Turning a Pest into a Natural Enemy: Removing Earwigs from Stone Fruit and Releasing Them in Pome Fruit Enhances Pest Control
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Earwig Mass-Trapping: Field Sites and Experimental Design
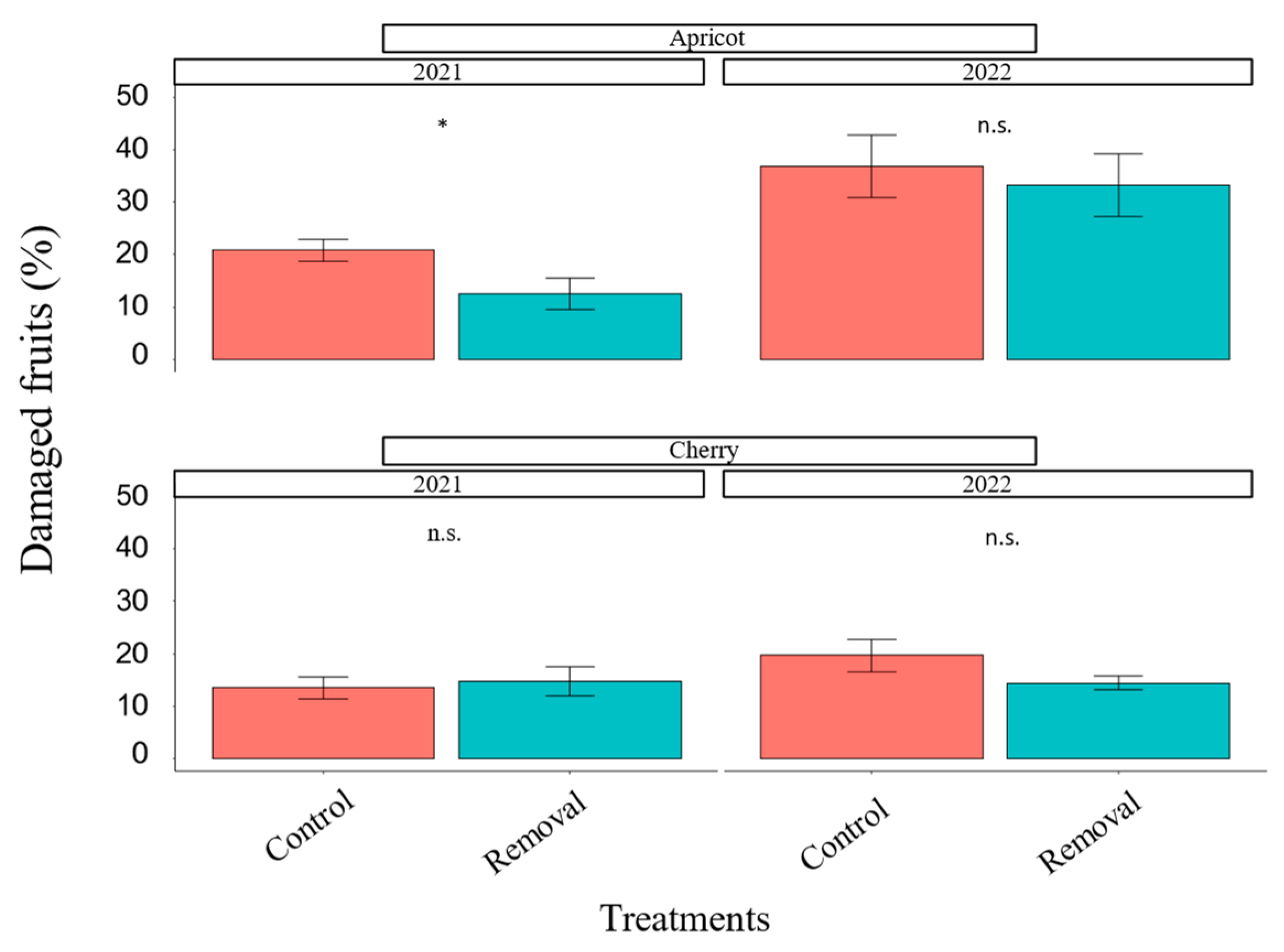
2.2. Earwig Mass-Trapping: Damage to Stone Fruit
2.3. Earwig Maintenance after Capture
2.4. Earwig Augmentation: Field Sites and Experimental Design
2.5. Earwig Augmentation: Establishment after Release
2.6. Earwig Augmentation: Pest Monitoring
2.7. Data Analysis
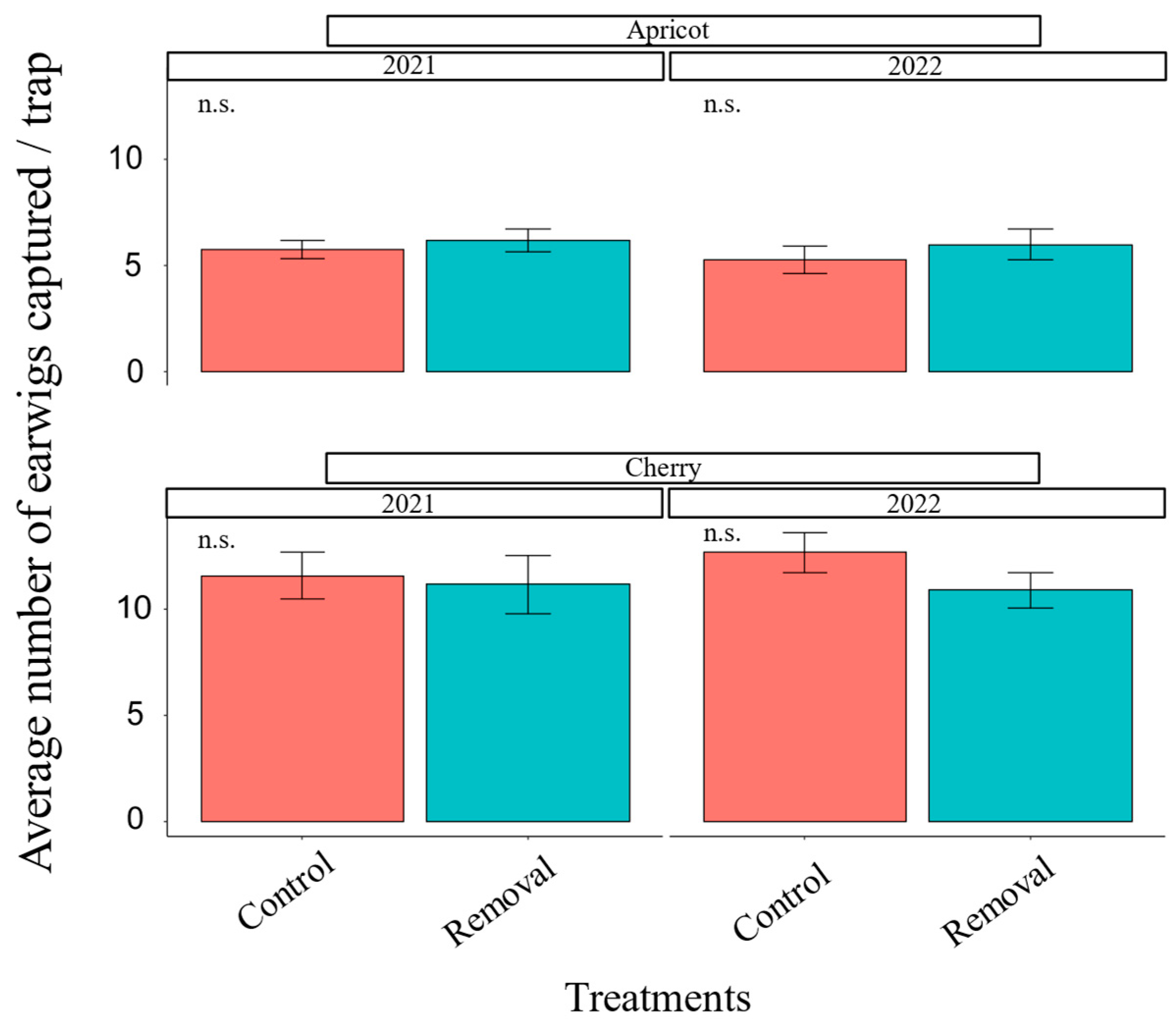
2.7.1. Earwig Mass-Trapping
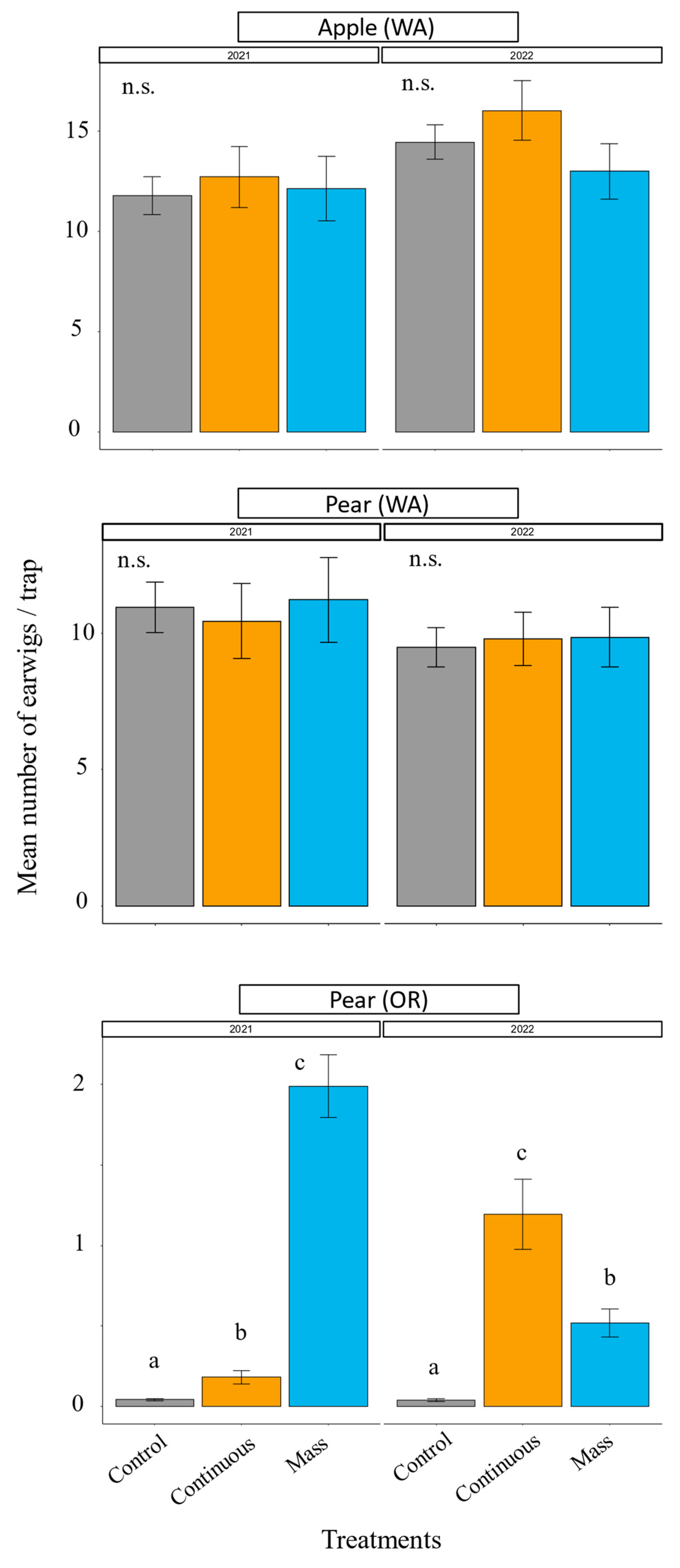
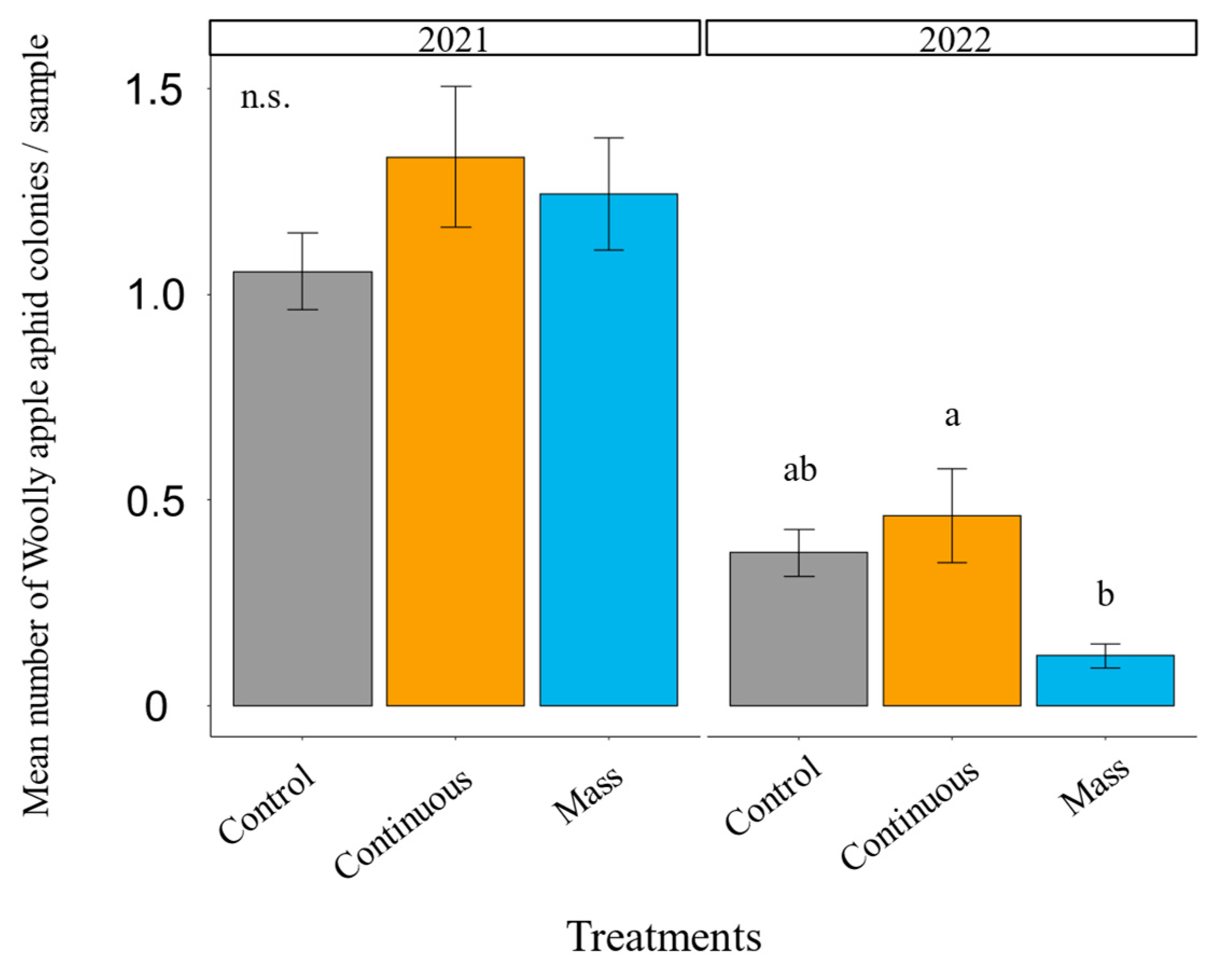
2.7.2. Earwig Augmentation
3. Results
3.1. Earwig Mass-Trapping
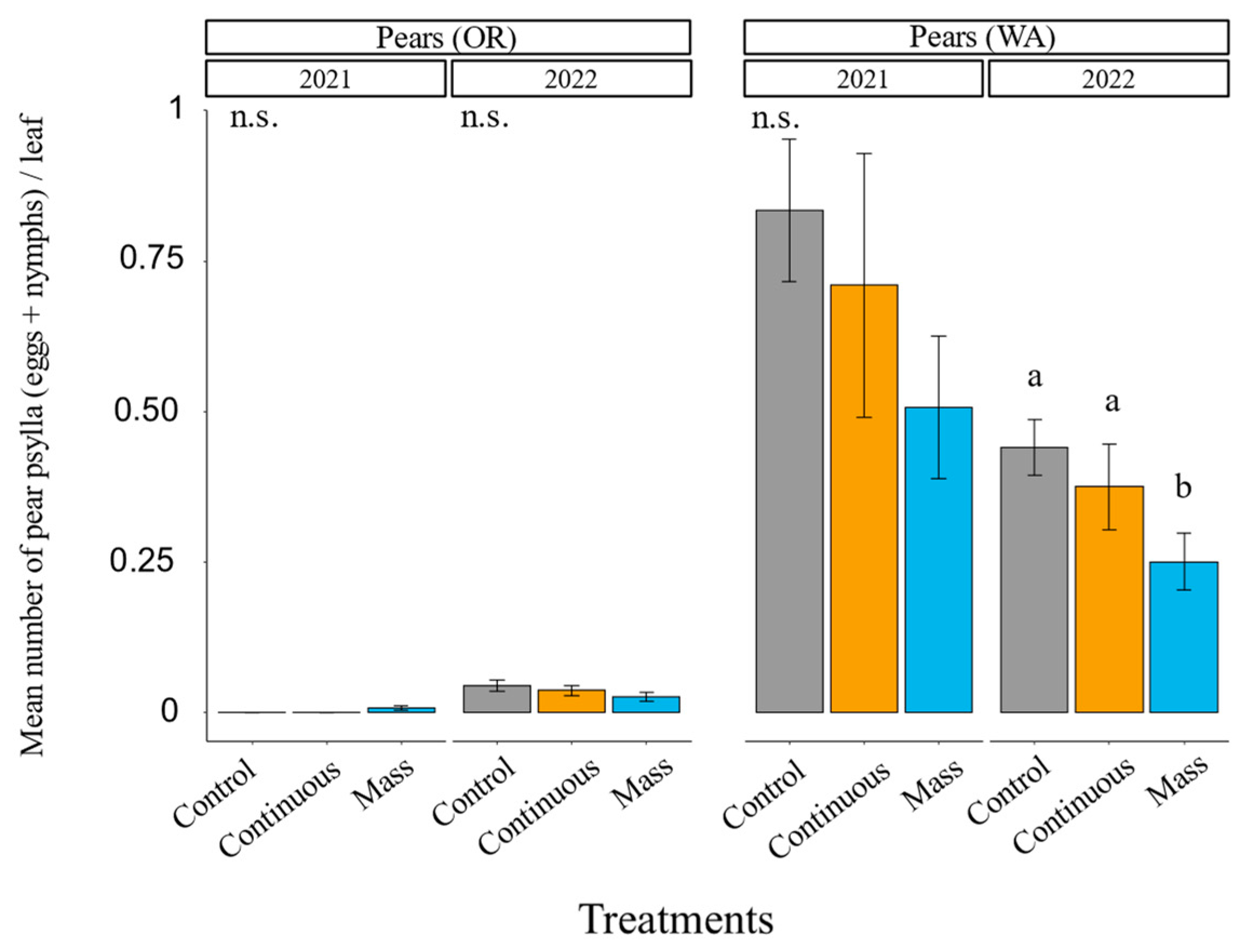
3.2. Earwig Augmentation
3.3. Earwig Augmentation: Pest Control
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naranjo, S.E.; Ellsworth, P.C.; Friswold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Bouvier, J.-C.; Debras, J.-F.; Sauphanor, B. Biodiversity and pest management in orchard systems. A review. Agron. Sustain. Dev. 2010, 30, 139–152. [Google Scholar] [CrossRef]
- DuPont, S.T.; Strohm, C.J. Integrated pest management programmes increase natural enemies of pear psylla in Central Washington pear orchards. J. Appl. Entomol. 2020, 144, 109–122. [Google Scholar] [CrossRef]
- Shaw, B.; Nagy, C.; Fountain, M.T. Organic Control Strategies for Use in IPM of Invertebrate Pests in Apple and Pear Orchards. Insects 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, R.K.; Brunner, J.F.; Castagnoli, S. Capturing the economic value of biological control in western tree fruit. Biol. Control 2016, 102, 93–100. [Google Scholar] [CrossRef]
- DuPont, S.T.; Strohm, C.; Nottingham, L.; Rendon, D. Evaluation of an integrated pest management program for central Washington pear orchards. Biol. Control 2021, 152, 104390. [Google Scholar] [CrossRef]
- González–Miguéns, R.; Muñoz–Nozal, E.; Jiménez–Ruiz, Y.; Mas–Peinado, P.; Ghanavi, H.R.; García–París, M. Speciation patterns in the Forficula auricularia species complex: Cryptic and not so cryptic taxa across the western Palaearctic region. Zoo. J. Linn. Soc. 2020, 190, 788–823. [Google Scholar] [CrossRef]
- Crumb, S.E.; Eide, P.M.; Bonn, A.E. The European earwig. USDA Tech. Bull. 1941, 766, 76. [Google Scholar]
- Orpet, R.J.; Crowder, D.W.; Jones, V.P. Biology and Management of European Earwig in Orchards and Vineyards. J. Integr. Pest Manag. 2019, 10, 21. [Google Scholar] [CrossRef]
- Orpet, R.J.; Goldberger, J.R.; Crowder, D.W.; Jones, V.P. Field evidence and grower perceptions on the roles of an omnivore, European earwig, in apple orchards. Biol. Control 2019, 132, 189–198. [Google Scholar] [CrossRef]
- Ravensberg, W.J. The natural enemies of the woolly apple aphid, Eriosoma lanigerum (Hausm.) (Homoptera: Aphididae), and their susceptibility to diflubenzuron. Meded. Fac. Landbouwwet. Univ. Gent. 1981, 46, 437–442. [Google Scholar]
- Carroll, D.P.; Hoyt, S.C. Augmentation of European Earwigs (Dermaptera: Forficulidae) for Biological Control of Apple Aphid (Homoptera: Aphididae) in an Apple Orchard. J. Econ. Entomol. 1984, 77, 738–740. [Google Scholar] [CrossRef]
- Wearing, C.H.; Attfield, B.A.; Colhoun, K. Biological control of woolly apple aphid, Eriosoma lanigerum (Hausmann), during transition to integrated fruit production for pipfruit in Central Otago, New Zealand. N. Z. J. Crop Hortic. 2010, 38, 255–273. [Google Scholar] [CrossRef]
- Beers, E.H.; Horton, D.R.; Miliczky, E. Pesticides used against Cydia pomonella disrupt biological control of secondary pests of apple. Biol. Control 2016, 102, 35–43. [Google Scholar] [CrossRef]
- Carroll, D.P.; Walker, J.T.S.; Hoyt, S.C. European Earwigs (Dermaptera: Forficulidae) Fail to Control Apple Aphids on Bearing Apple Trees and Woolly Apple Aphids (Homoptera: Aphididae) in Apple Rootstock Stool Beds. J. Econ. Entomol. 1985, 78, 972–974. [Google Scholar] [CrossRef]
- Dib, H.; Jamon, M.; Sauphanor, B.; Capowiez, Y. Individual and combined effects of the generalist Forficula auricularia and the specialist Episyrphus balteatus on Dysaphis plantaginea—Are two predators better than one? Entomol. Exp. Appl. 2016, 161, 1–10. [Google Scholar] [CrossRef]
- Lenfant, C.; Lyoussoufi, A.; Faivre d’Arcier, C.; Sauphanor, B. Potentialités prédatrices de Forficula auricularia sur le psylle du poirier Cacopsylla pyri. Entomol. Exp. Appl. 1994, 73, 51–60. [Google Scholar] [CrossRef]
- Höhn, H.; Lahusen, A.; Eder, R.; Ackermann, T.; Franck, L.; Höpli, H.U.; Samietz, J. Résultats et observations de 2002 à 2006 en Suisse alémanique. Revue suisse Vitic. Arboric. Hortic. 2007, 39, 169–176. [Google Scholar]
- DuPont, S.T.; Strohm, C.; Korgan, C.; Hilton, R.; Nottingham, L.; Orpet, R. Pear psylla and natural enemy thresholds for successful integrated pest management in pears. J. Econ. Entomol. 2023, 116, 1249–1260. [Google Scholar] [CrossRef]
- Unruh, T.R.; Miliczky, E.R.; Horton, D.R.; Thomsen–Archer, K.; Rehfield–Ray, L.; Jones, V.P. Gut content analysis of arthropod predators of codling moth in Washington apple orchards. Biol. Control 2016, 102, 85–92. [Google Scholar] [CrossRef]
- Cross, J.; Fountain, M.; Markó, V.; Nagy, C. Arthropod ecosystem services in apple orchards and their economic benefits. Ecol. Entomol. 2015, 40, 82–96. [Google Scholar] [CrossRef]
- Beliën, T.; Moerkens, R.; Leirs, H.; Peusens, G.; Bylemans, D. ‘Earwig management tool’: Transferring knowledge of population dynamics and side effects on earwigs (Forficula auricularia L.) into practical sustainable plant protection strategies in pip fruit growing. IOBC–WPRS Bulletin 2013, 91, 411–418. [Google Scholar]
- Beers, E.; Mills, N.J.; Shearer, P.W.; Horton, D.R.; Milickzy, E.R.; Amarasekare, K.G.; Gontijo, L.M. Nontarget effects of orchard pesticides on natural enemies: Lessons from the field and laboratory. Biol. Control 2016, 102, 44–52. [Google Scholar] [CrossRef]
- Lamb, R.J. Effects of dispersion, travel, and environmental heterogeneity on populations of the earwig Forficula auricularia L. Can. J. Zool. 1975, 53, 1855–1867. [Google Scholar] [CrossRef]
- Phillips, M.L. The Ecology of the Common Earwig Forficula auricularia in Apple Orchards. Ph.D. Dissertation, University of Bristol, Bristol, UK, 1981. [Google Scholar]
- Helsen, H.; Vaal, F.; Blommers, L. Phenology of the common earwig Forficula auricularia L. (Dermaptera: Forficulidae) in an apple orchard. Int. J. Pest Manag. 1998, 44, 75–79. [Google Scholar] [CrossRef]
- Walker, K.A.; Jones, T.H.; Fell, R.D. Pheromonal basis of aggregation in European earwig, Forficula auricularia L. (Dermaptera: Forficulidae). J. Chem. Ecol. 1993, 19, 2029–2038. [Google Scholar] [CrossRef]
- Quarrell, S.R.; Davies, N.W.; Walker, P.W.; Corkrey, R.; Smith, J.A.; Allen, G.R. Identification of the putative aggregation pheromone components emitted by the European earwig, Forficula auricularia. Chemoecology 2016, 26, 173–186. [Google Scholar] [CrossRef]
- Sauphanor, B.; Lenfant, C.; Sureau, F. Effets d’un extrait de neem (Azadirachta indica A. Juss) sur le développement de Forficula auricularia L. (Dermaptera). J. Appl. Entomol. 1995, 119, 215–219. [Google Scholar] [CrossRef]
- Quarrell, S.R.; Corkrey, R.; Allen, G.R. Cherry damage and the spatial distribution of European earwigs, (Forficula auricularia L.) in sweet cherry trees. Pest. Manag. Sci. 2020, 77, 159–167. [Google Scholar] [CrossRef]
- Santini, L.; Caroli, L. Damage to fruit crops by European earwig (Forficula auricularia L.). In. Fitopatol. 1992, 42, 35–38. [Google Scholar]
- Jones, V.P.; Steffan, S.A.; Wiman, N.G.; Horton, D.R.; Miliczky, E.; Zhang, Q.H.; Baker, C.C. Evaluation of herbivore–induced plant volatiles for monitoring green lacewings in Washington apple orchards. Biol. Control 2011, 56, 98–105. [Google Scholar] [CrossRef]
- Braasch, J.; Kaplan, I. Over what distance are plant volatiles bioactive? Estimating the spatial dimensions of attraction in an arthropod assemblage. Entomol. Exp. Appl. 2012, 145, 115–123. [Google Scholar] [CrossRef]
- Moerkens, R.; Leirs, H.; Peusens, G.; Gobin, B. Dispersal of single– and double–brood populations of the European earwig, Forficula auricularia: A mark–recapture experiment. Entomol. Exp. Appl. 2010, 137, 19–27. [Google Scholar] [CrossRef]
- Markó, V.; Jenser, G.; Kondorosy, E.; Ábrahám, L.; Balázs, K. Flowers for better pest control? The effects of apple orchard ground cover management on green apple aphids (Aphis spp.) (Hemiptera: Aphididae), their predators and the canopy insect community. Biocontrol Sci. Technol. 2013, 23, 126–145. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R–project.org/ (accessed on 26 September 2022).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi–Level/Mixed) Regression Models; Version 0.4.3; R Package: Madison, WI, USA, 2022. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility among Packages for Zero–Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Cranshaw, W.S. European Earwigs. Available online: https://extension.colostate.edu/topic-areas/insects/european-earwigs-5-533/ (accessed on 10 October 2023).
- Alston, D.G.; Tebeau, A. European Earwig (Forficula auricularia); UTAH Pests Fact Sheets ENT-145-11; Utah State University Extension and Utah Plant Pest Diagnostic Laboratory: Logan, UT, USA, 2011; pp. 1–3. [Google Scholar]
- Saladini, M.A.; Asteggiano, L.; Pansa, M.G.; Giodani, L.; Serre, L.; Vittone, G.; Tavella, L.; Tedeschi, R. Glue barriers reduce earwig damage on apricots in north–western Italy. Int. J. Pest Manag. 2016, 62, 214–221. [Google Scholar] [CrossRef]
- Nicholas, A.H.; Spooner–Hart, R.N.; Vickers, R.A. Abundance and natural control of the woolly aphid Eriosoma lanigerum in an Australian apple orchard IPM program. BioControl 2005, 50, 271–291. [Google Scholar] [CrossRef]
- Solomon, M.G.; Cross, J.V.; Fitzgerald, J.D.; Campbell, C.A.M.; Jolly, R.L.; Olszak, R.W.; Niemczyk, E.; Vogt, H. Biocontrol of Pests of Apples and Pears in Northern and Central Europe–3. Predators. Biocontrol Sci. Technol. 2000, 10, 91–128. [Google Scholar] [CrossRef]
- Chang, G.C.; Kareiva, P. The case for indigenous generalists in biological control. In Theoretical Approaches to Biological Control; Hawkins, B.A., Cornell, H.V., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 103–115. [Google Scholar]
- Piñol, J.; Espadaler, X.; Pérez, N.; Beven, K. Testing a new model of aphid abundance with sedentary and non–sedentary predators. Ecol. Model. 2009, 220, 2469–2480. [Google Scholar] [CrossRef]
- Mueller, T.F.; Blommers, L.H.M.; Mols, P.J.M. Earwig (Forficula auricularia) predation on the woolly apple aphid, Eriosoma lanigerum. Entomol. Exp. Appl. 1988, 47, 145–152. [Google Scholar] [CrossRef]
- Mols, P.J.M. Do natural enemies control woolly apple aphid? IOBC/WPRS Bull. 1996, 19, 203–207. [Google Scholar] [CrossRef]
- Quarrell, S.R.; Corkrey, R.; Allen, G.R. Predictive thresholds for forecasting the compatibility of Forficula auricularia and Aphelinus mali as biological control agents against woolly apple aphid in apple orchards. BioControl 2017, 62, 243–256. [Google Scholar] [CrossRef]
- Dib, H.; Simon, S.; Sauphanor, B.; Capowiez, Y. The role of natural enemies on the population dynamics of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in organic apple orchards in south–eastern France. Biol. Control 2010, 55, 97–109. [Google Scholar] [CrossRef]
- Miñarro, M.; Fernández-Mata, G.; Medina, P. Role of ants in structuring the aphid community on apple. Ecol. Entomol. 2010, 35, 206–215. [Google Scholar] [CrossRef]
- Moerkens, R.; Gobin, B.; Peusens, G.; Helsen, H.; Hilton, R.; Dib, H.; Suckling, D.M.; Leirs, H. Optimizing biocontrol using phenological day degree models: The European earwig in pipfruit orchards. Agric. For. Entomol. 2011, 13, 301–312. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Sabelis, M.W.; Janssen, A. Biological control of aphids in the presence of thrips and their enemies. BioControl 2013, 58, 45–55. [Google Scholar] [CrossRef]
- Silva, D.B.; Hanel, A.; Franco, F.P.; Silva-Filho, M.C.; Bento, J.M.S. Two in one: The neotropical mirid predator Macrolophus basicornis increases pest control by feeding on plants. Pest Manag. Sci. 2022, 78, 3314–3323. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.P. Predatory bugs. In Orchard Pest Management: A Resource Book for the Pacific Northwest; Beers, E.H., Brunner, J.F., Willett, M.J., Warner, G.M., Eds.; Good Fruit Grower: Yakima, WA, USA, 1993; pp. 218–222. [Google Scholar]
| Experiment | Site Location | Crop | Cultivar | Management Type | Row Spacing (m) | Tree Spacing (m) |
|---|---|---|---|---|---|---|
| Mass trapping | Yakima Co., WA, USA | Apricot | Unknown | Conventional | 3 | 1.5 |
| Mass trapping | Benton Co., WA, USA | Cherry | Chelan | Organic | 4 | 2 |
| Augmentation | Yakima Co., WA, USA | Pear | Bartlett | Organic | 6 | 6 |
| Augmentation | Jackson Co., OR, USA | Pear | Comice | Conventional | 4.5 | 2.3 |
| Augmentation | Yakima Co., WA, USA | Apple | Pink Lady | Organic | 3 | 0.5 |
| First Date of Earwig Release | ||
|---|---|---|
| Orchard | 2021 | 2022 |
| Apple—WA | 21 June | 28 June |
| Pear—WA | 17 June | 21 June |
| Pear—OR | 7 June | 8 June |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanel, A.; Orpet, R.J.; Hilton, R.; Nottingham, L.; Northfield, T.D.; Schmidt-Jeffris, R. Turning a Pest into a Natural Enemy: Removing Earwigs from Stone Fruit and Releasing Them in Pome Fruit Enhances Pest Control. Insects 2023, 14, 906. https://doi.org/10.3390/insects14120906
Hanel A, Orpet RJ, Hilton R, Nottingham L, Northfield TD, Schmidt-Jeffris R. Turning a Pest into a Natural Enemy: Removing Earwigs from Stone Fruit and Releasing Them in Pome Fruit Enhances Pest Control. Insects. 2023; 14(12):906. https://doi.org/10.3390/insects14120906
Chicago/Turabian StyleHanel, Aldo, Robert J. Orpet, Richard Hilton, Louis Nottingham, Tobin D. Northfield, and Rebecca Schmidt-Jeffris. 2023. "Turning a Pest into a Natural Enemy: Removing Earwigs from Stone Fruit and Releasing Them in Pome Fruit Enhances Pest Control" Insects 14, no. 12: 906. https://doi.org/10.3390/insects14120906
APA StyleHanel, A., Orpet, R. J., Hilton, R., Nottingham, L., Northfield, T. D., & Schmidt-Jeffris, R. (2023). Turning a Pest into a Natural Enemy: Removing Earwigs from Stone Fruit and Releasing Them in Pome Fruit Enhances Pest Control. Insects, 14(12), 906. https://doi.org/10.3390/insects14120906







