Mosquitoes Possess Specialized Cuticular Proteins That Are Evolutionarily Related to the Elastic Protein Resilin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sequence Analyses
2.3. cDNA Cloning
2.4. Construction of Expression Plasmids and Preparation of Recombinant Proteins
2.5. Chitin-Binding Assay
3. Results
3.1. Resilin Homologous Proteins in Holometabolous Insects
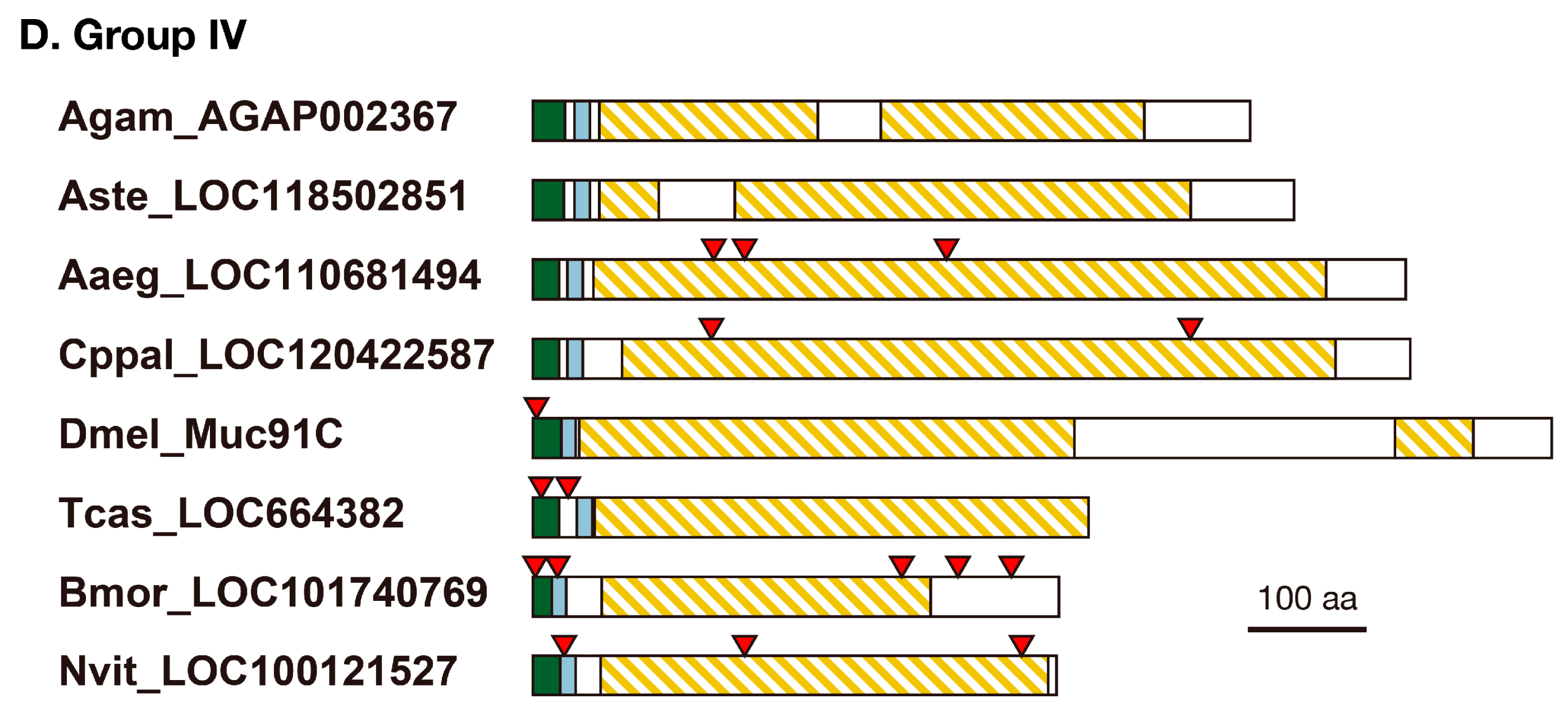
3.2. Structure of Resilin Homologous Proteins
3.3. Chitin-Binding Activity of Resilin and Resilin-Related
3.4. Other Cuticular Proteins with Repetitive Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, S.O.; Hojrup, P.; Roepstorff, P. Insect cuticular proteins. Insect Biochem. Mol. Biol. 1995, 25, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.H.; Iconomidou, V.A.; Smith, R.F.; Hamodrakas, S.J. Cuticular proteins. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier Ltd.: Oxford, UK, 2005; Volume 4, pp. 79–109. [Google Scholar]
- Willis, J.H.; Papandreou, N.C.; Iconomidou, V.A.; Hamodrakas, S.J. Cuticular Proteins. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 134–166. [Google Scholar]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle formation and pigmentation in beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rebers, J.E.; Willis, J.H. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem. Mol. Biol. 2001, 31, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Togawa, T.; Nakato, H.; Izumi, S. Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2004, 34, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Weis-Fogh, T. A rubber-like protein in insect cuticle. J. Exp. Biol. 1960, 37, 889–907. [Google Scholar] [CrossRef]
- Andersen, S.O.; Weis-Fogh, T. Resilin. A rubberlike protein in arthropod cuticle. Adv. Insect Physiol. 1964, 2, 1–65. [Google Scholar]
- Qin, G.; Hu, X.; Cebe, P.; Kaplan, D.L. Mechanism of resilin elasticity. Nat. Commun. 2012, 3, 1003. [Google Scholar] [CrossRef]
- Ardell, D.H.; Andersen, S.O. Tentative identification of a resilin gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2001, 31, 965–970. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C.; Lucey, E.C. The jump of the flea: A study of the energetics and a model of the mechanism. J. Exp. Biol. 1967, 47, 59–67. [Google Scholar] [CrossRef]
- Andersen, S.O. Structure and function of resilin. In Elastomeric Proteins: Structures, Biomechanical Properties and Biological Roles; Shewry, P.R., Tatham, A.S., Bailey, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 259–278. [Google Scholar]
- Andersen, S.O. Studies on resilin-like gene products in insects. Insect Biochem. Mol. Biol. 2010, 40, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Su, R.S.; Kim, Y.; Liu, J.C. Resilin: Protein-based elastomeric biomaterials. Acta Biomater. 2014, 10, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.E.; Wong, D.C.; Kim, M.; Lekieffre, N.; Huson, M.G.; Vuocolo, T.; Merritt, D.J.; Nairn, K.M.; Dudek, D.M.; Colgrave, M.L.; et al. Molecular and functional characterisation of resilin across three insect orders. Insect Biochem. Mol. Biol. 2011, 41, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Cornman, R.S.; Togawa, T.; Dunn, W.A.; He, N.; Emmons, A.C.; Willis, J.H. Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genom. 2008, 9, 22. [Google Scholar] [CrossRef]
- Willis, J.H. Structural cuticular proteins from arthropods: Annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gislason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- R Core Team. R Version 4.0.3: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Schneider, T.D.; Stephens, R.M. Sequence logos: A new way to display consensus sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Lerch, S.; Zuber, R.; Gehring, N.; Wang, Y.; Eckel, B.; Klass, K.D.; Lehmann, F.O.; Moussian, B. Resilin matrix distribution, variability and function in Drosophila. BMC Biol. 2020, 18, 195. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Hiromasa, Y.; Tomich, J.M.; Lu, N.; Beeman, R.W.; Kramer, K.J.; Kanost, M.R. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J. Proteome Res. 2012, 11, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Lapidot, S.; Numata, K.; Hu, X.; Meirovitch, S.; Dekel, M.; Podoler, I.; Shoseyov, O.; Kaplan, D.L. Expression, cross-linking, and characterization of recombinant chitin binding resilin. Biomacromolecules 2009, 10, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Badgett, M.J.; Bowen, J.H.; Vannini, L.; Orlando, R.; Willis, J.H. Distribution of cuticular proteins in different structures of adult Anopheles gambiae. Insect Biochem. Mol. Biol. 2016, 75, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Keime, N.; Fong, C.; Kraemer, A.; Fassbinder-Orth, C. Resilin Distribution and Abundance in Apis mellifera across Biological Age Classes and Castes. Insects 2023, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, J.A.; Stemmer, W.P. Directed evolution of proteins by exon shuffling. Nat. Biotechnol. 2001, 19, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Casola, C.; Betran, E. The Genomic Impact of Gene Retrocopies: What Have We Learned from Comparative Genomics, Population Genomics, and Transcriptomic Analyses? Genome Biol. Evol. 2017, 9, 1351–1373. [Google Scholar] [CrossRef] [PubMed]
- Togawa, T.; Dunn, W.A.; Emmons, A.C.; Nagao, J.; Willis, J.H. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 2008, 38, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Vannini, L.; Willis, J.H. Immunolocalization of cuticular proteins in Johnston’s organ and the corneal lens of Anopheles gambiae. Arthropod Struct. Dev. 2016, 45, 519–535. [Google Scholar] [CrossRef]
- Elvin, C.M.; Carr, A.G.; Huson, M.G.; Maxwell, J.M.; Pearson, R.D.; Vuocolo, T.; Liyou, N.E.; Wong, D.C.; Merritt, D.J.; Dixon, N.E. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005, 437, 999–1002. [Google Scholar] [CrossRef]
- Lyons, R.E.; Lesieur, E.; Kim, M.; Wong, D.C.; Huson, M.G.; Nairn, K.M.; Brownlee, A.G.; Pearson, R.D.; Elvin, C.M. Design and facile production of recombinant resilin-like polypeptides: Gene construction and a rapid protein purification method. Protein Eng. Des. Sel. 2007, 20, 25–32. [Google Scholar] [CrossRef]
- Nairn, K.M.; Lyons, R.E.; Mulder, R.J.; Mudie, S.T.; Cookson, D.J.; Lesieur, E.; Kim, M.; Lau, D.; Scholes, F.H.; Elvin, C.M. A synthetic resilin is largely unstructured. Biophys. J. 2008, 95, 3358–3365. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohkubo, S.; Shintaku, T.; Mine, S.; Yamamoto, D.S.; Togawa, T. Mosquitoes Possess Specialized Cuticular Proteins That Are Evolutionarily Related to the Elastic Protein Resilin. Insects 2023, 14, 941. https://doi.org/10.3390/insects14120941
Ohkubo S, Shintaku T, Mine S, Yamamoto DS, Togawa T. Mosquitoes Possess Specialized Cuticular Proteins That Are Evolutionarily Related to the Elastic Protein Resilin. Insects. 2023; 14(12):941. https://doi.org/10.3390/insects14120941
Chicago/Turabian StyleOhkubo, Sakura, Tohki Shintaku, Shotaro Mine, Daisuke S. Yamamoto, and Toru Togawa. 2023. "Mosquitoes Possess Specialized Cuticular Proteins That Are Evolutionarily Related to the Elastic Protein Resilin" Insects 14, no. 12: 941. https://doi.org/10.3390/insects14120941
APA StyleOhkubo, S., Shintaku, T., Mine, S., Yamamoto, D. S., & Togawa, T. (2023). Mosquitoes Possess Specialized Cuticular Proteins That Are Evolutionarily Related to the Elastic Protein Resilin. Insects, 14(12), 941. https://doi.org/10.3390/insects14120941




