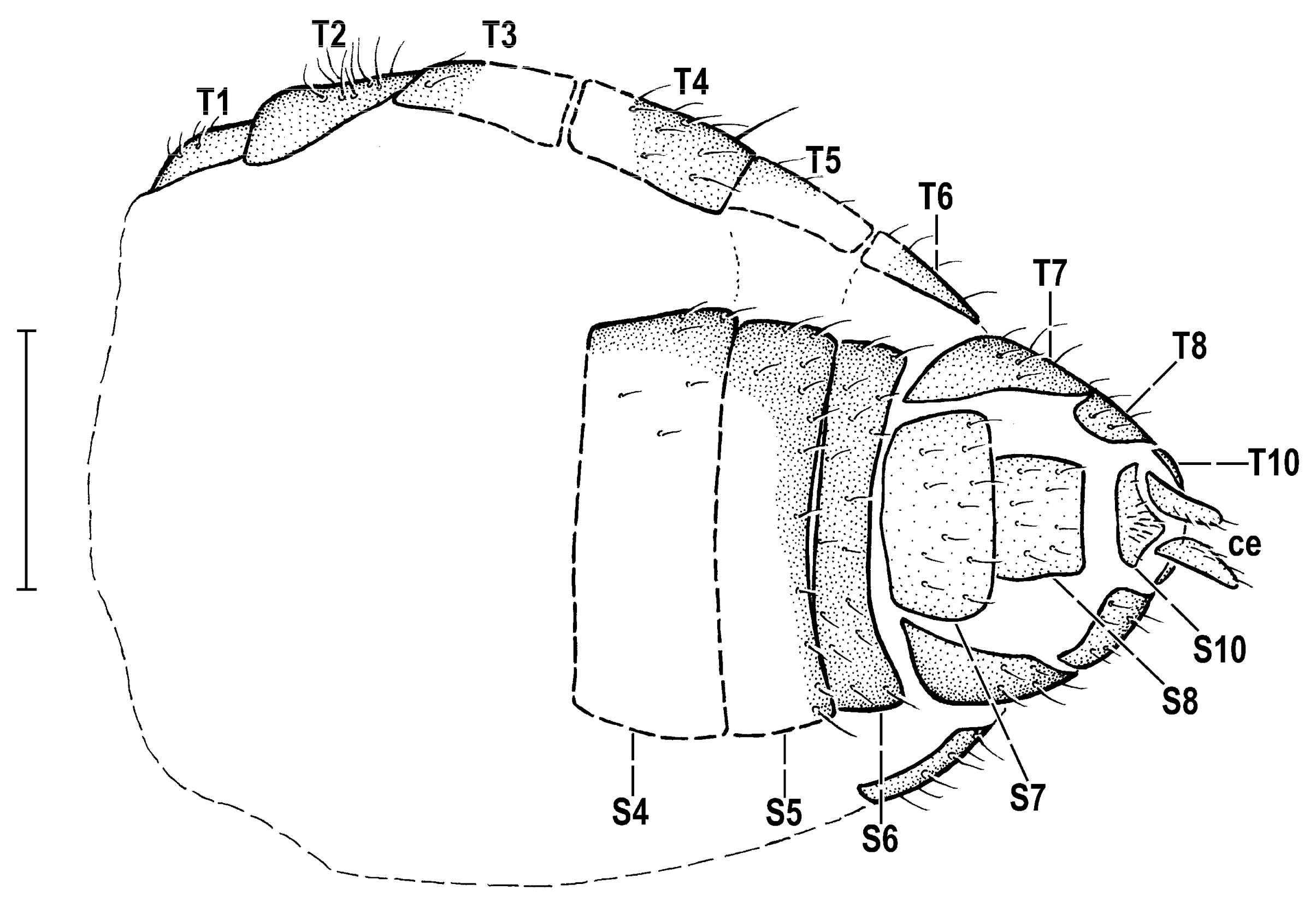
4. Discussion
4.1. Relationships and Classification of Hemilauxania
4.2. Palaeohabitat and Palaeobiology of Hemilauxania
4.3. Age of Hemilauxania and Other Oldest Fossils in Schizophora
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaimari, S.D.; Miller, R.M. Lauxaniidae (Lauxaniidae Flies). In Manual of Afrotropical Diptera; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; Brachycera–Cyclorrhapha; South African National Biodiversity Institute: Pretoria, South Africa, 2021; Volume 3, pp. 1757–1781. [Google Scholar]
- Hennig, W. Die Acalyptratae des Baltischen Bernsteins und ihre Bedeutung für die Erforschung der phylogenetischen Entwicklung dieser Dipteren-Gruppe. Stuttg. Beiträge Nat. 1965, 145, 1–215. [Google Scholar]
- Von Tschirnhaus, M.; Hoffeins, C. Fossil flies in Baltic amber—Insight in the diversity of Tertiary Acalyptratae (Diptera, Schizophora), with new morphological characters and a key based on 1000 collected inclusions. Denisia 2009, 26, 171–212. [Google Scholar]
- Hoffeins, C. (Independent Researcher, Hamburg, Germany). Personal communication, 2023.
- Hong, Y.-C. Eocene Fossil Diptera Insecta in Amber of Fushun Coalfield; Geological Publishing House: Beijing, China, 1981; pp. 1–166 + plates 1–27. (In Chinese) [Google Scholar]
- Hong, Y.-C. Amber Insects of China; Huayu Nature Book Trade Co., Ltd.: Beijing, China, 2002; pp. 1–653 + plates 1–48. (In Chinese) [Google Scholar]
- Hong, Y.-C. Atlas of Amber Insects of China; Henan Scientific and Technological Publishing House: Zhengzhou, China, 2002; pp. 1–394. (In Chinese) [Google Scholar]
- Melander, A.L. A report on some Miocene Diptera from Florissant, Colorado. Am. Mus. Novit. 1949, 1407, 1–63. Available online: http://npshistory.com/publications/flfo/amn-n1407-1949.pdf (accessed on 26 September 2023).
- Scudder, S.H. The insects of the Tertiary beds at Quesnel (British Columbia). Rep. Prog. Geol. Surv. Terr. 1877, 1875–1876, 266–280. Available online: https://publications.gc.ca/collections/collection_2019/rncan-nrcan/M41-1-1-1876-eng.pdf (accessed on 26 September 2023).
- Shewell, G.E. Lauxaniidae. In Manual of Nearctic Diptera; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Wood, D.M., Eds.; Agriculture Canada Monograph No. 28; Minister of Supply and Services: Ottawa, ON, Canada, 1987; Volume 2, pp. 993–1006. [Google Scholar]
- Evenhuis, N.L. Catalogue of the Fossil Flies of the World (Insecta: Diptera); Backhuys Publisher: Leiden, The Netherlands, 1994; pp. 1–600. [Google Scholar]
- Brasero, N.; Nel, A.; Michez, D. Insects from the Early Eocene amber of Oise (France): Diversity and palaeontological significance. Denisia 2009, 26, 41–52. [Google Scholar]
- Doitteau, G.; Nel, A. Chironomid midges from early Eocene amber of France (Diptera: Chironomidae). Zootaxa 2007, 1404, 1–66. [Google Scholar]
- Blagoderov, V.; Hippa, H.; Nel, A. Parisognoriste, a new genus of Lygistorrhinidae (Diptera, Sciaroidea) from the Oise amber with redescription of Palaeognoriste Meunier. ZooKeys 2010, 50, 79–90. [Google Scholar] [CrossRef][Green Version]
- Myskowiak, J.; Nel, A. A new genus and species of ibis fly in the Lowermost Eocene amber of Oise (France) (Diptera: Athericidae). Zootaxa 2014, 3869, 372–382. [Google Scholar] [CrossRef]
- Garrouste, R.; Azar, D.; Nel, A. The oldest accurate record of Scenopinidae in the Lowermost Eocene amber of France (Diptera: Brachycera). Zootaxa 2016, 4093, 444–450. [Google Scholar] [CrossRef]
- Nel, A.; Perreau, Z.; Doitteau, G. The oldest representative of the modern snipe fly genus Symphoromyia (Diptera: Rhagionidae). Zootaxa 2016, 4196, 144–150. [Google Scholar] [CrossRef]
- Bramuzzo, S.; Coty, D.; Nel, A. A new species of Ferneiella from the Eocene French amber (Diptera: Scatopsidae). Zootaxa 2017, 4350, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bramuzzo, S.; Nel, A. Youngest representative of the extinct genus Microphorites in the Eocene amber of France (Diptera: Dolichopodidae: Microphorinae). Zootaxa 2017, 4231, 590–594. [Google Scholar] [CrossRef]
- Myskowiak, J.; Garrouste, R.; Nel, A. Eodromyia pumilio gen. et sp. nov., a new empidoid fly from the Earliest Eocene amber of France (Diptera: Hybotidae: Tachydromiinae). Zootaxa 2018, 4379, 279–286. [Google Scholar] [CrossRef]
- Camier, M.; Nel, A. First Mycetobiinae of the lowermost Eocene Oise amber (France) (Diptera: Anisopodidae). Palaeoentomology 2019, 2, 245–250. [Google Scholar] [CrossRef]
- Camier, M.; Nel, A. The oldest fungus gnat of the tribe Exechiini in the lowermost Eocene Oise amber (Diptera: Mycetophilidae). Zootaxa 2020, 4722, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Roháček, J.; Hoffeins, C. Clusiomitidae, a new family of Eocene fossil Acalyptratae, with revision of Acartophthalmites Hennig and Clusiomites gen. nov. (Diptera). Insects 2021, 12, 1123. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Singh, H. The extinct genus Pareuthychaeta in Eocene ambers (Diptera: Schizophora: Ephydroidea). Can. Entomologist 2012, 144, 17–28. [Google Scholar] [CrossRef]
- Rust, J.; Singh, H.; Rana, R.S.; McCanna, T.; Singh, L.; Anderson, K.; Sarkare, N.; Nascimbene, P.C.; Stebner, F.; Thomas, J.C.; et al. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene of India. Proc. Nat. Acad. Sci. USA 2010, 107, 18360–18365. [Google Scholar] [CrossRef] [PubMed]
- Roháček, J.; Hammel, J.U.; Baranov, V. Christelenkidae, a new extinct family based on a new taxon from Eocene Baltic amber (Diptera: Acalyptratae), with X-ray synchrotron microtomography imaging of its structures. Arthropod Syst. Phyl. 2023, 81, 475–498. [Google Scholar] [CrossRef]
- Nel, A.; Garrouste, R.; Engel, M.S. The earliest Pupipara (Diptera: Hippoboscoidea): A new genus and species from the lower Eocene of the Green River Formation. Palaeoentomology 2023, 6, 58–63. [Google Scholar] [CrossRef]
- Winkler, I.S.; Labandeira, C.C.; Wappler, T.; Wilf, P. Distinguishing Agromyzidae (Diptera) leaf mines in the fossil record: New taxa from the Paleogene of North America and Germany and their evolutionary implications. J. Paleontol. 2010, 84, 935–954. [Google Scholar] [CrossRef]
- Donovan, M.P.; Wilf, P.; Labandeira, C.C.; Johnson, K.R.; Peppe, D.J. Novel Insect Leaf-Mining after the End-Cretaceous Extinction and the Demise of Cretaceous Leaf Miners, Great Plains, USA. PLoS ONE 2014, 9, e103542. [Google Scholar] [CrossRef] [PubMed]
- Ohlhoff, R. Bernstein aus Frankreich. In Bernstein Magazin, Ausgabe 2022; Ribbecke, H.-W., Ed.; Verein zur Förderung des Geologisch-Paläontologischen Museums der Universität Hamburg e.V. “Arbeitskreis Bernstein”: Hamburg, Germany, 2022; pp. 40–41. [Google Scholar]
- Hoffeins, H.W. On the preparation and conservation of amber inclusions in artificial resin. Pol. Pismo Entomol. 2001, 70, 215–219. [Google Scholar]
- Roháček, J. New amber fossil Anthomyzidae (Diptera): An unexpected Eocene diversity. J. Syst. Palaeontol. 2013, 11, 431–473. [Google Scholar] [CrossRef]
- Cumming, J.M.; Wood, D.M. Adult morphology and terminology. In Manual of Afrotropical Diptera. Introductory Chapters and Keys to Diptera Families; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; Suricata 4—South African National Biodiversity Institute: Pretoria, South Africa, 2017; Volume 1, pp. 89–133. [Google Scholar]
- Hennig, W. Neue Übersicht über die aus dem Baltischen Bernstein bekannten Acalyptratae (Diptera: Cyclorrhapha). Stuttg. Beiträge Nat. 1969, 209, 1–42. [Google Scholar]
- Hennig, W. Neue Untersuchungen über die Familien der Diptera Schizophora. Stuttg. Beiträge Nat. 1971, 226, 1–76. [Google Scholar]
- Papp, L.; Shatalkin, A.I. Family Lauxaniidae. In Contribution to a Manual of Palaearctic Diptera; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 1998; Volume 3, pp. 383–400. [Google Scholar]
- Semelbauer, M. Lauxaniidae—Tieňovkovité (Diptrera, Cyclorrhapha); Veda: Bratislava, Slovakia, 2016; pp. 1–183. (In Slovak) [Google Scholar]
- Gaimari, S.D.; Silva, V.C. Lauxaniidae (Lauxaniid flies). In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; NRC Research Press: Ottawa, ON, Canada, 2010; Volume 2, pp. 971–995. [Google Scholar]
- Miller, R.M. Ecology of Lauxaniidae (Diptera: Acalyptratae) I. Old and new rearing records with biological notes and discussion. Ann. Natal Mus. 1977, 23, 215–238. [Google Scholar]
- Alekseev, V.I.; Alekseev, P.I. New approaches for reconstruction of the ecosystem of an Eocene amber forest. Biol. Bull. 2016, 43, 75–86. [Google Scholar]
- Szwedo, J. Life in the Eocene forests. In Proceedings of the World Amber Council Seminar, Gdańsk the World Amber Capital, Gdańsk, Poland, 18–19 May 2012; Pytlos, R., Szadziewski, R., Zbierska, E., Adamska, G., Dmowska, A., Eds.; Mayor’s Office for City Promotion, City Hall of Gdańsk: Gdańsk, Poland, 2012; pp. 56–70. [Google Scholar]
- Słodkowska, B.; Kramarska, R.; Kasiński, J.R. The Eocene Climatic Optimum and the formation of the Baltic amber deposits. In Proceedings of the International Amber Researcher Symposium Amber, Deposits–Collections–the Market, Gdańsk, Poland, 22–23 March 2013; Kosmowska-Ceranowicz, B., Gierlowski, W., Sontag, E., Eds.; Gdańsk International Fair Co. Amberif: Gdańsk, Poland, 2013; pp. 28–32. [Google Scholar]
- Sadowski, E.-M.; Schmidt, A.R.; Denk, T. Staminate inflorescences with in situ pollen from Eocene Baltic amber reveal high diversity in Fagaceae (oak family). Willdenowia 2020, 50, 405–517. [Google Scholar] [CrossRef]
- Nel, A.; Brasero, N. Oise Amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Penney, D., Ed.; Siri Scientific Press: Manchester, UK, 2010; pp. 137–148. [Google Scholar]
- Nohra, Y.; Perrichot, V.; Jeanneau, L.; Le Pollès, L.; Azar, D. Chemical characterization and botanical origin of French ambers. J. Nat. Prod. 2015, 78, 1284–1293. [Google Scholar] [CrossRef]
- Ritzkowski, S. Das geologische Alter der bernsteinführenden Sedimente in Sambia (Bezirk Kaliningrad), bei Bitterfeld (Sachsen-Anhalt) und bei Helmstedt (SE-Niedersachsen). In Investigations into Amber, Proceedings of the International Interdisciplinary Symposium: Baltic Amber and Other Fossil Resins, Gdańsk, Poland, 2–6 September 1997; Kosmowska-Ceranowicz, B., Paner, H., Eds.; The Archaeological Museum in Gdansk, Museum of the Earth, Polish Academy of Sciences: Gdańsk, Poland, 1999; pp. 33–40. [Google Scholar]
- Wolfe, A.P.; McKellar, R.C.; Tappert, R.; Sodhi, R.N.S.; Muehlenbachs, K. Bitterfeld amber is not Baltic amber: Three geochemical tests and further constraints on the botanical affinities of succinite. Rev. Palaeobot. Palynol. 2016, 225, 21–32. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; pp. 1–755. [Google Scholar]
- Renne, P.R.; Deino, A.L.; Hilgen, F.J.; Kuiper, K.F.; Mark, D.F.; Mitchell, W.S., III; Morgan, L.E.; Mundil, R.; Smit, J. Time Scales of Critical Events Around the Cretaceous-Paleogene Boundary. Science 2013, 339, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, B.M.; Trautwein, M.D.; Winkler, I.S.; Barr, N.B.; Kim, J.-W.; Lambkin, C.; Bertone, M.A.; Cassel, B.K.; Bayless, K.M.; Heimberg, A.M.; et al. Episodic radiations in the fly tree of life. Proc. Nat. Acad. Sci. USA 2011, 108, 5690–5695. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roháček, J.; Hoffeins, C. A New Species of Hemilauxania Hennig (Lauxaniidae) from the Lower Eocene Oise Amber—The Oldest Record of Schizophora (Diptera)? Insects 2023, 14, 835. https://doi.org/10.3390/insects14110835
Roháček J, Hoffeins C. A New Species of Hemilauxania Hennig (Lauxaniidae) from the Lower Eocene Oise Amber—The Oldest Record of Schizophora (Diptera)? Insects. 2023; 14(11):835. https://doi.org/10.3390/insects14110835
Chicago/Turabian StyleRoháček, Jindřich, and Christel Hoffeins. 2023. "A New Species of Hemilauxania Hennig (Lauxaniidae) from the Lower Eocene Oise Amber—The Oldest Record of Schizophora (Diptera)?" Insects 14, no. 11: 835. https://doi.org/10.3390/insects14110835
APA StyleRoháček, J., & Hoffeins, C. (2023). A New Species of Hemilauxania Hennig (Lauxaniidae) from the Lower Eocene Oise Amber—The Oldest Record of Schizophora (Diptera)? Insects, 14(11), 835. https://doi.org/10.3390/insects14110835






