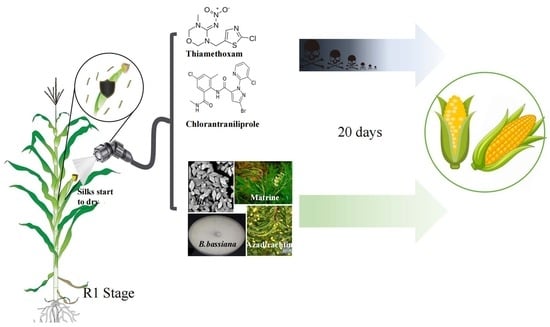
The Efficiency of Pest Control Options against Two Major Sweet Corn Ear Pests in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Pests Population Monitoring
2.2. Insecticide Test
2.3. Insecticide Residue Analysis
2.4. Statistical Analysis
2.5. Meteorological Data
3. Results
3.1. Dynamic Occurrence of Predominant Pests in Ear Stage of Sweet Corn
3.2. Insecticide Application Time Exerted a Significant Influence on Control Efficiency
3.3. Screening of High-Efficiency Synthetic Insecticides and Safety Check
3.4. Screening of High-Efficiency Biopesticide
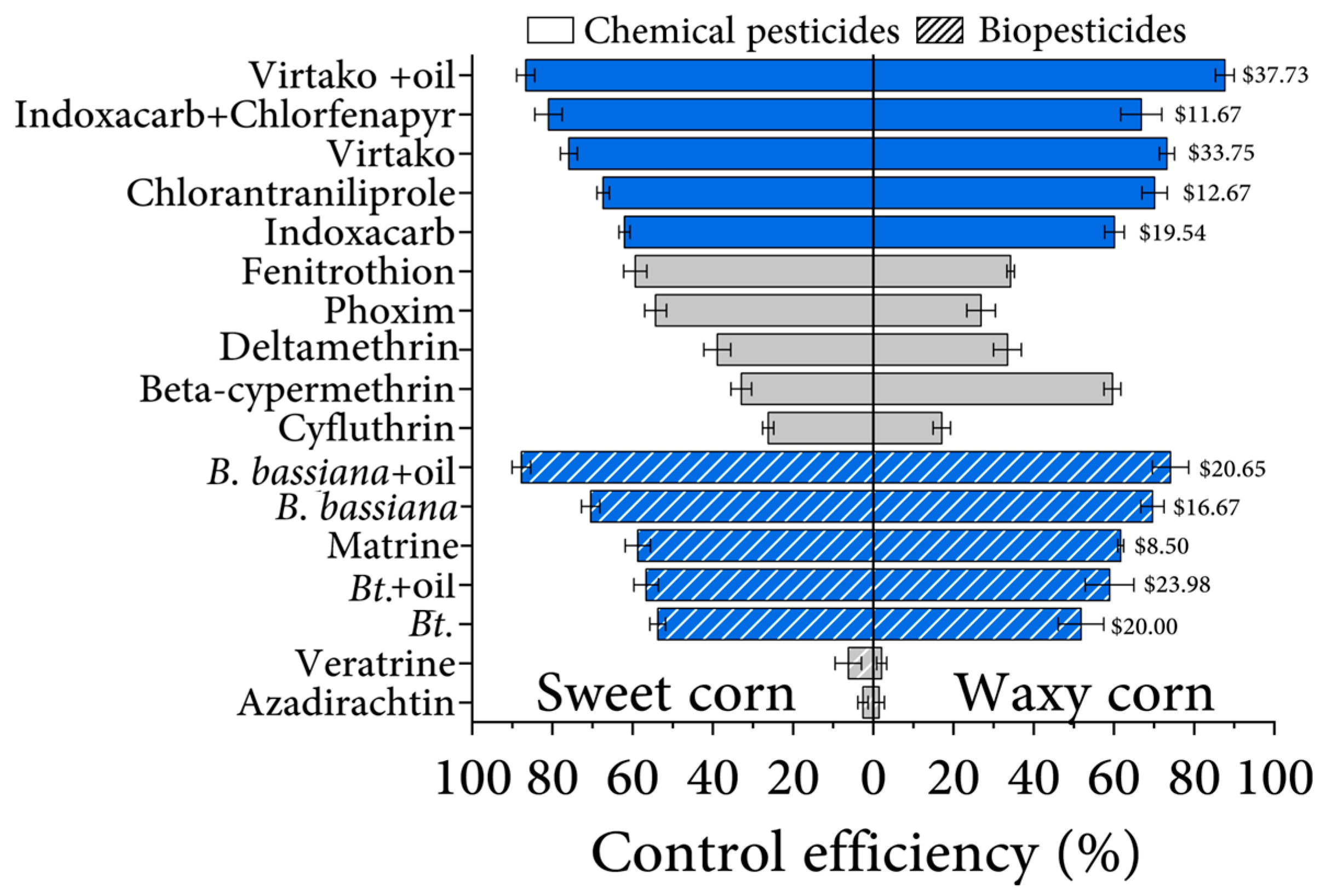
3.5. Efficiency and Benefits Comparison of Different Insecticides among Corn Varieties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Central People’s Government of the People’s Republic of China. National Grain and Oil Information Center: China’s Corn Yield Is Expected to Be 216 Million Tons This Year, a Slight Increase Year-on-Year. Available online: http://www.gov.cn/xinwen/2018-09/13/content_5321671.htm (accessed on 11 November 2022).
- Central People’s Government of the People’s Republic of China. Corn Production in Liaoning Is Expected to 29 Billion Jin and the Trading Is Stable. Available online: http://www.gov.cn/xinwen/2017-12/11/content_5245903.htm (accessed on 25 August 2022).
- Li, X.; Liu, Y.; Fu, J.; Ban, L. Current State of Green Control Technology of Asian Corn Borer Ostrinia furnacalls in the Production of Fresh Corn. Acta Agretia Sin. 2022, 30, 825–834. [Google Scholar]
- Jie, L.; Juan, Z.; Yuying, J. Occurring characteristics and related factors of major maize diseases and insect pests in China in 2018. China Plant Prot. 2019, 39, 43–49. [Google Scholar]
- Sparks, N.A.; Kemp, K.E. Efficacy and economics of different spray techniques for controlling corn earworm (Lepidoptera: Noctuidae) in sweet corn. J. Econ. Entomol. 1995, 88, 1033–1037. [Google Scholar] [CrossRef]
- Baio, F.H.R.; Antuniassi, U.R.; Castilho, B.R.; Teodoro, P.E.; da Silva, E.E. Factors affecting aerial spray drift in the Brazilian Cerrado. PLoS ONE 2019, 14, e0217957. [Google Scholar] [CrossRef] [PubMed]
- French, B.W.; Beckler, A.A.; Chandler, L.D. Landscape features and spatial distribution of adult northern corn rootworm (Coleoptera: Chrysomelidae) in the south Dakota Areawide management site. J. Econ. Entomol. 2004, 97, 1943–1957. [Google Scholar] [CrossRef] [PubMed]
- Carlos, C.; Goncalves, F.; Oliveira, I.; Torres, L. Is a biofix necessary for predicting the flight phenology of Lobesia botrana in Douro Demarcated Region vineyards? Crop Prot. 2018, 110, 57–64. [Google Scholar] [CrossRef]
- Yoo, B.H.; Kim, K.S.; Park, J.Y.; Moon, K.H.; Ahn, J.J.; Fleisher, D.H. Spatial portability of random forest models to estimate site-specific air temperature for prediction of emergence dates of the Asian Corn Borer in North Korea. Comput. Electron. Agric. 2022, 199, 23–35. [Google Scholar] [CrossRef]
- Zhifeng, H.; Chengping, L.; Tongshu, Z.; Zhe, Y. Occurrence Quantity Forecast Model Research of Maize Borer and Trichogramma in Shenyang District. In Proceedings of the 11th National Member Congress and 2013 Annual Conference of Chinese Plant Protection Society, Qingdao, China, 15 June 2014; p. 451. [Google Scholar]
- Guo, X.J.; Yu, Q.Q.; Chen, D.F.; Wei, J.N.; Yang, P.C.; Yu, J.; Wang, X.H.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Jacobson, M. Corn Silk Volatiles Attract Many Pest Species of Moths. J. Environ. Sci. Health A 1979, 14, 695–707. [Google Scholar] [CrossRef]
- Roelofs, W.L. Chemistry of Sex Attraction. Proc. Natl. Acad. Sci. USA 1995, 92, 44–49. [Google Scholar] [CrossRef]
- Shuxin, W.; YaHui, G.; YongShuo, F.; YuMei, L. Phenological synchrony between summer maize and the Asian corn borer. Sci. Sin. 2021, 51, 462–471. [Google Scholar]
- Chowanski, S.; Adamski, Z.; Marciniak, P.; Rosinski, G.; Buyukguzel, E.; Buyukguzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Masry, S.H.D.; El-Wakeil, N. Egg Parasitoid Production and Their Role in Controlling Insect Pests; Springer: Cham, Switzerland, 2020; pp. 3–47. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, S.; Wang, G.; Fu, J.; Lan, Y. Biological control technology and application based on agricultural unmanned aerial vehicle (UAV) intelligent delivery of insect natural enemies (Trichogramma) carrier. Pest Manag. Sci. 2021, 77, 3259–3272. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sui, L.; Gao, P.; Zhang, R.; Wang, Z.; Zhang, Z.; Li, Q. Study and application of wettable powder of Beauveria bassiana to control corn borer. Chin. J. Biol. Control 2020, 36, 862–865. [Google Scholar] [CrossRef]
- Xin, L.; Darong, Z. Effects of moisture on pupation of overwintering larvae of revived Asian corn borer, Ostrinia furnicalis. J. Plant Prot. 2012, 3, 213–217. [Google Scholar] [CrossRef]
- Xin, L.; Darong, Z. Effects of humidity on overwintering larvae of corn borer, Ostrinia furnicalis after resuscated. Plant Prot. 1999, 1, 3–5. [Google Scholar]
- European Union Law Documents Exchange. Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing Directive 79/700/EEC (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002L0063 (accessed on 13 November 2022).
- European Commission. EU Legislation on MRLs. Available online: https://food.ec.europa.eu/plants/pesticides/maximum-residue-levels/eu-legislation-mrls_en (accessed on 15 November 2022).
- Jiang, X.C.; Dong, W.X.; Chen, B.; Xiao, C.; Gui, F.R.; Yan, N.S.; Qian, L.; Li, Z.Y. Electrophysiological and oviposition responses of Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae), to compounds rinsed from the surfaces of sugarcane and maize leaves. Eur. J. Entomol. 2015, 112, 295–301. [Google Scholar] [CrossRef]
- Binder, B.F.; Robbins, J.C. Effect of terpenoids and related compounds on the oviposition behavior of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Agr. Food Chem. 1997, 45, 980–984. [Google Scholar] [CrossRef]
- Xingchuan, J.; Xingwei, X.; Yuqing, S. The adult orientated behavior response of Asian corn borer, Ostrinia furnacalis to corn volatile. J. Environ. Entomol. 2018, 40, 873–879. [Google Scholar]
- Godfrey, L.D.; Holtzer, T.O. Influence of Temperature and Humidity on European Corn-Borer (Lepidoptera, Pyralidae) Egg HatchabiliAty. Env. Entomol. 1991, 20, 8–14. [Google Scholar] [CrossRef]
- Kawakita, S.; Takahashi, H. Time-series analysis of population dynamics of the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae), using an ARIMAX model. Pest Manag. Sci. 2022, 78, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Ge, X. Functional study of Pheromono Binding Proteins in Yellow Peach Month Conogethes punctiferalis (Guenee). Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. [Google Scholar]
- Ravikumar, A.; Basavaraj, K.; Kumar, V.A. Evaluation of cypermethrin 10% + Indoxacarb 10% SC against fall armyworm, Spodoptera frugiperda and stem borer in maize. J. Exp. Zool. India 2022, 25, 259–265. [Google Scholar]
- Chen, Y.; Zheng, X.; Liu, J.; Wei, H.; Chen, Y.D.; Su, X.X.; Zhang, J. Appraisal of the impact of three insecticides on the principal rice pests and their predators in China. Fla. Entomol. 2016, 99, 210–220. [Google Scholar] [CrossRef]
- Haider, I.; Akhter, M.; Sabir, A.M. Evaluation of Different Insecticides Against Rice Leaffolder Cnaphalocrocis medinalis (Guenee) Under Field Conditions. Pak. J. Zool. 2014, 46, 1458–1461. [Google Scholar]
- Ho, G.T.T.; Le, C.V.; Nguyen, T.H.; Ueno, T.; Nguyen, D.V. Incidence of Yellow Rice Stem Borer Scirpophaga incertulas Walker in Haiphong, Vietnam and Control Efficacy of Egg Mass Removal and Insecticides. J. Fac. Agric. Kyushu Univ. 2013, 58, 301–306. [Google Scholar] [CrossRef]
- Bin, F.; Jiafu, C.; Yawei, L. The control efficiency of pesticide FUGE on sugarcane stem borer at different sugarcane planting time. Guangxi Sugarcane Ind. 2020, 32, 3–5. [Google Scholar]
- Shang, D. Study on Prevention and Control Technology of Pests in Fresh Corn Ear Stage; China Agricultural University: Beijing, China, 2019. [Google Scholar]
- Wu, S.H.; Kostromytska, O.S.; Koppenhofer, A.M. Synergistic Combinations of a Pyrethroid Insecticide and an Emulsifiable Oil Formulation of Beauveria bassiana to Overcome Insecticide Resistance in Listronotus maculicollis (Coleoptera: Curculionidae). J. Econ. Entomol. 2017, 110, 1794–1802. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Na, X. Study of Insecticidal Constitutions of Veratrum nigrum L. Master’s Thesis, Northwest Agriculture & Forestry University, Xianyang, China, 2018. [Google Scholar]
- Weiguo, C.; Ruihua, D.; Jianzhong, D.; Haiyan, S. Toxicity Determination of Azadirachtin to Silkworm and Detoxification Methoud. Acta Sericologica Sin. 2008, 2, 274–279. [Google Scholar]



| Name of Pesticide | Formulation | Content of Effective Constituent | Dosage (ha) | Cost ($/ha) | |
|---|---|---|---|---|---|
| 1 | Deltamethrin | EC. | 25 g L−1 | 600 mL | 6.93 |
| 2 | Fenitrothion | EC. | 6%Fenvalerate + 14% Fenitrothion | 900 mL | 6.40 |
| 3 | Phoxim | EC. | 40% | 750 mL | 4.43 |
| 4 | Chlorantraniliprole | EC. | 200 g L−1 | 150 mL | 12.67 |
| 5 | Virtako | WDG | 40% (20% Chlorantraniliprole + 20% thiamethoxam) | 120 g | 33.75 |
| 6 | Indoxacarb + Chlorfenapyr | EC. | 10% (5%Indoxacarb + 5%Chlorfenapyr) | 600 mL | 11.67 |
| 7 | Indoxacarb | EC. | 150 g L−1 | 375 mL | 19.54 |
| 8 | Beta-cypermethrin | AR. | 4.5% | 750 mL | 3.70 |
| 9 | Cyfluthrin | AR. | 5% | 300 mL | 5.12 |
| 10 | Beauveria bassiana | Pulv | 5 × 109 spores g−1 | 6000 g | 16.67 |
| 11 | Bacillus thuringiensis (Bt) | Pulv | 50,000 IU mg−1 | 900 g | 20 |
| 12 | Azadirachtin | EC. | 3% | 2250 mL | 73.20 |
| 13 | Matrine | Water aqua | 1.3% | 600 mL | 8.50 |
| 14 | Veratrine | SC. | 0.5% | 2250 mL | 3.65 |
| 15 | Mineral oil | Additives | 99 % | 3000 mL | 3.85 |
| Treatments | Jingketian (Sweet Corn) | Jingkenuo (Waxy Corn) | |
|---|---|---|---|
| 1 | Beta-cypermethrin | <LoD | <LoD |
| 2 | Indoxacarb | 0.20 ± 0.02 | 0.01 ± 0.01 |
| 3 | Indoxacarb + Chlorfenapyr | 0.37 ± 0.04 | 0.08 ± 0.01 |
| 4 | Chlorantraniliprole | 0.39 ± 0.02 | 0.60 ± 0.05 |
| 5 | Cyfluthrin | 0.05 ± 0.01 | <LoD |
| 6 | Virtako | 0.25 ± 0.03 | 5.22 ± 0.23 |
| 7 | Deltamethrin | <LoD | 1.20 ± 0.09 |
| 8 | Fenitrothion | <LoD | <LoD |
| 9 | Phoxim | 0.04 ± 0.01 | 0.07 ± 0.02 |
| 10 | Control | <LoD | <LoD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Pei, Z.; Tong, G.; Yue, J.; Li, J.; Dai, W.; Xu, H.; Shang, D.; Ban, L. The Efficiency of Pest Control Options against Two Major Sweet Corn Ear Pests in China. Insects 2023, 14, 929. https://doi.org/10.3390/insects14120929
Li X, Liu Y, Pei Z, Tong G, Yue J, Li J, Dai W, Xu H, Shang D, Ban L. The Efficiency of Pest Control Options against Two Major Sweet Corn Ear Pests in China. Insects. 2023; 14(12):929. https://doi.org/10.3390/insects14120929
Chicago/Turabian StyleLi, Xin, Yanqi Liu, Zhichao Pei, Guoxiang Tong, Jin Yue, Jin Li, Wenting Dai, Huizhong Xu, Dongliang Shang, and Liping Ban. 2023. "The Efficiency of Pest Control Options against Two Major Sweet Corn Ear Pests in China" Insects 14, no. 12: 929. https://doi.org/10.3390/insects14120929