A Study of the Toxic Effect of Plant Extracts against Philaenus spumarius (Hemiptera: Aphrophoridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Preparation of Plant Extracts
2.3. Toxicity Bioassays
2.4. Field Experiments
2.5. Statistical Analysis
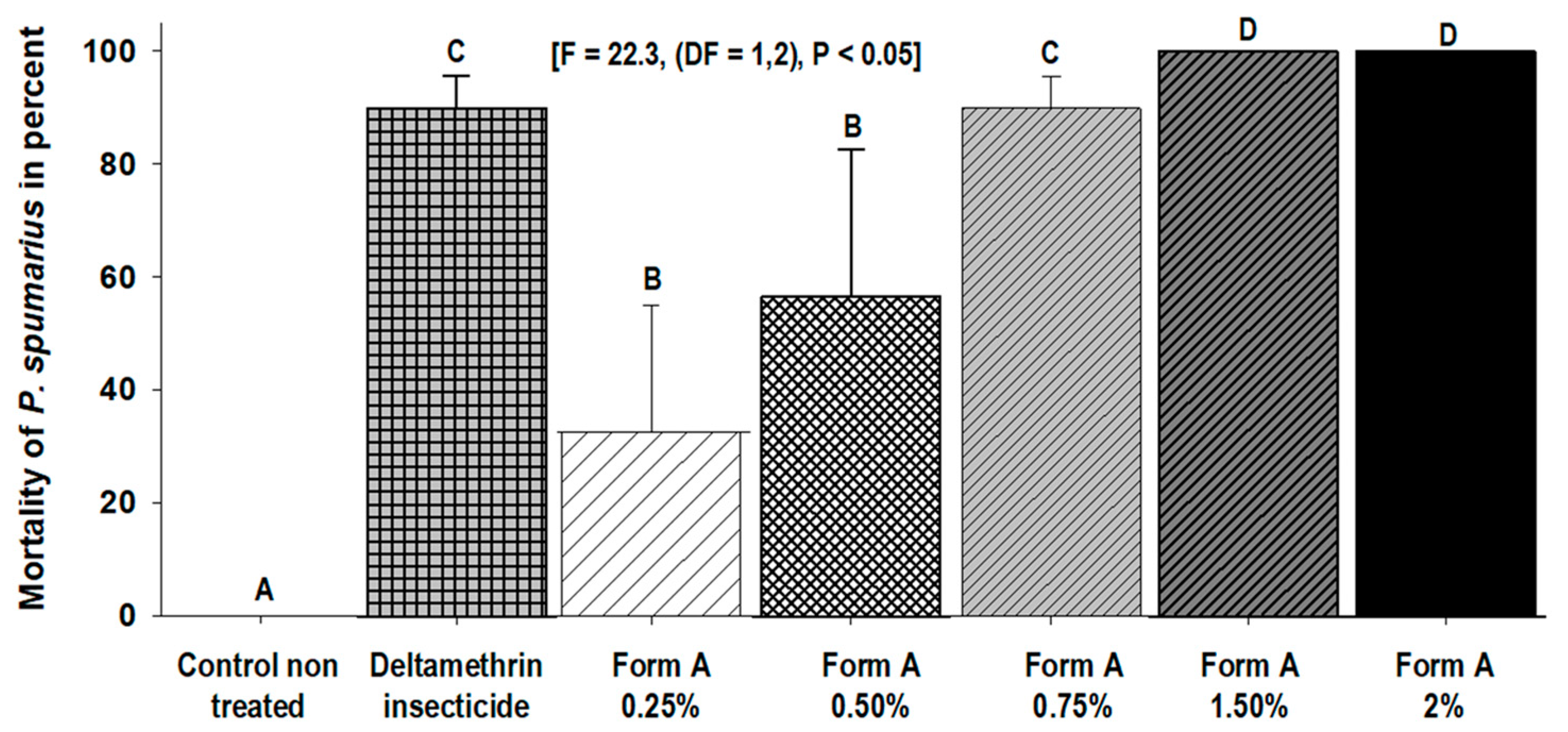
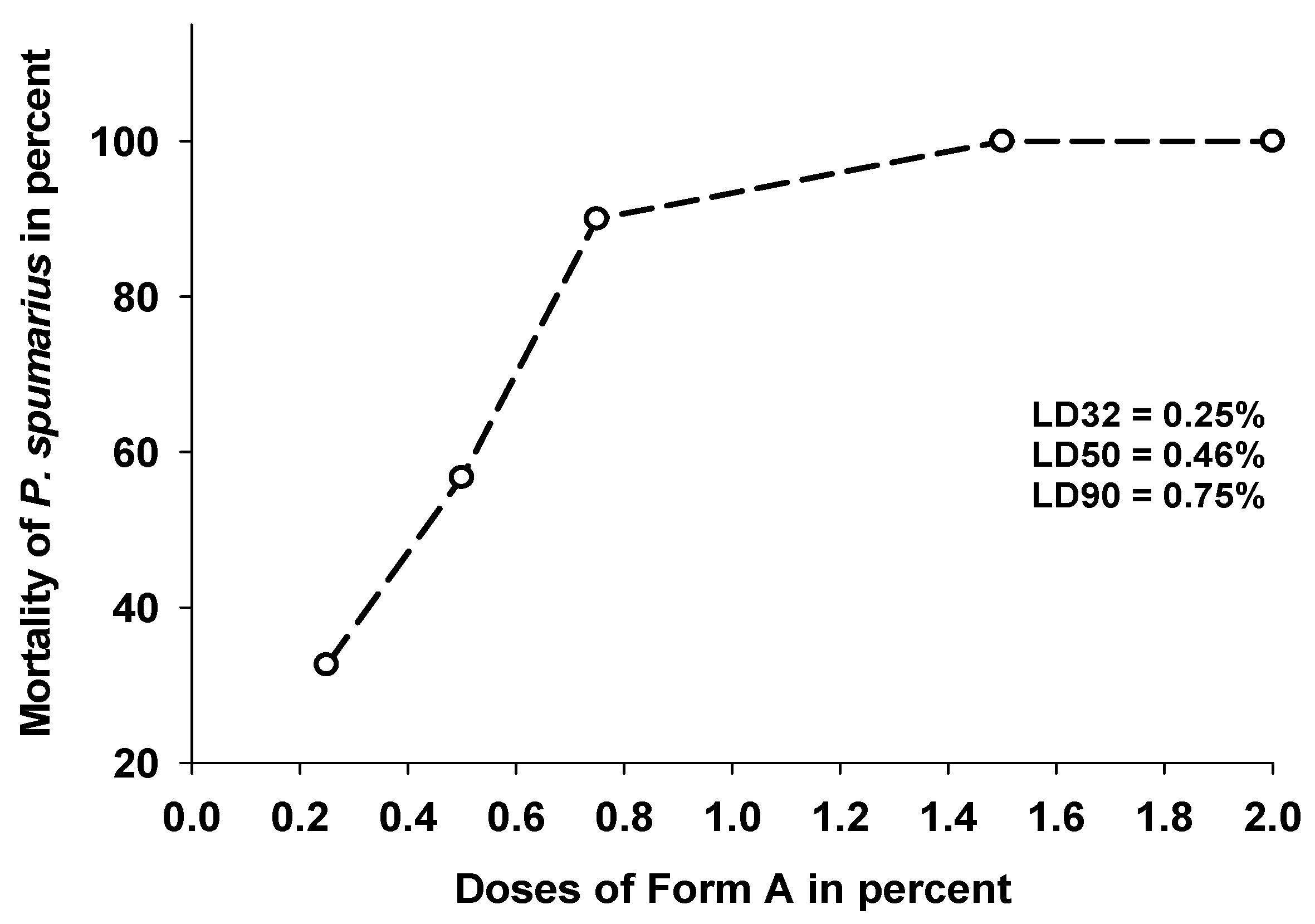
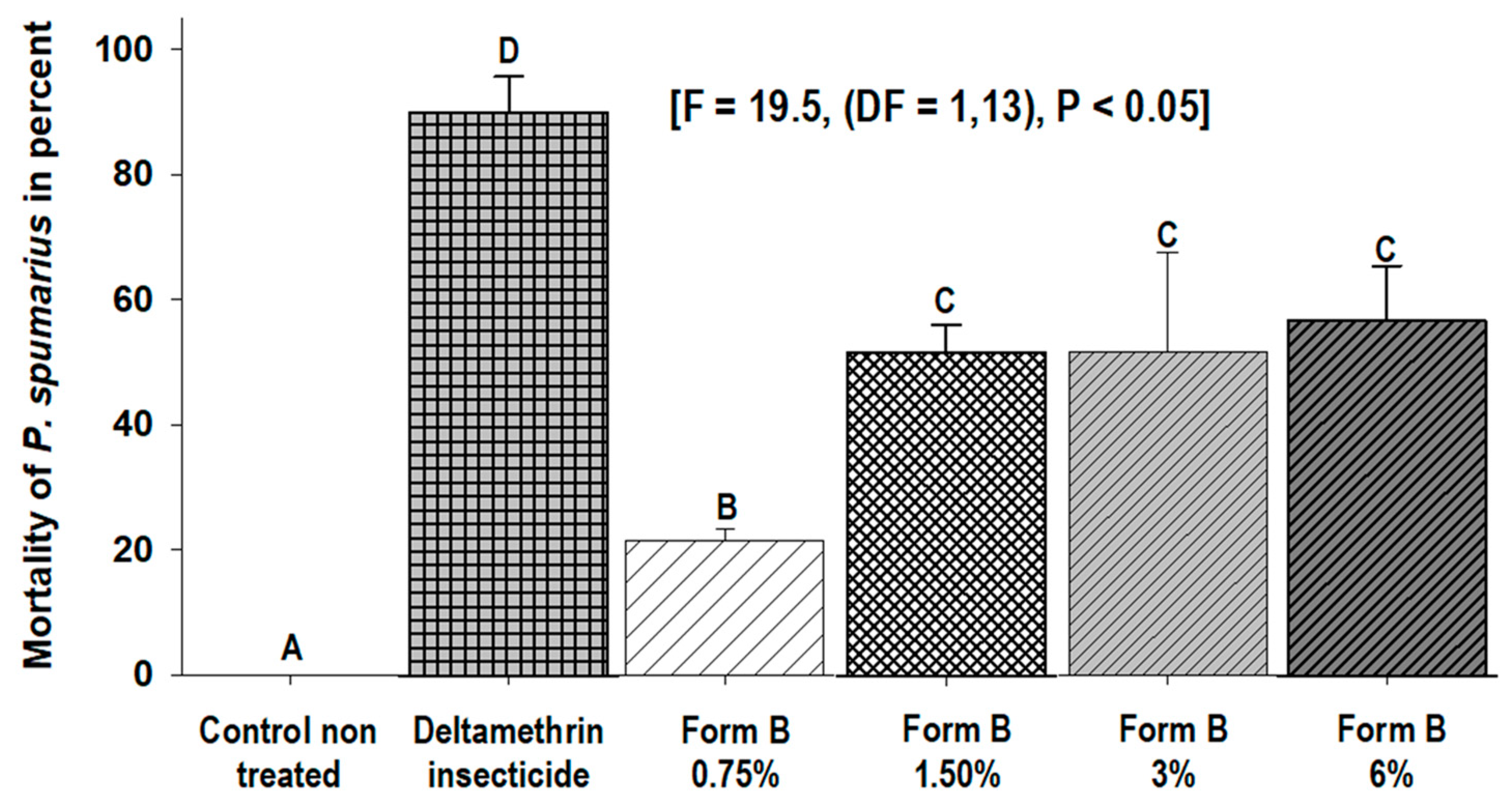
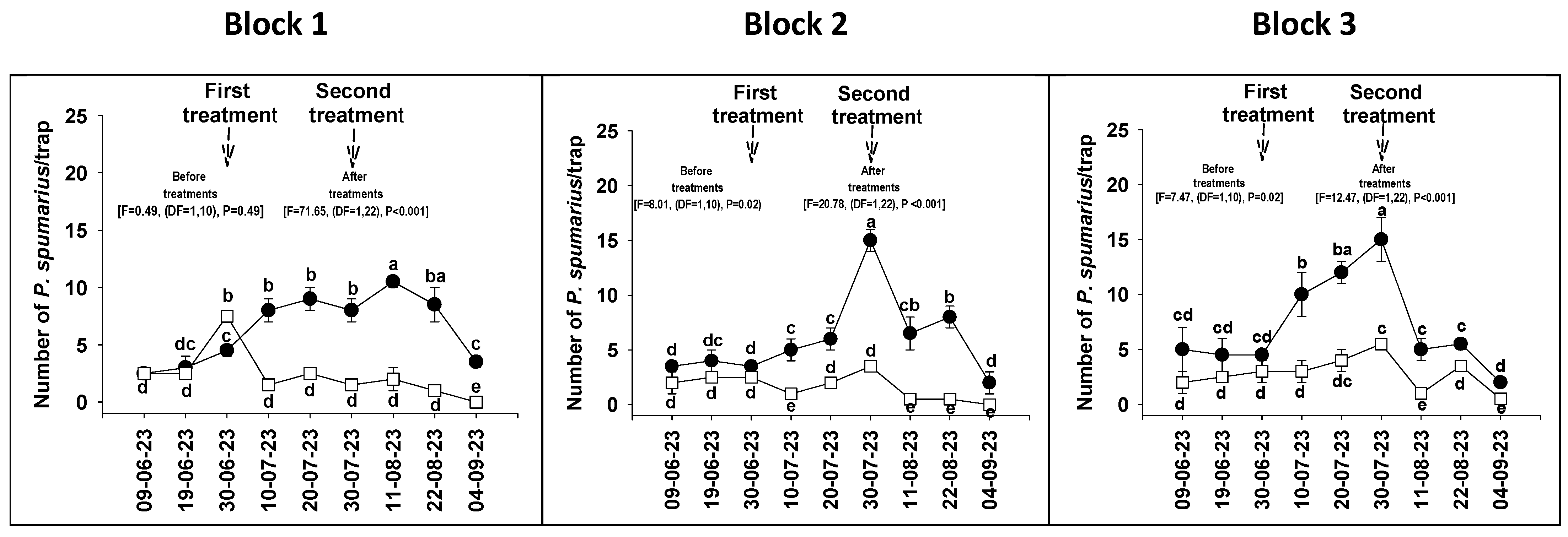
3. Results
3.1. Toxicity Bioassays
3.2. Field Experiments
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Plant Health. Scientific Opinion on the risk to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar]
- Scortichini, M. The epidemiology and control of “Olive Quick Decline Syndrome” in Salento (Apulia, Italy). Agronomy 2022, 12, 2475. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.P.P.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Bodino, N.; Demichelis, S.; Simonetto, A.; Volani, S.; Saladini, M.A.; Gilioli, G.; Bosco, D. Phenology, seasonal abundance, and host-plant association of spittlebugs (Hemiptera: Aphrophoridae) in vineyards of Northwestern Italy. Insects 2021, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Marra, M.; Morente, M.; Garzo, E.; Moreno, A.; Saponari, M.; Fereres, A. Feeding behavior in relation to spittlebug transmission of Xylella fastidiosa. J. Pest. Sci. 2020, 93, 1197–1213. [Google Scholar] [CrossRef]
- White, S.M.; Navas-Cortes, J.A.; Bullock, J.M.; Boscia, D.; Chapman, D.S. Estimating the epidemiology of emerging Xylella fastidiosa outbreaks in olives. Plant Pathol. 2020, 69, 1403–1413. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; Di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marin, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef]
- Martelli, G. The current status of the quick decline syndrome of olive in southern Italy. Phytoparasitica 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Saponari, M.; D’Attoma, G.; Abou Kubaa, R.; Loconsole, G.; Altamura, G.; Zicca, S.; Rizzo, D.; Boscia, D. A new variant of Xylella fastidiosa subspecies multiplex detected in different host plants in the recently emerged outbreak in the region of Tuscany, Italy. Eur. J. Plant Pathol. 2019, 154, 1195–1200. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Cavalieri, V. Evaluation of efficacy of different insecticides against Philaenus spumarius L., vector of Xylella fastidiosa in olive orchards in Southern Italy, 2015–2017. Arthropod Manag. Tests 2018, 43, tsy034. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Cavalieri, V. Evaluation of insecticides for the control of juveniles of Philaenus spumarius L., 2015–2017. Arthropod Manag. Tests 2018, 43, tsy073. [Google Scholar] [CrossRef]
- Vicente-Díez, I.; Blanco-Pérez, R.; González-Trujillo, M.M.; Pou, A.; Campos-Herrera, R. Insecticidal Effect of Entomopathogenic Nematodes and the Cell-Free Supernatant from Their Symbiotic Bacteria against Philaenus spumarius (Hemiptera: Aphrophoridae) Nymphs. Insects 2021, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Yousef-Yousef, M.; Morente, M.; Gonzalez-Mas, N.; Fereres, A.; Quesada-Moraga, E.; Moreno, A. Direct and indirect effects of two endophytic entomopathogenic fungi on survival and feeding behaviour of meadow spittlebug Philaenus spumarius. Biol. Control 2023, 186, 105348. [Google Scholar] [CrossRef]
- Ganassi, S.; Di Domenico, C.; Altomare, C.; Samuels, G.J.; Grazioso, P.; Cillo, P.D.; Pietrantonio, L.; De Cristofaro, A. Potential of fungi of the genus Trichoderma for biocontrol of Philaenus spumarius, the insect vector for the quarantine bacterium Xylella fastidosa. Pest Manag. Sci. 2023, 79, 719–728. [Google Scholar] [CrossRef]
- Ganassi, S.; Cascone, P.; Di Domenico, C.; Pistillo, M.; Formisano, G.; Giorgini, M.; Grazioso, P.; Germinara, G.S.; De Cristofaro, A.; Guerrieri, E. Electrophysiological and behavioural response of Philaenus spumarius to essential oils and aromatic plants. Sci. Rep. 2020, 10, 3114. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Mori, N.; Santoiemma, G.; D’Ascenzo, D.; Scotillo, M.A.; Marini, L. Ground cover management in olive groves reduces populations of Philaenus spumarius (Hemiptera: Aphrophoridae), vector of Xylella fastidiosa. J. Econ. Entomol. 2021, 114, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Morente, M.; Cornara, D.; Moreno, A.; Fereres, A. Parapause breakage as a key step for the continuous indoor rearing of Philaenus spumarius. J. Appl. Entomol. 2021, 145, 1062–1067. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in olive orchards of Greece with special reference to Xylella fastidiosa vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Strauß, J.; Stritih-Peljhan, N.; Nieri, R.; Virant-Doberlet, M.; Mazzoni, V. Communication by substrate-borne mechanical waves in insects: From basic to applied biotremology. In Advances in Insect Physiology; Jurenka, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 189–307. [Google Scholar] [CrossRef]
- Eriksson, A.; Anfora, G.; Lucchi, A.; Lanzo, F.; Virant-Doberlet, M.; Mazzoni, V. Exploitation of insect vibrational signals reveals a new method of pest management. PLoS ONE 2012, 7, e32954. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, T.; Uechi, N.; Tatsuta, H. Vibrations in hemipteran and coleopteran insects: Behaviors and application in pest management. Appl. Entomol. Zool. 2019, 54, 21–29. [Google Scholar] [CrossRef]
- Avosani, S.; Nieri, R.; Mazzoni, V.; Anfora, G.; Hamouche, Z.; Zippari, C.; Vitale, M.L.; Verrastro, V.; Tarasco, E.; D’Isita, I.; et al. Intruding into a conversation: How behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J. Pest Sci. 2023. [Google Scholar] [CrossRef]
- Dáder, B.; Viñuela, E.; Moreno, A.; Plaza, M.; Garzo, E.; Del Estal, P.; Fereres, A. Sulfoxaflor and natural Pyrethrin with Piperonyl Butoxide are effective alternatives to Neonicotinoids against juveniles of Philaenus spumarius, the european vector of Xylella fastidiosa. Insects 2019, 10, 225. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. (Previously Cent. Eur. J. Biol.) 2015, 10, 409–416. [Google Scholar] [CrossRef]
- Watkinson, I.A.; Wiseman, J.; Robinson, J. A simple test kit for field evaluation of the susceptibility of insect pests to insecticides. Br. Crop Prot. Conf. Pest Dis. 1984, 6, 559–564. [Google Scholar]
- Strona, G.; Carstens, C.J.; Beck, P.S.A. Network analysis reveals why Xylella fastidiosa will persist in Europe. Sci. Rep. 2017, 7, 71. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, 5665. [Google Scholar] [CrossRef]
- Marder, M.; Viola, H.; Wasowski, C.; Wolfman, C.; Waterman, P.G.; Medina, J.H.; Paladini, A.C. Cirsiliol and caffeic acid ethyl ester, isolated from Salvia guaranitica, are competitive ligands for the central benzodiazepine receptors. Phytomedicine 1996, 3, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafeek, K.A.; Abdelrahem, F.; Elwahsh, M.A.; Abdelkhalek, I.A. Investigation of the flavonoidal constituents and insecticidal activity of Teucrium zanonii. Pharmacogn. Res. 2009, 1, 410–416. [Google Scholar]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C.P. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol-Gel Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]
- Masanori, M.; Sumiko, K.; Kiochiro, K. Study of flavonoids and antifeedant activity of Teucrium species. J. Agric. Food Chem. 2000, 48, 1888–1894. [Google Scholar] [CrossRef]
- Williams, L.A.D.; Mansingh, A. Pesticidal Potentials of Tropical Plants—I. Insecticidal Activity in Leaf Extracts of Sixty Plants. Int. J. Trop. Insect. Sci. 1993, 14, 697–700. [Google Scholar] [CrossRef]
- Okonkwo, C.O.; Ohaeri, O.C. Insecticidal potentials of some selected plants. J. Chem. Pharm. Res. 2013, 5, 370–376. [Google Scholar]
- Huang, Y.; Ho, S.H.; Lee, H.C.; Yap, Y.L. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2002, 38, 403–412. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anithole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2009, 65, 241–248. [Google Scholar] [CrossRef]
- Dancewicz, K.; Gabrys, B.; Dams, I.; Wawrzeńczykm, C. Enantiospecific effect of pulegone and pulegone-derived lactones on Myzus persicae (Sulz.) settling and feeding. J. Chem. Ecol. 2008, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.A.; Dibble, J.E.; Flint, M.L.; Marer, P.J.; Guye, A. Managing Insects and Mites with Spray Oils; University of California, Integrated Pest Management Program: Oakland, CA, USA, 1991; p. 3347. [Google Scholar]
- Castillo, L.; González-Coloma, A.; González, A.; Díaz, M.; Santos, E.; Alonso-Paz, E.; Bassagoda, M.J.; Rossini, C. Screening of Uruguayan plants for deterrent activity against insects. Ind. Crops Prod. 2009, 29, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, M.; Shi, R.; Ye, M. Insecticidal Activity of Natural Capsaicinoids Against Several Agricultural Insects. Nat. Prod. Commun. 2019, 14, 7. [Google Scholar] [CrossRef]
- Siam, A.; Othman, E. Field evaluation of botanicals extracts for suppressing the mango scale insect, Aulacaspis tubercularis (Newstead) (Hemiptera: Diaspididae). Egypt. J. Biol. Pest Control 2020, 30, 22. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Bosco, D.; Porcelli, F.; Cavalieri, V.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Saponari, M. Project Deliverable 9.1: Practical Solution for Management and Containment of Xf. POnTE 2020. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5cdd715c6&appId=PPGMS (accessed on 7 June 2023).
- Rongai, D.; Cerato, C.; Lazzeri, L.; Palmieri, S.; Patalano, G. Vegetable oil formulation as biopesticide to control California red scale (Aonidiella aurantii Maskell). J. Pest Sci. 2008, 81, 179–185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rongai, D.; Cesari, E.; Bertin, S. A Study of the Toxic Effect of Plant Extracts against Philaenus spumarius (Hemiptera: Aphrophoridae). Insects 2023, 14, 939. https://doi.org/10.3390/insects14120939
Rongai D, Cesari E, Bertin S. A Study of the Toxic Effect of Plant Extracts against Philaenus spumarius (Hemiptera: Aphrophoridae). Insects. 2023; 14(12):939. https://doi.org/10.3390/insects14120939
Chicago/Turabian StyleRongai, Domenico, Erica Cesari, and Sabrina Bertin. 2023. "A Study of the Toxic Effect of Plant Extracts against Philaenus spumarius (Hemiptera: Aphrophoridae)" Insects 14, no. 12: 939. https://doi.org/10.3390/insects14120939





