Simple Summary
The aphid Aphis gossypii Glover is a serious pest that inflicts severe damage upon goji berry plants in China. The current prevailing approach to pest prevention involves the utilization of chemical insecticides, which presents potential risks to both human health and the environment. The improper use of pesticides leads to the emergence of drug-resistant pests, thereby contributing to the resurgence of rampant pest populations. Therefore, the conservation and management of predators in agricultural ecosystems should receive increased attention, given their crucial role. Ladybirds have previously been identified as the primary predators of aphid species. However, their effectiveness as biological agents against aphids on goji berry plants remains uncertain. We found that the potential of ladybirds in controlling the aphid populations is promising, thus possibly making a contribution to environmental protection. By conducting an analysis of functional responses, intraspecific competition, and a semi-field study, we have determined that H. axyridis and C. septempunctata exhibit greater potential as biocontrol agents against aphids in comparison to H. variegata. Notably, future field studies will play a pivotal role in ensuring the effective implementation of a biological control program.
Abstract
The aphid, Aphis gossypii Glover, is identified as a significant pest that causes severe damage to goji berries in China. To analyze the ladybird consumption of aphids, the functional responses of three ladybird species, Harmonia axyridis, Coccinella septempunctata, and Hippodamia variegata, and intraspecific competition among ladybird individuals were evaluated under laboratory conditions. Moreover, the practical impact of ladybirds on aphid population reduction was investigated in semi-field conditions. We found that all adult ladybirds of the three species exhibited a type II functional response toward aphids. According to Holling’s disc equation, H. axyridis exhibited the highest searching efficiency (a = 0.79), while C. septempunctata had the shortest handling time (Th = 5.07 min) among the three ladybird species studied. Additionally, intraspecific competition had a greater impact on H. variegata (m = 0.41) compared to the other two ladybird species. The semi-field study demonstrated that H. axyridis (83.9% reduction) and C. septempunctata (78.7% reduction) exhibited higher efficacy in reducing aphid populations compared to H. variegata (27.3% reduction). This study suggests that H. axyridis and C. septempunctata exhibit potential as effective biological control agents against aphids on goji berry plants and highlights the importance of considering intraspecific competition. However, the results obtained from laboratory and semi-field studies cannot be directly extrapolated to field conditions due to the simplification of these experimental systems. Future field studies are crucial in ensuring the effective implementation of a biological control program.
1. Introduction
The goji berry, Lycium barbarum L. (Solanaceae: Lycium Linn.), is a prominent plant endemic to China. The efficacy of goji berry has been extensively investigated and empirically validated in terms of its capacity to augment sleep quality and provide a diverse array of supplementary health advantages [1,2,3]. Because of its flourishing leaf growth, sweet fruit, and nutrient-rich composition, goji berries are highly susceptible to pest infestations and disease outbreaks. The vegetative and reproductive growth periods of goji berry exhibit a high overlap. The duration of the young fruit stage in goji berry is extended, whereas the periods of fruit expansion and maturation are comparatively brief. Pest control in goji berry cultivation poses a formidable challenge [4,5]. Furthermore, inadequate chemical control not only seldom attains the desired level of efficacy, but it also readily gives rise to significant concerns such as pesticide residue and environmental pollution [6,7,8,9,10]. The frequent occurrence of pests, influenced by recent climatic factors, has led to significant losses in both the quantity and quality of goji berries. Particularly, the presence of aphid populations is frequently observed on goji berry plants [11].
The majority of this aphid population has been identified as Aphis gossypii Glover (Homoptera: Aphididae), which is commonly observed in goji berry-growing regions, exhibiting a broad geographical distribution [12]. The pest exhibits clustering behavior and inflicts damage on newly emerged shoots, stems, and leaf surfaces through its sap-sucking activity. This results in the stunted growth of new shoots and the development of narrow brown scorched margins on leaves followed by withering and aberrant fruit formation [13,14]. The leaf surfaces become coated with honeydew, which significantly impairs photosynthetic efficiency, disrupts reproductive processes, and inhibits plant blooming [15].
The majority of previous studies pertaining to the aphid have primarily focused on its occurrence and population dynamics, as well as the utilization of parasitoids and their host acceptance behavior [16,17]. Additionally, research has been conducted on toxicity tests in relation to the aphid, along with strategies aimed at enhancing insect resistance [18]. Regarding its predators, functional responses of ladybird larvae to A. gossypii have been investigated both in laboratory settings and in melon fields using molecular gut content analysis to assess predation [19,20,21,22,23]. However, there is currently limited research on the potential of adult ladybirds for controlling aphids on goji berry plants, and our study aimed to establish a theoretical foundation for their biological control. In addition, the current predominant approach to pest prevention involves the utilization of chemical pesticides, which pose potential risks to both human health and the environment [24,25,26]. The misuse of pesticides also results in the emergence of drug resistance, thereby contributing to the resurgence of rampant pest populations [7,8,27,28]. Therefore, the conservation and management of predators in agricultural ecosystems warrants greater attention. Ladybirds have been previously reported as the primary predators of aphid species [29]; however, their effectiveness as biological agents against this aphid species remains uncertain. The potential of ladybirds in controlling invasive pest populations is highly promising, suggesting that they could make a contribution to environmental protection.
In order to assess the ladybird consumption of aphids on goji berry plants, we selected three common predatory ladybird species: Harmonia axyridis Pallas, Coccinella septempunctata Linnaeus, and Hippodamia variegata Goeze. We aimed to address three key questions: (1) the functional response of adult ladybirds preying on aphids, (2) the intraspecific competition among adult ladybirds, and (3) the practical effect of ladybirds in reducing aphid populations in a semi-field setting.
2. Materials and Methods
2.1. Insect Rearing
The colonies of the ladybird species H. axyridis, C. septempunctata, and H. variegata, as well as the aphid A. gossypii, were obtained from the Dadi eco-cultivation bases of goji berry in Zhongning County, Ningxia Province, China. The aphids were reared and propagated on goji berry leaves under controlled conditions of 16 L: 8 D, 25 °C and 40–60% RH. Approximately 20 larvae or adults belonging to the same ladybird species were reared per plastic container (16 cm × 22 cm × 8 cm). The ladybird colonies selected were transferred into Petri dishes (9 cm diameter) containing goji berry leaves infested with aphids. The neonates of the ladybirds were promptly separated upon hatching to mitigate sibling cannibalism. To ensure a thriving ladybird population, aphids were provided at 12 h intervals. In the experiments, newly emerged (<12 h) adult ladybirds were initially subjected to a 24 h period of fasting, followed by placement in Petri dishes containing recently hatched (<12 h) adult aphids. The dishes were preserved under the same climatic conditions as previously described. Adult aphids remained unchanged throughout the experiments, while nymphs originating from the adult aphids were gently removed every 2 h using gentle brushes.
2.2. Functional Response
To investigate the functional response of adult ladybirds to aphids, we conducted an experiment employing a fully factorial design in which we independently manipulated three ladybird species (H. axyridis, C. septempunctata, or H. variegata) and five levels of initial aphid density (100, 150, 200, 250, or 300 individuals). The appropriate number of aphids was placed on a moistened paper disc in a 9 cm diameter Petri dish to establish our experimental treatments. Subsequently, a single adult ladybird was introduced into each Petri dish, followed by an examination of aphid consumption by the ladybirds using a binocular microscope after 24 h. The control treatments were implemented to account for the inherent mortality of adult aphids and the fecundity of newborn nymphs, thereby adjusting for ladybird predation on the aphids. Each treatment was replicated five times.
2.3. Intraspecific Competition
To evaluate the influence of intraspecific competition on aphid consumption by adult ladybirds, three ladybird species (H. axyridis, C. septempunctata, or H. variegata) were independently manipulated. In a Petri dish, 100, 200, 300, 400 or 500 aphids were provided to 1, 2, 3, 4 or 5 ladybirds, respectively (5 treatments), maintaining a prey-to-predator ratio of 100. The intensity of intraspecific competition among ladybirds for space exhibited a positive correlation with the abundance of ladybirds in a Petri dish. The parameter assessing intraspecific competition was determined by quantifying the number of surviving aphids after a 24 h period. Each treatment was replicated 5 times.
2.4. Semi-Field Study
The practical efficacy of the biological control program against aphids was evaluated through a semi-field study. The optimal aphid-to-predator ratio was initially estimated based on the theoretical maximum consumption derived from the functional response model mentioned above. The initial aphid abundance was recorded on a goji berry plant within a mesh cage (60 mesh, 1 m diameter, 1.5 m height). This study involved the collection of a total of 60 leaves, with three layers and four directions from the tree. Subsequently, the average number of aphids per leaf was calculated and multiplied by the total number of leaves on the plant to determine the aphid abundance. The adult ladybirds (H. axyridis, C. septempunctata, or H. variegata) were introduced into the cage in an appropriate number, maintaining the optimal prey-to-predator ratio. The aphid abundance within the mesh cage was reassessed after a 30-day period. Control treatments were implemented without the presence of ladybirds to account for natural fluctuations in the aphid populations and to accurately assess the efficacy of the biological control program. All treatments were each replicated 3 times for a total of 12 plants.
2.5. Statistical Analysis
2.5.1. Functional Response
The functional responses of ladybirds feeding on aphids on goji berry plants were determined through a two-stage analysis [30]. The first step involved conducting cubic logistic regression analysis to determine the shape (type II or type III) of the functional responses by examining the proportion of aphids consumed as a function of their initial density:
The equation is defined as follows: Na represents the number of aphids consumed, while N0 denotes the initial aphid density. P0, P1, P2 and P3 correspond to the intercept, linear, quadratic, and cubic parameters, respectively. The presence of negative or positive linear parameters (P1) indicates a type II or type III functional response, respectively. If the parameters of a cubic equation are not statistically significant, it is recommended to simplify the model by removing the quadratic and cubic parameters from Equation (1) and to retest the remaining parameters. The logistic regression analysis revealed that our data exhibited a type II functional response in each case; thus, subsequent analyses were exclusively focused on the type II functional response. Holling’s disc (Equation (2)) [31] was employed to depict the correlation between the consumption of aphids (Na) and their initial density (N0):
where Na and N0 are defined in Equation (1), T represents the total duration, which is 24 h in this case, a denotes the ladybird’s searching efficiency (i.e., the area covered per unit time), and Th signifies the handling time required for processing one prey [32]. The parameters a and Th were estimated using a nonlinear regression procedure (NLR) based on the Levenberg–Marquardt method. The initial values of a and Th required by the NLR procedure were determined through the linear regression analysis of 1/Na against 1/N0. The resulting y intercept serves as the initial estimation of Th, while the reciprocal of the regression parameter provides an estimate of a [33]. The theoretical maximum consumption by ladybirds (Nmax) can be obtained as N0 approaches infinity.
2.5.2. Intraspecific Competition
This experiment was conducted to quantify the parameter of intraspecific competition among ladybird individuals during predation events. The estimation of the parameters for intraspecific competition was conducted through nonlinear regression analysis by fitting Equation (3) [34]:
E = qP−m
The equation is defined as follows: E represents the per capita consumption rate of ladybirds, P denotes the ladybird abundance, m signifies the parameter of intraspecific competition, and q indicates the maximum per capita consumption rate. The model was established for conducting regression analysis on a power function curve.
The descriptive statistics are presented as the mean values accompanied by their corresponding standard errors of the mean. All data were checked for a normal distribution and homoscedasticity. The differences between the natural mortality rate and zero were assessed using a one sample t-test; significance was determined at p < 0.05. The remaining data were subjected to one-way ANOVA analysis, followed by the Tukey HSD test for significance at a 5% level of statistical significance. The statistical analysis was conducted using SPSS 20.0 software (IBM, Armonk, NY, USA), while the regression analyses were performed utilizing SigmaPlot 13.0 software (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Functional Response
Regardless of the aphid density, the natural mortality rates of the aphids did not exhibit a significant deviation from zero (t-test, p > 0.05) due to the limited production of newborn nymphs during the experiments. Consequently, both the mortality rates and the number of newborn nymphs among aphids remained inconsequential throughout the experimental trials. The parameter estimates derived from the logistic model (Equation (1)) for the proportion of aphids consumed by ladybirds within a 24 h period, in relation to the aphid density, are presented in Table 1. The estimated values of the linear parameter P1 were found to be significantly negative for all three species of ladybirds, indicating a type II response of these three ladybird species according to the logistic model analysis.

Table 1.
Maximum likelihood estimates (±SE) for parameters of the logistic model fit to the proportion of aphids consumed versus the initial aphid density.
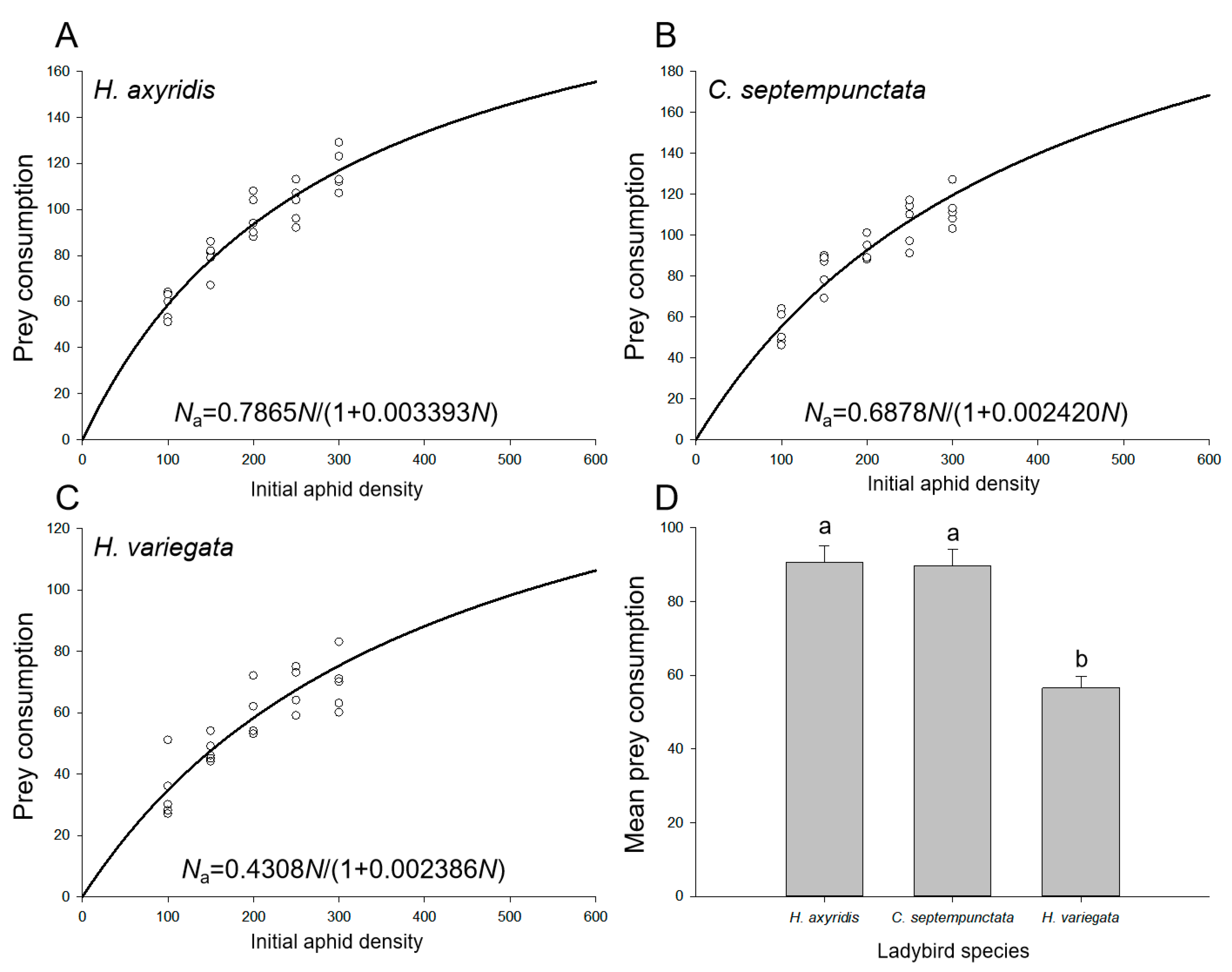
The functional response data for aphid consumption by adult ladybirds over a 24 h period exhibited a strong fit to Holling’s disc model (Equation (2)), as evidenced by the results presented in Table 2. This confirms the presence of a type II response across all three ladybird species. The consumption of aphids gradually increased with an increase in the initial aphid densities (Figure 1A–C), and both H. axyridis (90.6 ± 4.4) and C. septempunctata (89.6 ± 4.5) exhibited significantly higher mean aphid consumption compared to H. variegata (56.6 ± 3.1) (F2,72 = 22.874, p < 0.001; Figure 1D). The numerical values of the parameters for search efficiency (a) and handling time (Th) exhibited this relationship, with asymptotic 95% confidence intervals excluding zero, across all three ladybird species. We found that H. axyridis (a = 78.6%) displayed a significantly greater rate of spatial coverage per unit time than both C. septempunctata (a = 68.8%) and H. variegata (a = 43.1%), whereas C. septempunctata (Th = 5.07 min) exhibited a notably shorter duration for processing each individual aphid when compared with H. axyridis (Th = 6.21 min) and H. variegata (Th = 7.97 min). Moreover, the theoretical maximum aphid consumption by C. septempunctata (Nmax = 284.2) exceeded that of H. axyridis (Nmax = 231.8) and H. variegata (Nmax = 180.6).

Table 2.
Parameter estimates of Holling’s disc equation for ladybirds preying on aphids on goji berry plants.
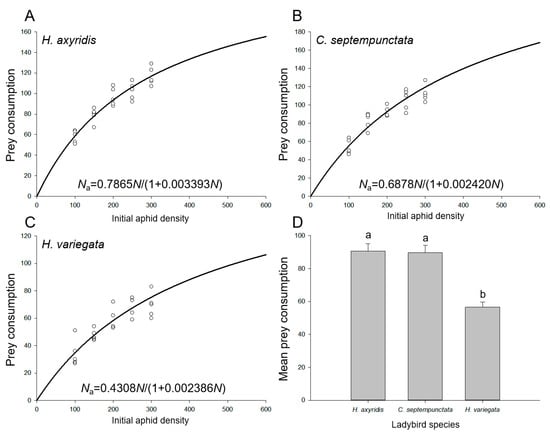
Figure 1.
Ladybird consumption of aphids on goji berry plants. Functional response of (A) H. axyridis, (B) C. septempunctata, and (C) H. variegata. Solid lines show the functional response curves of ladybirds attacking aphids obtained by fitting Holling’s disc model (Equation (2)). Circles indicate the number of aphids consumed at each aphid density. (D) Mean consumption by the three ladybird species. Different letters indicate significant differences among the ladybird species (mean separation by Tukey’s HSD, p < 0.05).
3.2. Intraspecific Competition
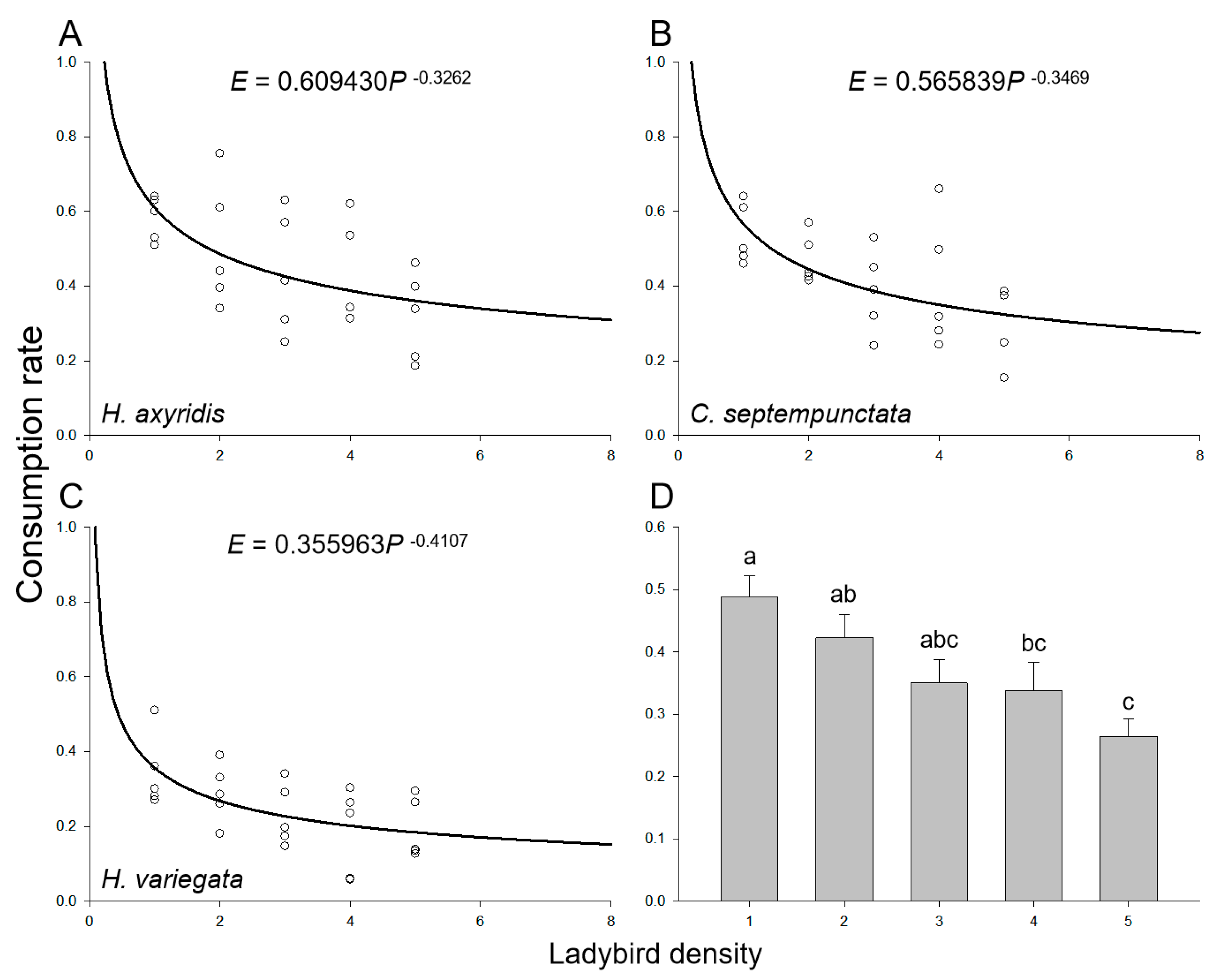
As the population sizes of the introduced aphids and ladybirds increased, there was a gradual augmentation in the total consumption of aphids in a Petri dish while maintaining a ratio of 100 aphids to ladybirds. However, irrespective of the ladybird species, the consumption rate of the ladybirds declined with increasing densities of both aphids and ladybirds due to intraspecific competition arising from spatial limitations (Figure 2A–C). The overall consumption rate by ladybirds demonstrated a significant decline with increasing densities of aphids and ladybirds (F4,70 = 5.368, p = 0.001; Figure 2D), with 48.8% ± 3.4%, 42.3% ± 3.8%, 35.0% ± 3.8%, 33.8% ± 4.5%, and 26.4% ± 2.8% when the density of ladybirds varied from one to five, respectively. Besides, the mean consumption rates of H. axyridis (45.5% ± 3.1%) and C. septempunctata (41.5% ± 2.7%) were higher than that of H. variegata (24.7% ± 2.1%) (F2,72 = 17.208, p < 0.001). The intraspecific competition model (Equation (3)) effectively captured the consumption rate across various ladybird densities, irrespective of the species (Table 3). The asymptotic 95% confidence intervals for the maximum consumption rate (q) and the parameter of intraspecific competition (m) of all three ladybird species did not include zero. The maximum consumption rate of H. axyridis (q = 60.9%) or C. septempunctata (q = 56.6%) was nearly twice that of H. variegata (q = 35.6%). The impact of intraspecific competition on H. variegata (m = 0.41) was more pronounced compared to H. axyridis (m = 0.33) and C. septempunctata (m = 0.35).
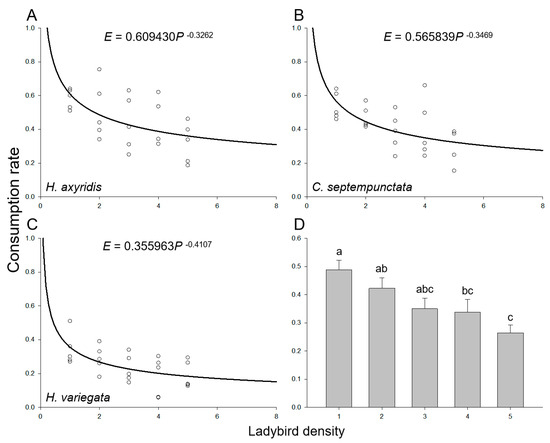
Figure 2.
Consumption rate of ladybirds preying on aphids on goji berry plants. Intraspecific competition among adult individuals of (A) H. axyridis, (B) C. septempunctata, and (C) H. variegata. Circles represent the mean consumption rate per ladybird at each ladybird density. The curve was fit using the intraspecific competition model (Equation (3)). (D) Mean consumption rate at various ladybird densities. Different letters indicate significant differences among treatments (mean separation by Tukey’s HSD, p < 0.05).

Table 3.
Parameter estimates of the intraspecific competition equation of the consumption rate of ladybirds at various densities.
3.3. Semi-Field Study
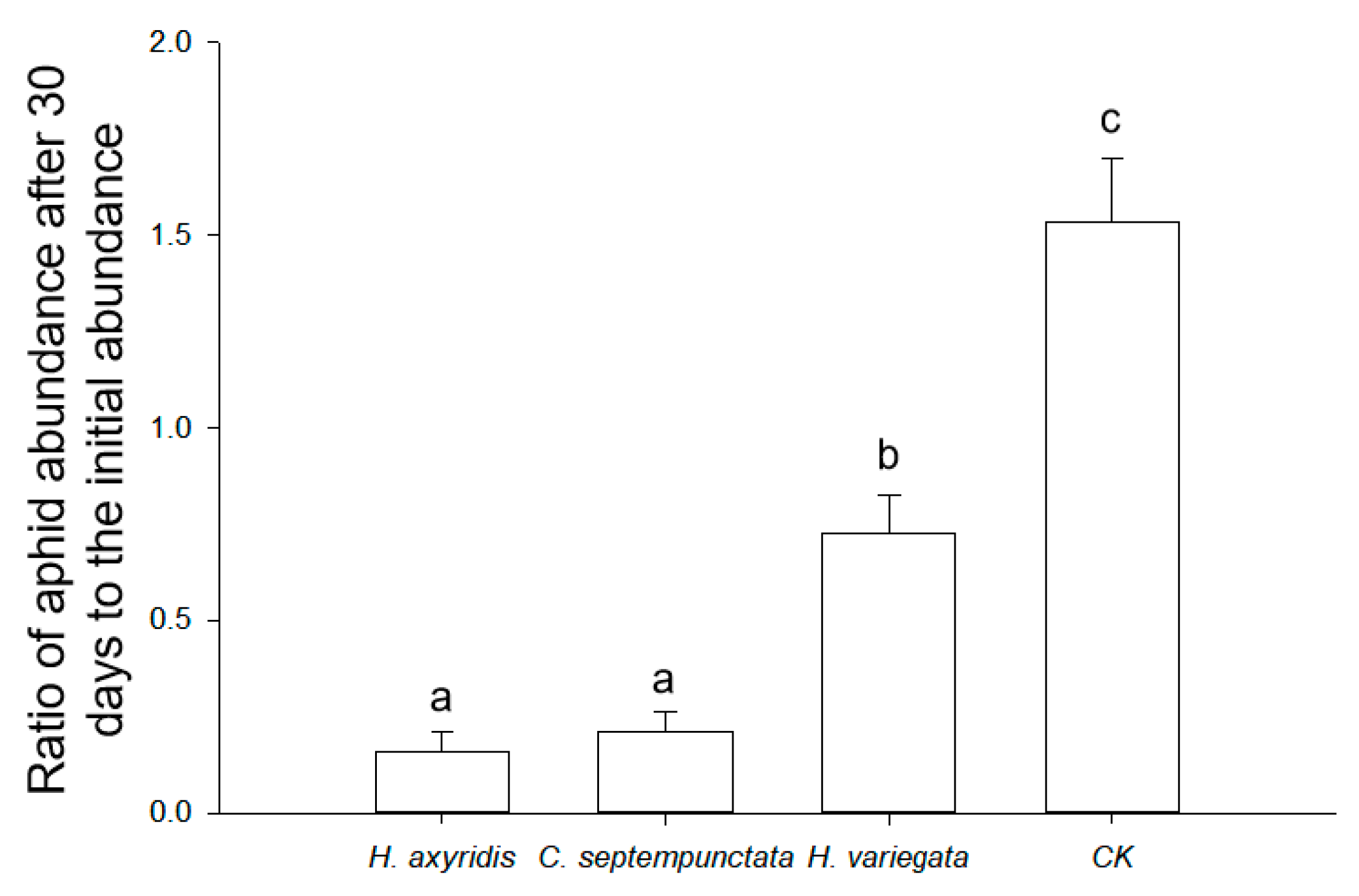
The optimal prey-to-predator ratios for ladybirds feeding on aphids were determined to be 231.8 (H. axyridis), 284.2 (C. septempunctata), and 180.6 (H. variegata), based on the theoretical maximum consumption (Nmax) calculated using Equation (2). The utilization of ladybirds in practical applications was demonstrated as an effective approach to managing aphid populations (F3,8 = 38.772, p < 0.001; Figure 3). In the absence of ladybirds, the aphid population increased by 53.6% ± 16.4% in 30 days. In contrast, H. axyridis (83.9% ± 5.2%) and C. septempunctata (78.7% ± 5.0%) exhibited a more pronounced reduction in the aphid abundance compared to H. variegata (27.3% ± 9.9%) within a span of 30 days.
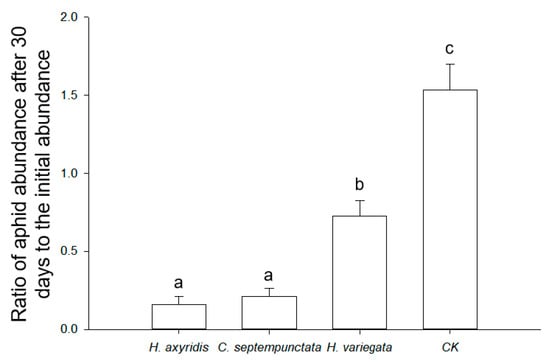
Figure 3.
Ratio of aphid abundance after 30 days to the initial abundance in semi-field conditions. The treatments include the presence of H. axyridis, C. septempunctata, or H. variegata, and the absence of ladybirds (CK). Different letters indicate significant differences among treatments (mean separation by Tukey’s HSD, p < 0.05).
4. Discussion
Our study demonstrated that ladybirds should be recognized as natural enemies of aphids and potentially valuable agents in biological control programs by decision-makers in goji berry orchards. Both our laboratory and field studies provided evidence of the ability of ladybirds to suppress aphids on goji berry plants. The pest pressure of aphids was not exceptionally high during our study. However, the abundance of aphids per foliage reached a maximum average of 9.2 in the ladybird-free plots of the most-infested orchard. This level of infestation would have exceeded the tolerance threshold for farmers. The plots introduced with ladybirds consistently exhibited an average of less than two aphids, a level that would be deemed acceptable by most standards. Our findings support previous studies indicating the significant role of ladybirds as effective predators of aphid species [29]. Additionally, our results emphasize the potential underestimation of ladybirds by decision-makers in goji berry orchards, who could benefit from considering their inclusion in integrated pest management strategies.
We observed a type II functional response against aphids by all adult ladybirds of three species. Relevant studies have also documented type II functional response curves in ladybird predation on diverse prey species [35,36,37,38,39]. Holling’s disc equation is widely used as the predominant model for analyzing the type II functional response [40,41]. The results of our study also revealed that, for three species of ladybirds, the consumption of aphids by ladybirds increased proportionally with the number of aphids provided. However, once a certain threshold was reached, the consumption gradually slowed down and remained below this threshold level. This pattern can be accurately described using Holling’s disc model. These findings were consistent with previous research, indicating that Holling’s disc model is an appropriate framework for describing the ladybird consumption of various prey species [42,43,44,45].
The mean aphid consumption by H. axyridis and C. septempunctata exceeded that of H. variegata, potentially attributed to the relatively smaller size of H. variegata. However, the DNA-based analysis of gut contents suggests that H. variegata exhibits a higher predation rate on A. gossypii compared to C. septempunctata (90.6% vs. 70.9%) in melon crops [23], which can be attributed to factors such as the ladybug’s digestive capacity and feeding timing, potentially resulting in contrasting outcomes. Additionally, the reduced predation by H. variegata on goji berry plants may be attributed to the disparate nutritional value of A. gossypii when feeding on goji berries as opposed to melons [29]. The efficiency of H. axyridis in locating aphids was found to be the highest, potentially attributed to the invasive nature of H. axyridis, which allows for greater adaptability and more flexibility [46]. Predators exhibit exceptional sensitivity to the direction and angle of prey, leading them to efficiently locate their target [47,48]. In this regard, H. axyridis demonstrates rapid recognition of prey location based on reference objects in its surroundings. The mobility of H. axyridis facilitates its detection, thereby augmenting its searching efficiency on aphids. The handling time required for C. septempunctata to process one aphid was the shortest, possibly due to its higher nutritional requirements for reproduction [49]. The larger size of C. septempunctata may indicate a higher level of aggression and voracity compared to the other two species, and these characteristics may also result in a potentially greater maximum theoretical consumption by C. septempunctata.
The findings of our study revealed that, despite maintaining a constant aphid-to-ladybird ratio of 100, the consumption rate of ladybirds exhibited a progressive decline with increasing ladybird density in the Petri dish. The consumption rate of ladybirds was negatively affected at high ladybird densities due to an increased likelihood of intraspecific competition or mutual interference resulting from resource or space limitations [50]. The intraspecific competition model for ladybirds has been proven to yield the parameters q (maximum consumption rate) and m (parameter of intraspecific competition) [51,52].
The maximum consumption rate of H. axyridis or C. septempunctata was found to be twice as high as that of H. variegata, while the influence of intraspecific competition on H. variegata exceeded that on H. axyridis or C. septempunctata. However, the presence of H. variegata is likely to be further reduced due to the hypothetical artificial increase of both H. axyridis and C. septempunctata, as they are asymmetric intraguild predators of H. variegata [23,53]. The consumption rate of ladybirds, being digestive-limited predators, exhibits a limitation due to predator satiation [54]. The activity of the ladybirds increased significantly following the consumption of a large number of aphids, which likely promoted intraspecific competition. Therefore, it is possible that a considerable amount of time was dedicated to intraspecific competition when the ladybirds had reached a low level of satiation [55,56]. Therefore, the stronger intraspecific competition among H. variegata individuals may be attributed to their lower maximum consumption rate, given their small size. This reduction may be insignificant for aphid control if the impact of H. variegata is low, but this may not be the case considering that this species is recognized for its biocontrol potential in the goji berry field. If the efficacy of larvae is indeed confirmed, one could consider releasing H. variegata as a less aggressive intraguild predator. Alternatively, the introduction of H. axyridis or C. septempuctata could be proposed, but strategies for mitigating the risk of intraguild predation on H. variegata should also be taken into account.
In the semi-field study, all three species of ladybirds demonstrated high efficacy in controlling aphid populations. The absence of ladybirds on plants resulted in a 53% increase in aphid populations within 30 days, whereas H. axyridis, C. septempunctata, and H. variegata exhibited the potential to cause reductions ranging from 27.3% to 83.9%. Comparatively, the biological control efficiency of H. axyridis and C. septempunctata against the aphids was superior to that of H. variegata, indicating that H. axyridis and C. septempunctata may be more suitable choices for controlling aphids in the goji berry field. However, the suitability of H. variegata as a predator for aphids can still be observed under specific circumstances [57]. Significantly, the field release of ladybird eggs has demonstrated cost-effectiveness by obviating the necessity for larval mass rearing and providing convenience in terms of their transportation, storage, and portability [58].
The estimation of a predator’s potential as a biological control agent relies heavily on consumption traits such as searching efficiency and handling time [59], and prey–predator dynamics can be assessed through the utilization of a mathematical model [60]. Thus, the aphid–ladybird dynamics can be established by analyzing the functional response curves, and the consumption ability of ladybirds is contingent upon the aphid density in their natural habitats. However, the validation of aphid–ladybird dynamics requires field studies, as quantitative models developed in laboratory or semi-field studies seem to have limited applicability in assessing foraging abilities under actual field conditions, likely due to variations in predator search efficiency under different conditions [61,62]. The spatial complexity, which plays a crucial role in the natural environment, cannot be replicated under simplified semi-field or laboratory conditions. These laboratory studies provide a parametric analysis of predator-dependent intraspecific competition models, but they are limited to a non-spatial scale. Therefore, it is crucial for future research to consider intraspecific competition in a spatial context as it is closer to natural conditions [63,64,65]. The presence of intraspecific competition has the potential to disrupt the foraging capacities quantified by a functional response. Therefore, the comprehension of not only prey–predator but also predator–predator interactions is crucial for a reliable aphid control strategy based on ladybirds.
The semi-field study further confirmed the significant potential of H. axyridis and C. septempunctata as promising candidates for aphid control in goji berry orchards, but the selection between them may also depend on the specific ladybird’s environmental adaptability. Therefore, it is crucial to choose the dominant ladybird species based on the prevailing environmental conditions. However, H. variegata also exhibits potential as a biological control agent due to its enhanced fitness, enabling it to effectively respond to fluctuations in pest density. The availability of pollen and nectar from flowers as supplementary food sources in the marginal vegetation of crops can enhance the reproductive performance of H. variegata, particularly when prey is scarce [66]. The indoor feeding of ladybirds on a large scale is indispensable if they are to be released into fields in the future. Therefore, disparities in feeding efficiency between naturally occurring and laboratory-reared ladybirds must also be considered, given that the ladybirds utilized in this study were all collected from the field. Moreover, the influence of natural stochasticity and intraguild predation should be taken into account in future field experiments investigating intraspecific competition among ladybirds [67,68]. The consideration of plant characteristics is also essential due to their influence on the feeding efficiency of ladybirds [69]. Future field studies associated with prey–predator dynamics are crucial for the successful implementation of ladybird release, as it has the potential to minimize pesticide usage and enhance the predator population [70].
5. Conclusions
The aphid A. gossypii is a significant pest that inflicts severe damage upon goji berry plants in China. To assess the potential of ladybirds for aphid biological control, we conducted laboratory experiments to evaluate the functional responses of three ladybird species: H. axyridis, C. septempunctata, and H. variegata. Additionally, we examined intraspecific competition among ladybird individuals. The practical efficacy of ladybirds in reducing aphid populations was investigated under semi-field conditions. A functional response model accurately described the foraging behaviors of ladybirds, while an intraspecific competition model indicated the presence of mutual interference among ladybird individuals that impacted their prey consumption. Therefore, conducting a comprehensive analysis of the functional response and intraspecific competition will contribute to enhancing our understanding of prey–predator and predator–predator interactions in the context of biological control against aphids on goji berry plants. The functional response study suggested the efficacy of ladybirds as efficient biological control agents for managing aphids, and the semi-field study further confirmed the significant potential of H. axyridis and C. septempunctata as promising candidates. The consideration of an appropriate density is also crucial to mitigate intraspecific competition when releasing ladybirds in the field, as an insufficient or excessive number of released ladybirds may compromise the effectiveness of the biological control program. However, future field studies are crucial in ensuring the effective implementation of a biological control program.
Author Contributions
Conceptualization, P.W. and R.Z.; methodology, P.W. and H.D.; investigation, P.W. and J.H.; writing—original draft preparation, P.W.; writing—review and editing, P.W., J.H., H.D. and R.Z.; supervision, P.W. and R.Z.; funding acquisition, P.W. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32102206), the National Key Research and Development Program (2021YFC2600100), and the Research and Development Program of Ningxia Hui Autonomous Region (2021BEF03002).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Siqin Ge for providing the greenhouse. We also thank Haoyong Ouyang and Baoxu Ma for their investigations, thank Jiaqi Xu and Ruchen Fu for formatting, and thank Yanan Wang and Qiaoling Lin for greenhouse management. All individuals mentioned above are affiliated with the Institute of Zoology, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vidovi, B.B.; Milini, D.D.; Mareti, M.D.; Djuri, J.D.; Ilic, T.D.; Kosti, A.; Pesic, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.M.; Badwal, T.S.; Xu, B.J. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Magalhaes, V.; Silva, B.; Zhang, X.Y.; Silva, A.R.; Dias, A.C.P. Comparative studies on the anti-neuroinflammatory and antioxidant activities of black and red goji berries. J. Funct. Foods 2022, 92, 105038. [Google Scholar] [CrossRef]
- Wu, P.X.; Ge, Y.; He, J.; Haseeb, M.; Zhang, R.Z. Positive Interactions between Aceria pallida and Bactericera gobica on Goji Berry Plants. Insects 2022, 1, 577. [Google Scholar] [CrossRef]
- Wu, P.X.; Ma, B.X.; Wu, F.M.; Ouyang, H.Y.; Fan, J.Y.; Xu, J.; Zhang, R.Z. The hyperparasitoid Marietta picta mediates the coexistence of primary parasitoids of goji berry psyllid. Entomol. Gen. 2020, 2, 187–194. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shen, S.; Zhi, H.; Li, W. Pesticides residues on Goji berry: A characteristic minor crop in China. J. Food Compos. Anal. 2023, 120, 105342. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, R.; Sharma, S. Impact of chemical- and bio-pesticides on bacterial diversity in rhizosphere of Vigna radiata. Ecotoxicology 2013, 22, 1479–1489. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, R.; Sharma, S. Impact of pesticides on plant growth promotion of Vigna radiata and non-target microbes: Comparison between chemical- and bio-pesticides. Ecotoxicology 2014, 23, 1015–1021. [Google Scholar] [CrossRef]
- Mohamed, A.K.A.; Pratt, J.A.P.; Nelson, F.R.S. Compatability of Metarhizium Anisopliae Var. Anisopliae with chemical pesticides. Mycopathologia 1987, 99, 99–105. [Google Scholar]
- Wilkinson, J.D.; Biever, K.D.; Ignoffo, C.M. Contact toxicity of some chemical and biological pesticides to several insect parasitoids and predators. Entomophaga 1975, 20, 113–120. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.; He, J.; Tian, Y.; Chen, J.; Zhang, R. Resistance of wolfberry aphid in Ningxia. J. Northwest A&F Univ. 2017, 45, 61–67. [Google Scholar]
- Zhang, R.Z.; Zhang, R. Aphids infested Lycium chinense Miller are Aphis gossypii Glover, Myzus persicae (Sulzer) and A. craccivora Koch. Chin. J. Appl. Entomol. 2016, 53, 218–222. [Google Scholar]
- Ebert, T.A.; Cartwright, B. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Shi, D.D.; Liang, P.Z.; Zhang, L.; Lv, H.X.; Gao, X.W.; You, H.; Li, J.H.; Ma, K.S. Susceptibility baseline of Aphis gossypii Glover (Hemiptera: Aphididae) to the novel insecticide afidopyropen in China. Crop Prot. 2022, 151, 105834. [Google Scholar]
- Watanabe, H.; Yano, E.; Higashida, K.; Hasegawa, S.; Takabayashi, J.; Ozawa, R. An attractant of the aphidophagous gall midge Aphidoletes aphidimyza from honeydew of Aphis gossypii. J. Chem. Ecol. 2016, 42, 149–155. [Google Scholar]
- Mahas, J.B.; Ray, C.; Kesheimer, A.; Conner, K.; Jacobson, A.L. Seasonal dynamics of aphid flights and cotton leafroll dwarf virus spread in Alabama. Insects 2023, 14, 604. [Google Scholar]
- Hopkinson, J.E.; Zalucki, M.P.; Murray, D.A.H. Host selection and parasitism behavior of Lysiphlebus testaceipes: Role of plant, aphid species and instar. Biol. Control 2013, 64, 283–290. [Google Scholar]
- Pan, Y.O.; Wen, S.Y.; Chen, X.W.; Gao, X.W.; Zeng, X.C.; Liu, X.M.; Tian, F.Y.; Shang, Q.L. UDP-glycosyltransferases contribute to spirotetramat resistance in Aphis gossypii Glover. Pestic. Biochem. Phys. 2020, 166, 104565. [Google Scholar]
- Xia, J.Y.; Van Der Werf, W.; Rabbinge, R. Temperature and prey density on bionomics of Coccinella septempunctata (Coleoptera: Coccinellidae) feeding on Aphis gossypii (Homoptera: Aphididae) on cotton. Environ. Entomol. 1999, 28, 307–314. [Google Scholar]
- Xia, J.Y.; Rabbinge, R.; Van Der Werf, W. Multistage functional responses in a ladybeetle-aphid system: Scaling up from the laboratory to the field. Environ. Entomol. 2003, 32, 151–162. [Google Scholar]
- Rondoni, G.; Ielo, F.; Ricci, C.; Conti, E. Intraguild predation responses in two aphidophagous coccinellids identify differences among juvenile stages and aphid densities. Insects 2014, 5, 974–983. [Google Scholar] [PubMed]
- Madadi, H.; Parizi, E.M.; Allahyari, H.; Enkegaard, A. Assessment of the biological control capability of Hippodamia variegata (Col.: Coccinellidae) using functional response experiments. J. Pest Sci. 2011, 84, 447–455. [Google Scholar]
- Rondoni, G.; Fenjan, S.; Bertoldi, V.; Ielo, F.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Ricci, C.; Conti, E. Molecular detection of field predation among larvae of two ladybird beetles is partially predicted from laboratory experiments. Sci. Rep. 2018, 8, 2594. [Google Scholar] [PubMed]
- Calvert, G.M.; Sanderson, W.T.; Barnett, M.; Blondell, J.M.; Mehler, L.N. Surveillance of pesticide-related illness and injury in humans. In Handbook of Pesticide Toxicology; Krieger, R.I., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 603–641. [Google Scholar]
- Bruce, N.A.; Margie, P.; Lois, S.G. Nature’s chemicals and synthetic chemicals: Comparative toxicology. Proc. Natl. Acad. Sci. USA 1990, 87, 7782–7786. [Google Scholar]
- Ejaz, S.; Akram, W.; Lim, C.W.; Lee, J.J.; Hussain, I. Endocrine disrupting pesticides: A leading cause of cancer among rural people. Exp. Oncol. 2004, 26, 98–105. [Google Scholar]
- Steinmann, K.P.; Minghua, Z.; Grant, J.A. Does use of pesticides known to harm natural enemies of spider mites (Acari: Tetranychidae) result in increased number of miticide applications? An examination of California walnut orchards. J. Econ. Entomol. 2011, 104, 1496–1501. [Google Scholar] [CrossRef]
- Smilanich, A.M.; Dyer, L.A. Effects of banana plantation pesticides on the immune response of lepidopteran larvae and their parasitoid natural enemies. Insects 2012, 3, 23–24. [Google Scholar]
- Wu, X.H.; Zhou, X.R.; Pang, B.P. Influence of five host plants of Aphis gossypii Glover on some population parameters of Hippodamia variegata (goeze). J. Pest Sci. 2010, 83, 77–83. [Google Scholar]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar]
- Poole, A.E.; Stillman, R.A.; Watson, H.K.; Norris, K.J. Searching efficiency and the functional response of a pause-travel forage. Funct. Ecol. 2007, 21, 784–792. [Google Scholar] [CrossRef]
- Livdahl, T.P.; Stiven, A.E. Statistical difficulties in the analysis of predator functional response data. Can. Entomol. 1983, 115, 1365–1370. [Google Scholar] [CrossRef]
- Hassell, M.P.; Varley, G.C. New inductive population model for insect parasites and its bearing on biological control. Nature 1969, 223, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Uiterwaal, S.F.; DeLong, J.P. Multiple factors, including arena size, shape the functional responses of ladybird beetles. J. Appl. Ecol. 2018, 55, 2429–2438. [Google Scholar] [CrossRef]
- Pervez, A.; Omkar. Functional responses of coccinellid predators: An illustration of a logistic approach. J. Insect Sci. 2005, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.X.; Zhang, J.; Haseeb, M.; Yan, S.; Kanga, L.; Zhang, R.Z. Functional responses and intraspecific competition in the ladybird Harmonia axyridis (Coleoptera: Coccinellidae) provided with Melanaphis sacchari (Homoptera: Aphididae) as prey. Eur. J. Entomol. 2018, 115, 232–241. [Google Scholar] [CrossRef]
- Wu, P.X.; Haseeb, M.; Liu, C.; Yan, S.; Xu, J.; Zhang, R.Z. Possible coexistence of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) and Diaeretiella rapae M’Intosh (Hymenoptera: Braconidae) in the biological control of Lipaphis erysimi (Homoptera: Aphididae). J. Asia-Pacific Entomol. 2019, 22, 250–255. [Google Scholar] [CrossRef]
- Koch, R.L.; Hutchison, W.D.; Venette, R.C.; Heimpel, G.E. Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 265–270. [Google Scholar] [CrossRef]
- Fan, Y.; Petitt, F.L. Parameter estimation of functional response. Environ. Entomol. 1994, 23, 785–794. [Google Scholar] [CrossRef]
- Fan, Y.; Petitt, F.L. Functional response, variance, and regression analysis: A reply to Williams and Juliano. Environ. Entomol. 1997, 26, 1–3. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Guo, X.H.; Hou, Z.R.; Wang, L.; Yin, Z.; Hao, S.D.; Guo, Y.Y.; Wang, J.Z.; Zhang, Z.Y. The predation functional response of Harmonia axyridis Pallas to Aphis craccivora. J. Environ. Entomol. 2014, 36, 965–970. [Google Scholar]
- Peixoto, M.D.; de Barros, L.C.; Bassanezi, R.C. Predator-prey fuzzy model. Ecol. Model. 2008, 214, 39–44. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Broufas, G.D.; Papachristos, D.P.; Pappas, M.L.; Kyriakaki, C.; Samaras, K.; Kypraios, T. On the mechanistic understanding of predator feeding behavior using the functional response concept. Ecosphere 2020, 11, e03147. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Zhang, F.; Liu, C.Z. A comparison of predatory function response in different search spaces of a French non-flying form Harmonia axyridis (Coleoptera: Coccinellidae) to Aphis carvines. J. Environ. Entomol. 2012, 34, 80–87. [Google Scholar]
- Tayeh, A.; Hufbauer, R.A.; Estoup, A.; Ravigne, V.; Frachon, L.; Facon, B. Biological invasion and biological control select for different life histories. Nat. Commun. 2015, 6, 7268. [Google Scholar] [CrossRef]
- Klarner, D.; Barth, F. Vibratory signals and prey capture in web spiders (Zygiella x-notata, Nephila clavipes). J. Comp. Physiol. 1982, 148, 445–455. [Google Scholar] [CrossRef]
- Hergenroder, R.; Barth, F.G. Vibratory signals and spider behavior: How do the sensory inputs from the eight legs interact in orientation? J. Comp. Physiol. 1983, 152, 361–371. [Google Scholar] [CrossRef]
- Omkar; Srivastava, S. Functional response of the seven-spotted lady beetle, Coccinella septempunctata Linnaeus on the mustard aphid, Lipaphis erysimi (Kaltenbach). Insect Sci. Appl. 2003, 23, 149–152. [Google Scholar]
- Hattingh, V.; Samways, M.J. Absence of intraspecific interference during feeding by the predatory ladybirds Chilocorus spp. (Coleoptera: Coccinellidae). Ecol. Entomol. 1990, 15, 385–390. [Google Scholar] [CrossRef]
- Zaviezo, T.; Soares, A.O.; Grez, A.A. Interspecific exploitative competition between Harmonia axyridis and other coccinellids is stronger than intraspecific competition. Biol. Control 2019, 131, 62–68. [Google Scholar] [CrossRef]
- Howe, A.G.; Ransijn, J.; Ravn, H.P. A sublethal effect on native Anthocoris nemoralis through competitive interactions with invasive Harmonia axyridis. Ecol. Entomol. 2015, 40, 639–649. [Google Scholar] [CrossRef]
- Katsanis, A.; Babendreier, D.; Nentwig, W.; Kenis, M. Intraguild predation between the invasive ladybird Harmonia axyridis and non-target European coccinellid species. Biocontrol 2013, 58, 73–83. [Google Scholar] [CrossRef][Green Version]
- Papanikolaou, N.E.; Milonas, P.G.; Demiris, N.; Papachristos, D.P.; Matsinos, Y.G. Digestion limits the functional response of an aphidophagous coccinellid. Ann. Entomol. Soc. Am. 2014, 107, 468–474. [Google Scholar] [CrossRef]
- Van Gils, J.A.; Piersma, T. Digestively constrained predators evade the cost of interference competition. J. Anim. Ecol. 2004, 73, 386–398. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Nikos, D.; Milonas, P.G.; Simon, P.; Theodore, K. Does mutual interference affect the feeding rate of aphidophagous coccinellids? A modeling perspective. PLoS ONE 2016, 11, e0146168. [Google Scholar] [CrossRef] [PubMed]
- Alizamani, T.; Razmjou, J.; Naseri, B.; Hassanpour, M.; Asadi, A.; Kerr, C. Effect of vermicompost on life history of Hippodamia variegata preying on Aphis gossypii Glover. J. Entomol. Res. Soc. 2017, 19, 51–60. [Google Scholar]
- Seko, T.; Miura, K. Functional response of the lady beetle Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) on the aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 2008, 43, 341–345. [Google Scholar] [CrossRef]
- Lucas, E.; Coderre, D.; Vincent, C. Voracity and feeding preferences of two aphidophagous coccinellids on Aphis citricola and Tetranychus urticae. Entomol. Exp. Appl. 1997, 85, 151–159. [Google Scholar] [CrossRef]
- Hassell, M.P. The Dynamics of Arthropod Predator–Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978; pp. 1–237. [Google Scholar]
- Gitonga, L.M.; Overholt, W.A.; Löhr, B.; Magambo, J.K.; Mueke, J.M. Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biol. Control. 2002, 24, 1–6. [Google Scholar] [CrossRef]
- Lee, J.; Kang, T. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the laboratory. Biol. Control 2004, 31, 306–310. [Google Scholar] [CrossRef]
- Sun, G.Q.; Jin, Z.; Liu, Q.X.; Li, L. Dynamical complexity of a spatial predator-prey model with migration. Ecol. Model. 2008, 219, 248–255. [Google Scholar] [CrossRef]
- Sun, G.Q.; Chakraborty, A.; Liu, Q.X.; Lin, Z.; Anderson, K.E.; Li, B.L. Influence of time delay and nonlinear diffusion on herbivore outbreak. Commun. Nonlinear Sci. Numer. Simulat. 2014, 19, 1507–1518. [Google Scholar] [CrossRef]
- Sun, G.Q.; Wang, S.L.; Ren, Q.; Jin, Z.; Wu, Y.P. Effects of time delay and space on herbivore dynamics: Linking inducible defenses of plants to herbivore outbreak. Sci. Rep. 2015, 5, 11246. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, I.; Núñez-Pérez, E.; Tizado, E.J. Effect of wild flowers on oviposition of Hippodamia variegata (Coleoptera: Coccinellidae) in the laboratory. J. Econ. Entomol. 2008, 101, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- De Villemereuil, P.B.; López-Sepulcre, A. Consumer functional responses under intra- and inter-specific interference competition. Ecol. Model. 2011, 222, 419–426. [Google Scholar] [CrossRef]
- Sentis, A.; Hemptinne, J.L.; Brodeur, J. How functional response and productivity modulate intraguild predation. Ecosphere 2013, 4, 46. [Google Scholar] [CrossRef]
- Hodek, I.; Honek, A. Ecology of Coccinellidae; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; p. 464. [Google Scholar]
- Xue, Y.; Bahlai, C.A.; Frewin, A.; Sears, M.K.; Schaafsma, A.W.; Hallett, R.H. Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae). Environ. Entomol. 2009, 38, 708–714. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).