A Thermophile-Fermented Compost Modulates Intestinal Cations and the Expression of a Juvenile Hormone-Binding Protein Gene in the Female Larvae of Hercules Beetle Dynastes hercules (Coleoptera: Scarabaeidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Rearing Conditions
2.2. Preparation of the Feces, Gut, and Fat Body
2.3. Determination of pH and Cation and Origanic Acid Concentrations
2.4. RNA Extraction and Transcriptomic Analysis
3. Results
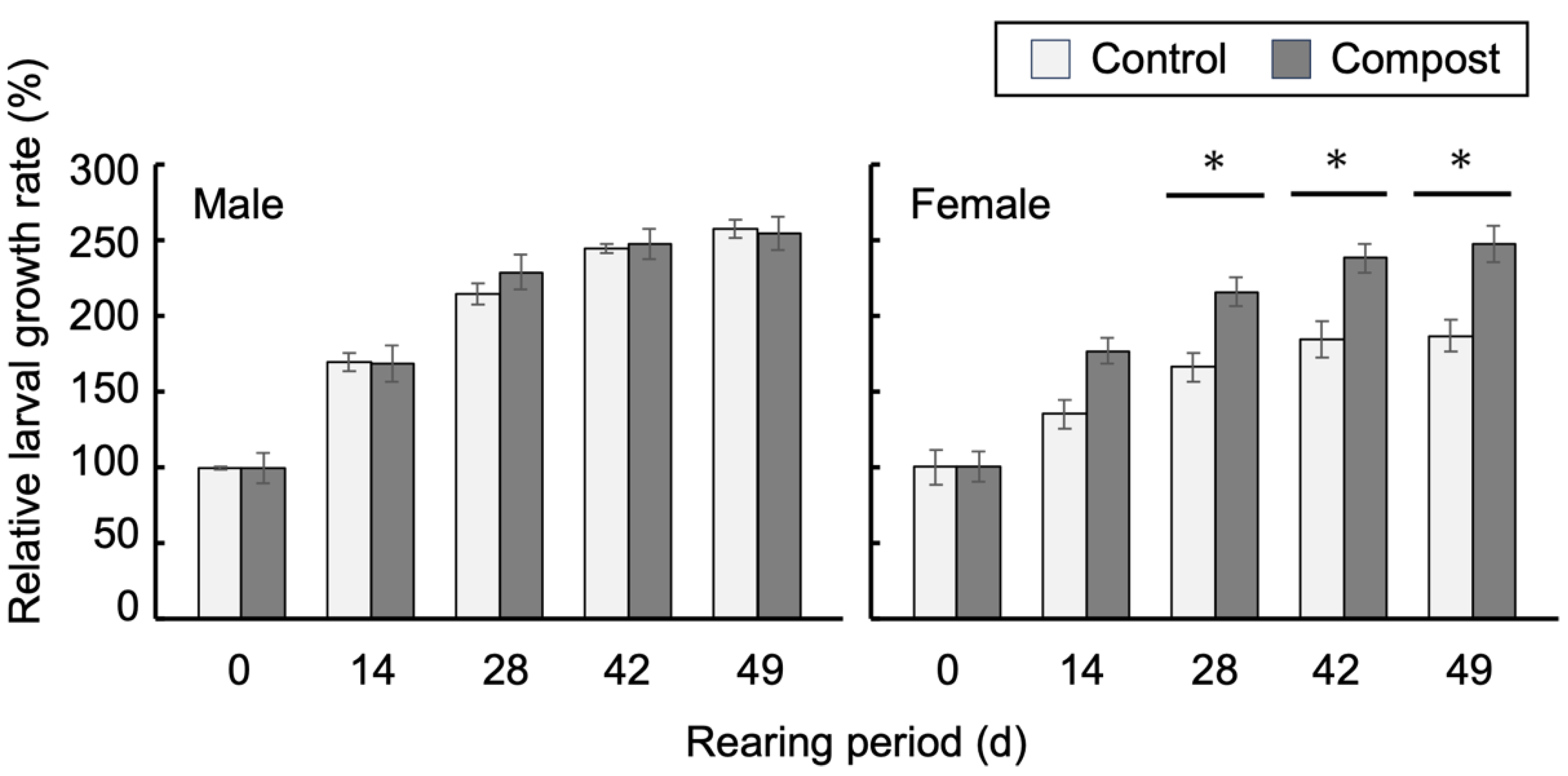
3.1. Improved Growth of Female Larvae by Compost
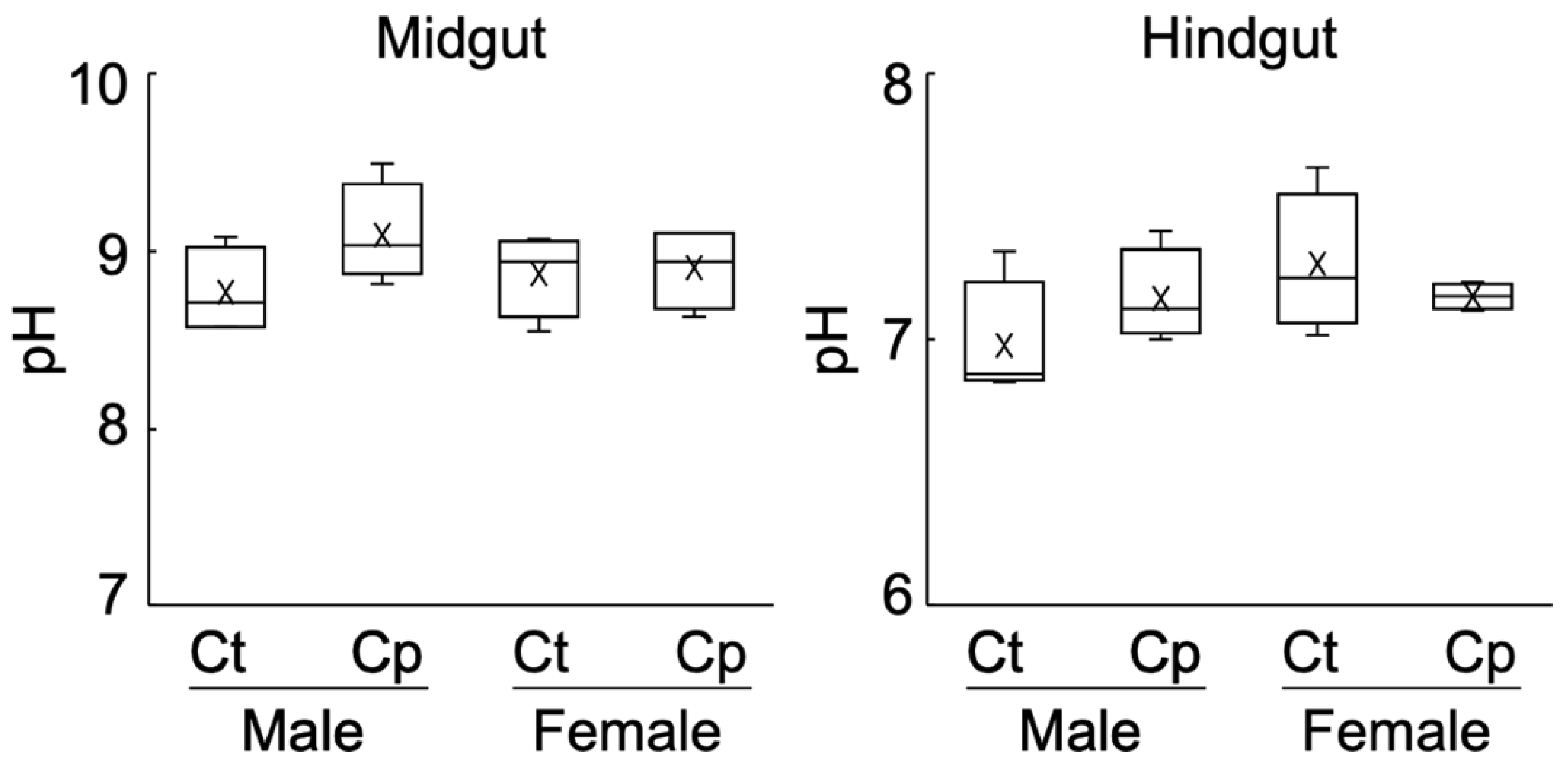
3.2. Alkalinity of the Midgut and Hindgut Contents
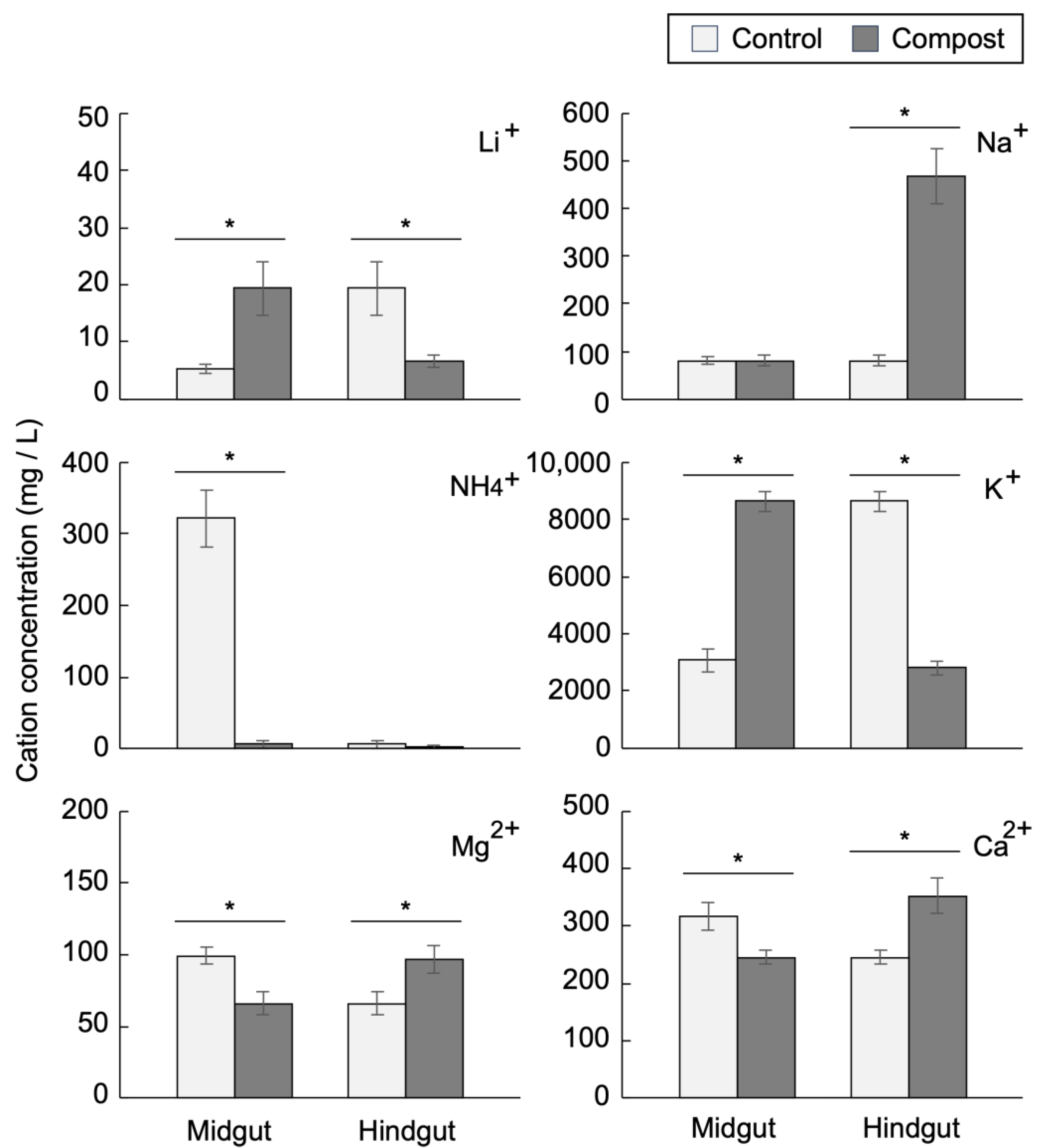
3.3. Midgut and Hindgut Cation Concentrations
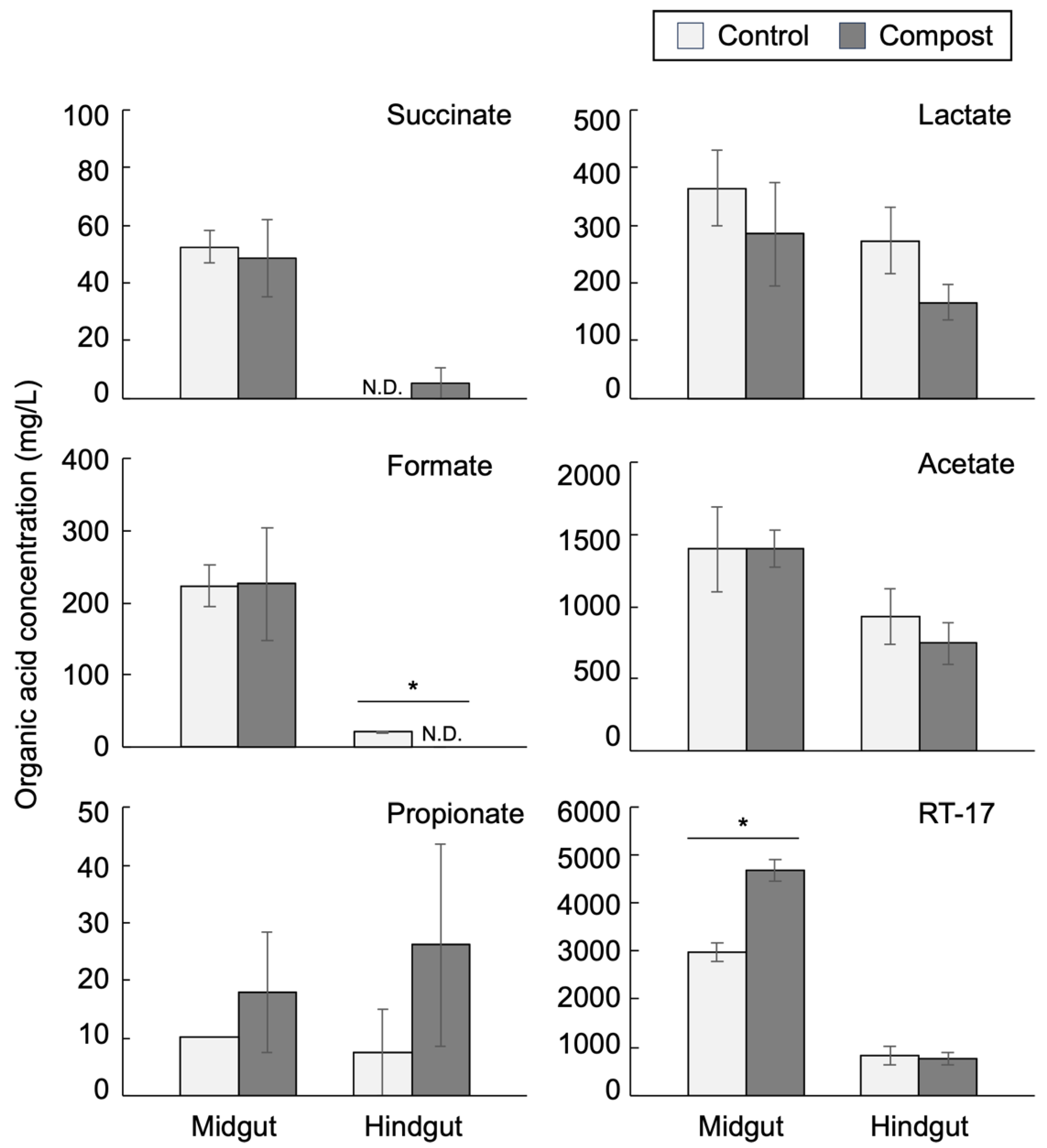
3.4. Intestinal Organic Acids
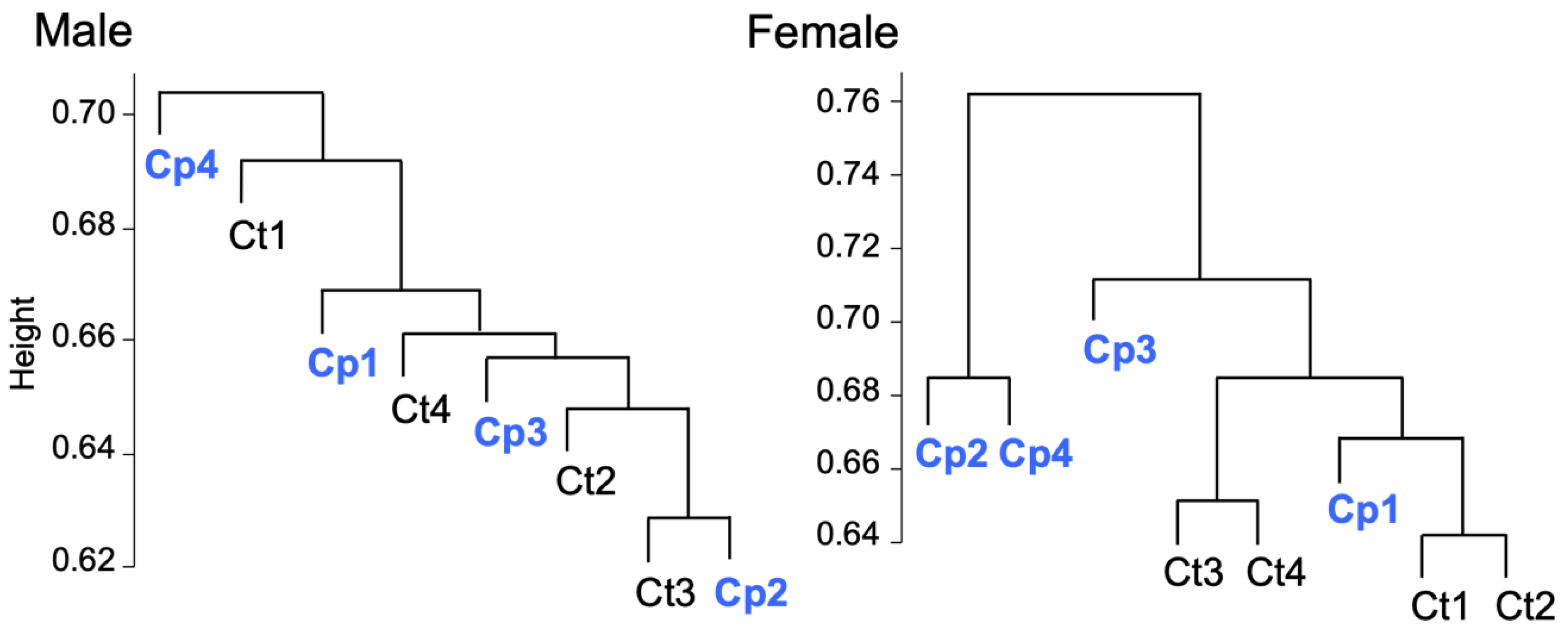
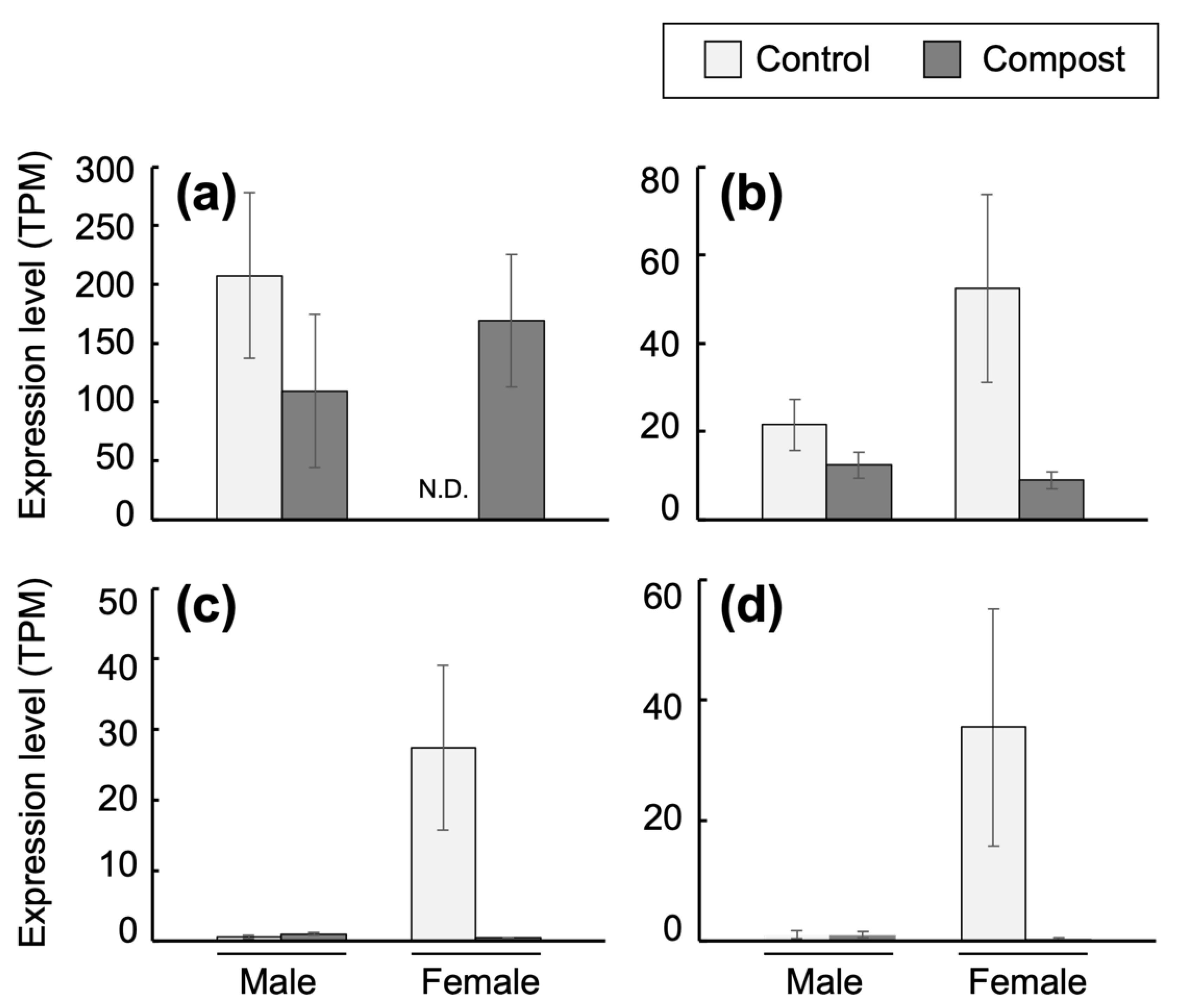
3.5. Transcriptomic Analysis of Fat Body
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karino, K.; Seki, N.; Chiba, M. Larval nutritional environment determines adult size in Japanese horned beetles Allomyrina dichotoma. Ecol. Res. 2004, 19, 663–668. [Google Scholar] [CrossRef]
- Chafino, S.; Ureña, E.; Casanova, J.; Casacuberta, E.; Franch-Marro, X.; Martín, D. Upregulation of E93 gene expression acts as the trigger for metamorphosis independently of the threshold size in the beetle Tribolium castaneum. Cell Rep. 2019, 27, 1039–1049.e2. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Asano, F.; Ishizawa, K.; Suda, W.; Miyamoto, H.; Tsuji, N.; Matsuura, M.; Tsuboi, A.; Ishii, C.; Nakaguma, T.; et al. A potential network structure of symbiotic bacteria involved in carbon and nitrogen metabolism of wood-utilizing insect larvae. Sci. Total Environ. 2022, 836, 155520. [Google Scholar] [CrossRef] [PubMed]
- Asano, F.; Tsuboi, A.; Moriya, S.; Kato, T.; Tsuji, N.; Nakaguma, T.; Ohno, H.; Miyamoto, H.; Kodama, H. Amendment of a thermophile-fermented compost to humus improves the growth of female larvae of the Hercules beetle Dynastes hercules (Coleoptera: Scarabaeidae). J. Appl. Microbiol. 2023, 134, xac006. [Google Scholar] [CrossRef] [PubMed]
- Bayon, C. Volatile fatty acids and methane production in relation to anaerobic carbohydrate fermentation in Oryctes nasicornis larvae (Coleoptera: Scarabaeidae). J. Insect Physiol. 1980, 26, 819–828. [Google Scholar] [CrossRef]
- Andert, J.; Geissinger, O.; Brune, A. Peptidic soil components are a major dietary resource for the humivorous larvae of Pachnoda spp. (Coleoptera: Scarabaeidae). J. Insect Physiol. 2008, 54, 105–113. [Google Scholar] [CrossRef]
- Hobbie, S.N.; Li, X.; Basen, M.; Stingl, U.; Brune, A. Humic substance-mediated Fe(III) reduction by a fermenting Bacillus strain from the alkaline gut of a humus-feeding scarab beetle larva. Syst. Appl. Microbiol. 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zborowska, M.; Waliszewska, H.; Waliszewska, B.; Borysiak, S.; Brozdowski, J.; Stachowiak-Wencek, A. Conversion of carbohydrates in lignocellulosic biomass after chemical pretreatment. Energies 2022, 15, 254. [Google Scholar] [CrossRef]
- Grayson, J.M.D. Digestive tract pH of six species of Coleoptera. Ann. Entomol. Soc. Am. 1958, 51, 403–405. [Google Scholar] [CrossRef]
- Dow, J.A. pH gradients in lepidopteran midgut. J. Exp. Biol. 1992, 172 Pt 1, 355–375. [Google Scholar] [CrossRef]
- Lemke, T.; Stingl, U.; Egert, M.; Friedrich, M.W.; Brune, A. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.G.; Logroño, W.; Rocha, U.N.d.; Harms, H.; Nikolausz, M. Enrichment of anaerobic microbial communities from midgut and hindgut of sun beetle larvae (Pachnoda marginata) on wheat straw: Effect of inoculum preparation. Microorganisms 2022, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Odelson, D.A.; Breznak, J.A. Volatile fatty acid production by the hindgut microbiota of xylophagous termites. Appl. Environ. Microbiol. 1983, 45, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Kayukawa, T.; Nagamine, K.; Ito, Y.; Nishita, Y.; Ishikawa, Y.; Shinoda, T. Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of broad-complex, a pupal specifier gene. J. Biol. Chem. 2016, 291, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Mirth, C.K.; Tang, H.Y.; Makohon-Moore, S.C.; Salhadar, S.; Gokhale, R.H.; Warner, R.D.; Koyama, T.; Riddiford, L.M.; Shingleton, A.W. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 7018–7023. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 2004, 6, 49–62. [Google Scholar]
- Gotoh, H.; Cornette, R.; Koshikawa, S.; Okada, Y.; Lavine, L.C.; Emlen, D.J.; Miura, T. Juvenile hormone regulates extreme mandible growth in male stag beetles. PLoS ONE 2011, 6, e21139. [Google Scholar] [CrossRef] [PubMed]
- Vea, I.M.; Tanaka, S.; Shiotsuki, T.; Jouraku, A.; Tanaka, T.; Minakuchi, C. Differential juvenile hormone variations in scale insect extreme sexual dimorphism. PLoS ONE 2016, 11, e0149459. [Google Scholar] [CrossRef]
- Niisawa, C.; Oka, S.; Kodama, H.; Hirai, M.; Kumagai, Y.; Mori, K.; Matsumoto, J.; Miyamoto, H.; Miyamoto, H. Microbial analysis of a composted product of marine animal resources and isolation of bacteria antagonistic to a plant pathogen from the compost. J. Gen. Appl. Microbiol. 2008, 54, 149–158. [Google Scholar] [CrossRef]
- Ito, T.; Miyamoto, H.; Kumagai, Y.; Udagawa, M.; Shinmyo, T.; Mori, K.; Ogawa, K.; Miyamoto, H.; Kodama, H. Thermophile-fermented compost extract as a possible feed additive to enhance fecundity in the laying hen and pig: Modulation of gut metabolism. J. Biosci. Bioeng. 2016, 121, 659–664. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ohmori, T.; Miyamoto, H.; Ito, T.; Kumagai, Y.; Sonodam, M.; Matsumoto, J.; Miyamoto, H.; Kodama, H. Denitrification in soil amended with thermophile-fermented compost suppresses nitrate accumulation in plants. Appl. Microbiol. Biotechnol. 2013, 97, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Iwabuchi, K. Relevant principal factors affecting the reproducibility of insect primary culture. In Vitro Cell Dev. Biol. Anim. 2017, 53, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.; Saido-Sakanaka, H.; Yang, J.; Sagisaka, A.; Yamakawa, M. Purification, cDNA cloning and modification of a defensin from the coconut rhinoceros beetle, Oryctes rhinoceros. Eur. J. Biochem. 1999, 266, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, J.; Kim, Y.J.; Bang, H.S.; Yun, E.Y.; Kim, S.R.; Suh, H.J.; Kang, B.R.; Nam, S.H.; Jeon, J.P.; et al. Isolation and characterization of a defensin-like peptide (coprisin) from the dung beetle, Copris tripartitus. Int. J. Pept. 2009, 2009, 136284. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Sanburg, L.L.; Kézdy, F.J.; Law, J.H. The juvenile hormone binding protein in the hemolymph of Manduca sexta Johannson (Lepidoptera: Sphingidae). Proc. Natl. Acad. Sci. USA 1974, 71, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Ritdachyeng, E.; Manaboon, M.; Tobe, S.S.; Singtripop, T. Molecular characterization and gene expression of juvenile hormone binding protein in the bamboo borer, Omphisa fuscidentalis. J. Insect Physiol. 2012, 58, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. Peritrophic Membranes; Springer: Berlin/Heidelberg, Germany, 1992; Volume 30, pp. 482–483. [Google Scholar]
- Zhao, D.; Liu, B.; Zhang, Y.K.; Guo, W.; Li, S.Y.; Lu, X.J.; Li, R.J. Structural and biochemical characteristics of chitin-binding protein SeCBP66 from Spodoptera exigua (Hübner). Genet. Mol. Res. 2016, 15, gmr.15038789. [Google Scholar] [CrossRef]
- Rodríguez-de la Noval, C.; Rodríguez-Cabrera, L.; Izquierdo, L.; Espinosa, L.A.; Hernandez, D.; Ponce, M.; Moran-Bertot, I.; Tellez-Rodríguez, P.; Borras-Hidalgo, O.; Huang, S.; et al. Functional expression of a peritrophin A-like SfPER protein is required for larval development in Spodoptera frugiperda (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 2630. [Google Scholar] [CrossRef]
- Han, G.; Li, X.; Zhang, T.; Zhu, X.; Li, J. Cloning and tissue-specific expression of a chitin deacetylase gene from Helicoverpa armigera (Lepidoptera: Noctuidae) and its response to Bacillus thuringiensis. J. Insect Sci. 2015, 15, 95. [Google Scholar] [CrossRef]
- Pacheco, C.A.; Alevi, K.C.C.; Ravazi, A.; de Azeredo Oliveria, M.T.V. Review: Malpighian tubule, an essential organ for insects. Entomol. Ornithol. Herpetol. 2014, 3, 1000122. [Google Scholar] [CrossRef]
- Silva, J.R.; Amaral, D.T.; Viviani, V.R. Comparison of the Malpighian tubules and fat body transcriptional profiles of Zophobas morio larvae (Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Khan, A.; O’Donnell, M.J.; Kolosov, D. Novel mechanisms of epithelial ion transport: Insights from the cryptonephridial system of lepidopteran larvae. Curr. Opin. Insect Sci. 2021, 47, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Blaesse, A.K.; Broehan, G.; Meyer, H.; Merzendorfer, H.; Weihrauch, D. Ammonia uptake in Manduca sexta midgut is mediated by an amiloride sensitive cation/proton exchanger: Transport studies and mRNA expression analysis of NHE7, 9, NHE8, and V-ATPase (subunit D). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Egert, M.; Stingl, U.; Bruun, L.D.; Pommerenke, B.; Brune, A.; Friedrich, M.W. Structure and topology of microbial communities in the major gut compartments of Melolontha melolontha larvae (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2005, 71, 4556–4566. [Google Scholar] [CrossRef]
- Yin, J.; Yang, S.; Li, K.; Guo, W.; Cao, Y. Identification and molecular characterization of a chitin-binding protein from the beet webworm, Loxostege sticticalis L. Int. J. Mol. Sci. 2014, 15, 19147–19161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, G.; Ma, L.; Jiang, T.; Xiao, H. Dissecting the role of juvenile hormone binding protein in response to hormone and starvation in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 1411–1417. [Google Scholar] [CrossRef]
- Hidayat, P.; Goodman, W.G. Juvenile hormone and hemolymph juvenile hormone binding protein titers and their interaction in the hemolymph of fourth stadium Manduca sexta. Insect Biochem. Mol. Biol. 1994, 24, 709–715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, F.; Miyahara, T.; Miyamoto, H.; Kodama, H. A Thermophile-Fermented Compost Modulates Intestinal Cations and the Expression of a Juvenile Hormone-Binding Protein Gene in the Female Larvae of Hercules Beetle Dynastes hercules (Coleoptera: Scarabaeidae). Insects 2023, 14, 910. https://doi.org/10.3390/insects14120910
Asano F, Miyahara T, Miyamoto H, Kodama H. A Thermophile-Fermented Compost Modulates Intestinal Cations and the Expression of a Juvenile Hormone-Binding Protein Gene in the Female Larvae of Hercules Beetle Dynastes hercules (Coleoptera: Scarabaeidae). Insects. 2023; 14(12):910. https://doi.org/10.3390/insects14120910
Chicago/Turabian StyleAsano, Futo, Taira Miyahara, Hirokuni Miyamoto, and Hiroaki Kodama. 2023. "A Thermophile-Fermented Compost Modulates Intestinal Cations and the Expression of a Juvenile Hormone-Binding Protein Gene in the Female Larvae of Hercules Beetle Dynastes hercules (Coleoptera: Scarabaeidae)" Insects 14, no. 12: 910. https://doi.org/10.3390/insects14120910





