Simple Summary
The deformed wing virus (DWV) has been detected in Apis florea and its ectoparasite Euvarroa sinhai since they invaded Taiwan. Using a reverse transcription-PCR with specific primers enabled us to ascertain that DWV type A was prevalent among A. florea samples. Homology analysis and maximum-likelihood phylogenetic tree between different genotypes disclosed a complete polyprotein nucleotide sequence identity of 88% between isolates from A. florea, E. sinhai, and DWV type A strains from GenBank. The results demonstrated that a novel variant of deformed wing viruses exists in the invasive species A. florea and E. sinhai.
Abstract
The invasion of Apis florea in Taiwan was first recorded in 2017. The deformed wing virus (DWV) has been identified as a common bee virus in apiculture around the world. Ectoparasitic mites are the main DWV vector for horizontal transmission. However, there are few studies about the ectoparasitic mite of Euvarroa sinhai, which has been found in A. florea. In this study, the prevalence of DWV among four hosts, including A. florea, Apis mellifera, E. sinhai, and Varroa destructor, was determined. The results showed that a high DWV-A prevalence rate in A. florea, ranging from 69.2% to 94.4%, was detected. Additionally, the genome of DWV isolates was sequenced and subjected to phylogenetic analysis based on the complete polyprotein sequence. Furthermore, isolates from A. florea and E. sinhai both formed a monophyletic group for the DWV-A lineage, and the sequence identity was 88% between the isolates and DWV-A reference strains. As noted above, two isolates could be the novel DWV strain. It cannot be excluded that novel DWV strains could pose an indirect threat to sympatric species, such as A. mellifera and Apis cerana.
1. Introduction
The deformed wing virus (DWV) has been studied extensively, as it is thought to cause colony losses in the Western honeybee Apis mellifera, thereby reducing the commercial values of honeybee pollination and products [1]. It is a single-stranded positive-stranded RNA of the Iflavirus virus in the family of Iflaviridae, which can prolong its life cycle through mass replication via honeybee pupae and adults or the ectoparasitic mite Varroa destructor [2]. Unsuccessful honeybee adults with wing deformities and exposed tongues have been found in front of hive comb cells [3], and even in eclosion survivals, the atrophy of the hypopharyngeal and mandible glands have been observed, which affects their abilities to forage and secrete royal jelly [4].
The DWV has the characteristics of rapid replication and high mutation rate and often exists in the host and in an environment with multiple genotypes or so-called quasispecies [5]. The genotype of the DWV-A lineage includes the Kakugo virus (KV) and the so-called deformed wing virus (DWV); it has been recognized that it is highly adaptive to various hosts [6], whereas DWV causes wing deformities in honeybees pathologically [7]. DWV-B was isolated from V. destructor [8], as it was the main variant of DWV and was originally called Varroa destructor virus-1 (VDV-1). Subsequent studies have shown at least four variants to be detected in Varroa mites [9,10]. The relevant studies also found that various bees, wasps, ants, and other insects incorporated with their symbionts were infected with various DWV genotypes. For example, DWV-A and DWV-C were found in the small hive beetle Aethina tumida and stingless bee Melipona subnitida [11,12,13].
The DWV genome length is about 10,140 nucleotides (nt) with a poly (A) tail, and 30 nm particle geometry is in icosahedral symmetry [8,14]. The genome structure is monopartite monocistronic with a single open reading frame (ORF) coding a single polyprotein and divided into two parts [15,16]. The major structural protein includes the capsid protein VP1–4 and the non-structural proteins RNA helicase, chymotrypsin-like 3C protease (3C-pro), and an RNA-dependent RNA polymerase (RdRp) which are mapped at the C-terminal region [14,15,16]. A previous study suggested that there were 95% shared amino acids and 84% shared nucleotide identities between DWV-A and DWV-B, and 89.1% and 79% between DWV-A and DWV-C; in addition, the phylogenetic tree showed that DWV-C was independent far from the main branch [17]. To date, though the fourth genotype of DWV-D has been classified, its relevant sequence has not been compared well with the known genotypes [18].
The dwarf honeybee A. florea is an invasive alien species (IAS) (Figure S1) that has been brought to attention by its remarkable aggressive expansion via import and export trade worldwide [18,19,20]. The infestation and population density of ectoparasite Euvarroa sinhai (Figure S2) in A. florea has been studied in terms of geographical origin, whereas little is known about its invasive populations [21,22,23,24]. A study has indicated that A. florea can also be infected with DWV [25]. To date, there have been no further reports involving the DWV genotype studies about A. florea and its ectoparasite E. sinhai in origin or invasive populations. Studies have shown that E. sinhai and V. destructor in honeybee colonies share life history traits [24,26]. These mites feed on the hemolymph of honeybee host pupae and undermine their immunity and health [27,28]. In addition, they act as a biological vector for the horizontal and vertical transmission of DWV, increasing the viral levels and reducing in-virus diversity [29,30,31,32,33]. Since 2017, A. florea has invaded Kaohsiung City, southern Taiwan [20], where many domesticated A. mellifera has been reared [32]. In the case of honeybees sharing food resources and interacting with each other in a sympatric area, pathogens carried by one may cause cross-infection through contact behaviors such as mite transfer or competition for nectars [29,30,31]. Thus, the purpose of this study was to detect the prevalence of DWV viruses in invasive A. florea and establish whole-genome sequences to compare the differences of viral strains among A. florea, A. mellifera, E. sinhai, and V. destructor.
2. Materials and Methods
The hot spots of the invasive A. florea located in the Fengshan, Gangshan, Gushan, Lingya, Qianzhen, Qijin, and Xiaogang districts in Kaohsiung city were reported by Hsu et al. [20]. From May 2020 to February 2022, 13 nests of A. florea were collected periodically in these areas, and between four and eight adult workers in each nest were sampled for monitoring DWV-A and DWV-B. In addition, sympatric A. florea and A. mellifera foragers were collected by sweeping nets periodically in the open spaces of these areas (Figure S3). A total of 72 and 78 adults of A. florea were sampled from nests and open spaces, and 28 of A. mellifera were from open spaces, respectively. No symptoms of the deformed wing were found in all the collected honeybee samples. For ectoparasitic mites, five fresh mites of E. sinhai were collected from drone cells of the above-mentioned A. florea nests, and seven mites of V. destructor from A. mellifera nests in the apiary of NCYU. Collection details for the sample are given in Table S1. All samples were deposited in a sample RNA protector (RNAlter) soon after collection [34] and then preserved in a −20 °C freezer until they were isolated.
The total RNA was isolated from single homogenized honeybee midgut or mite bodies’ tissue using RNAzol RT (Molecular Research Center Inc., Cincinnati, OH, USA) [33]. Summarily, mid-gut tissue was homogenized in a 1.5 mL microfuge tube with a disposable tissue grinding pestle in 500 μL RNAzol RT and 200 μL of added deionized water (diH2O); the resulting mixture was shaken vigorously and allowed to stand at room temperature for 10 min before the sample mixture was centrifuged at 12,000 rpm for 15 min. The supernatant liquid (500 μL) was then placed in a microfuge tube, and an equal volume of isopropanol (500 μL) was added, and after mixing, the sample solution was placed at room temperature for 15 min. The mixed solution was then centrifuged (12,000 rpm for 10 min), and the pellet was washed twice with 75% ethanol and then repelleted (4000 rpm for 3 min). The supernatant was removed, and the dried pellet was reconstituted in deionized water (diH2O) 50 μL.
Sample RNA was used to synthesize cDNA with an oligo (dT) 18 primer (Protech, Taipei, Taiwan) by a HiScript I TM First Strand cDNA Synthesis Kit (BIONOVAS Biotechnology, Toronto, Ontario, Canada). PCR reactions were performed in a 25 µL mixed solution using a 2× red PCR Master mix kit (Ampliqon). For DWV-A and DWV-B, the three primers used to sequence the RdRp-encoding region were DWV-F (GGATGTTATCTCTTGCGTGGA), DWV-R (CGATAATAATTTCGAACGCTGA) (412 bp) [14]; KV-F (GGACTGAACCAAATCCGATGTCATCACG), and KV-R (TCTCAAGTTCGGGACGCATTC) (378 bp) [7] and VDV-1-F (TGGCTAATCGACGTAAAGCA) and VDV-R (ACTAATCTCTGAGCCAACACGT) (195 bp) [35]. We chose a DWV, KV, and VDV-1 positive A. mellifera sample from the apiary of NCYU as a positive control.
Ten sets of primers were designed to amplify overlapping PCR products, which comprised the complete polyprotein genome sequence of targeted DWV isolates. The sequences, orientations, and locations of the primers, as well as the expected product sizes, are shown in Table 1. The thermal cycling program followed: one cycle at 94 °C for 5 min, 35 cycles at 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s, and an elongation cycle at 72 °C for 5 min. The reaction was then held at 4 °C. The PCR-amplified product was electrophoresed in a 2% agarose gel containing 0.5 μg/mL of ethidium bromide and was visualized under UV light.

Table 1.
The primers designed for overlapping sequences to ensure the complete polyprotein sequencing of the selected DWV genotypes.
PCR reactions used a Q5 High-Fidelity DNA Polymerase Kit (New England Biolabs, Ipswich, MA, USA) and 10 mM dNTPs. Each reaction contained a 5x Q5 buffer, 2.5 mM dNTPs, Q5 high-fidelity DNA polymerase, forward and reverse DWV-variant-specific primer (Table 1), diH2O, and cDNA template. The thermocycler was set to initial activation at 98 °C for 30 s, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 52 °C for 30 s, and extension at 72 °C for 3 min, followed by a final extension at 72 °C for 5 min. The purified gene fragments were separately cloned with a pGEM-T easy vector (Promega) kit and transformed into competent cells, Escherichia coli (DH5α, Smobio Champion E. coli Transformation Kit). The transformants (E. coli) were selected by blue-white selection on the plates containing 5% X-gal (20 mg/mL), Ampicillin (100 μg/mL), and IPTG (50 Mm)) and were screened as positive clones by colony PCR. The plasmid was isolated and then restricted the enzyme digestion of plasmid DNAs with EcoR1. For each virus, 3–5 clones were picked up for Sanger sequencing. The cDNA clones were reamplified from the respective plasmid with a pair of T7P and SP6 by automated DNA sequencer at Genomics BioSci & Tech Co., Ltd. Company (New Taipei City, Taiwan). All the sequences were submitted to GenBank (accession nos. OP889266–OP889269).
Based on the complete polyproteins to construct a homology tree and phylogenetic tree, the phylogenetic relationships among the strains of the honeybee viruses and other close groups available in GenBank were initially assessed using BLAST [36]. The identification of all the gene domains referred to the predicted protease sites for the DWV reference sequence (AJ489744.2) [14]. The nucleotide (nt) and amino acid (aa) sequences of the viruses were aligned with sequence analyses and phylogenetic analyses by using DNAMAN and MEGA X [37,38]. The maximum-likelihood phylogenetic tree (model TN93 + G; 1000 replicates) was also constructed from sequences of DWV-A, DWV-B, DWV-C, and sequences of virus isolates in this study (Table 2).

Table 2.
DWV sequences published in GenBank used to construct phylogenetic trees.
3. Results
3.1. Prevalence of DWV-A and DWV-B in Honeybees and Mites
All samples containing DWV were amplified by the PCR using the primers KV-F/-R and DWV-F/-R, and it was confirmed by gel electrophoresis that the KV primer showed higher sensitivity than DWV in A. florea, A. mellifera, and E. sinhai (Figure 1; Table 3). For DWV-A testing, A. florea showed partial/all positives in the samples collected from nests and open spaces in different locations, whereas only five locations were partial/all positive in A. mellifera (Table 3). Meanwhile, all the samples were negative in DWV-B testing. A total of 68 (94.4%) A. florea nest samples were positive for KV, and 54 (69.2%) were in open-space samples (Table 4). Though A. mellifera showed a lower portion of positive results (32.1%), all samples from E. sinhai and V. destructor were positive for KV.
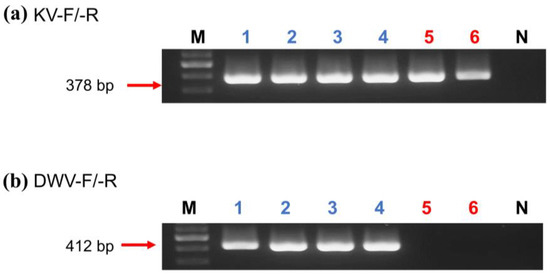
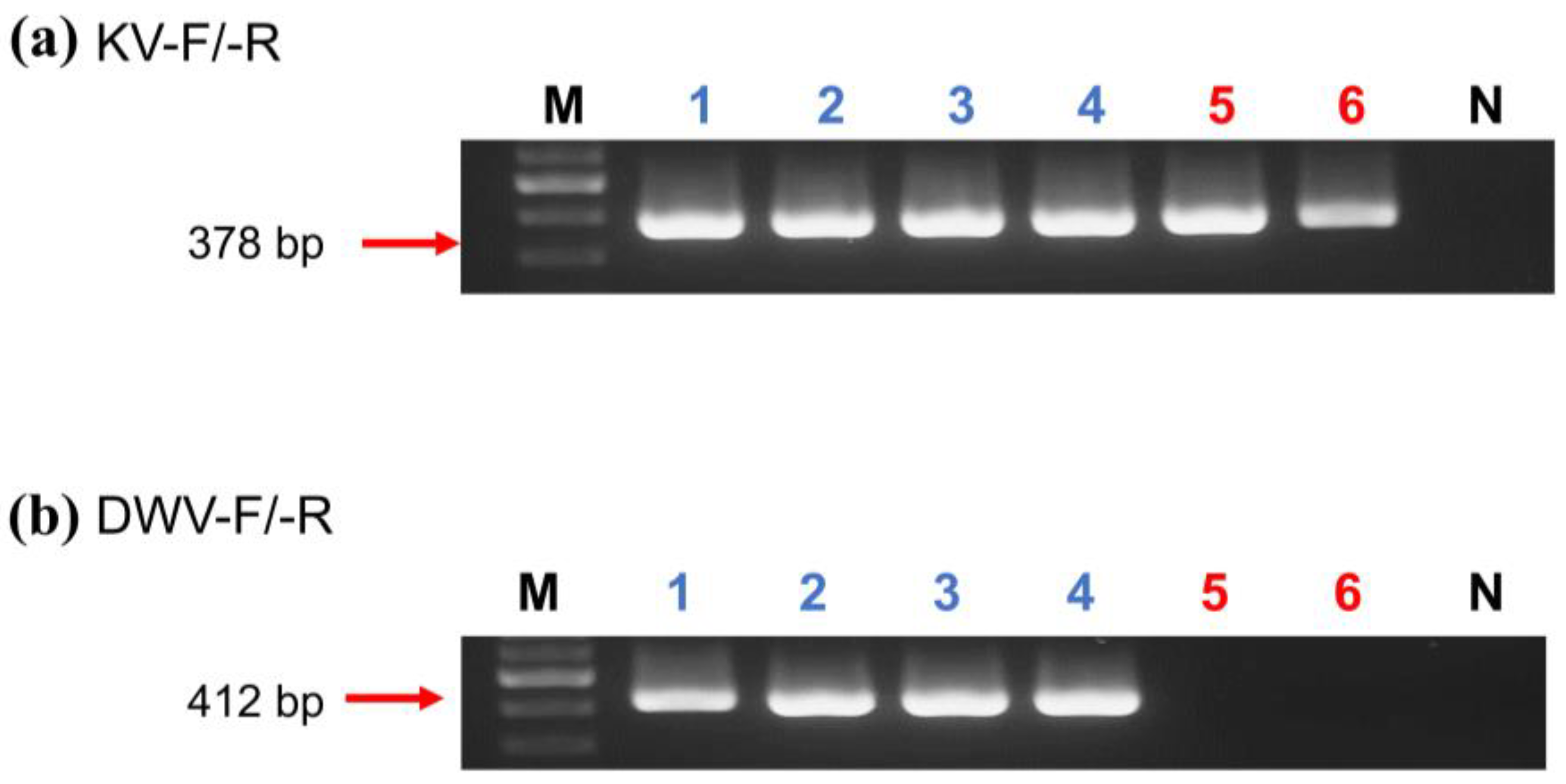
Figure 1.
Gel electrophoresis of KV ((a), 378 bp) and DWV ((b), 412 bp) products amplified by selective primers KV-F/-R and DWV-F/-R, respectively. (a,b) Lane M: 100 bp Nautia DNA Ladder (Nautia); Lane N: negative control; Lanes 1–3: A. mellifera; Lanes 4: V. destructor; Lanes 5: A. florea; and Lanes 6: E. sinhai.

Table 3.
Detection of DWV-A and DWV-B in workers of Apis florea and A. mellifera by RT-PCR.

Table 4.
Prevalence of DWV-A in Apis florea, Euvarroa sinhai, A. mellifera and Varroa destructor.
3.2. Genome Sequence of DWV Variant in A. florea and E. sinhai
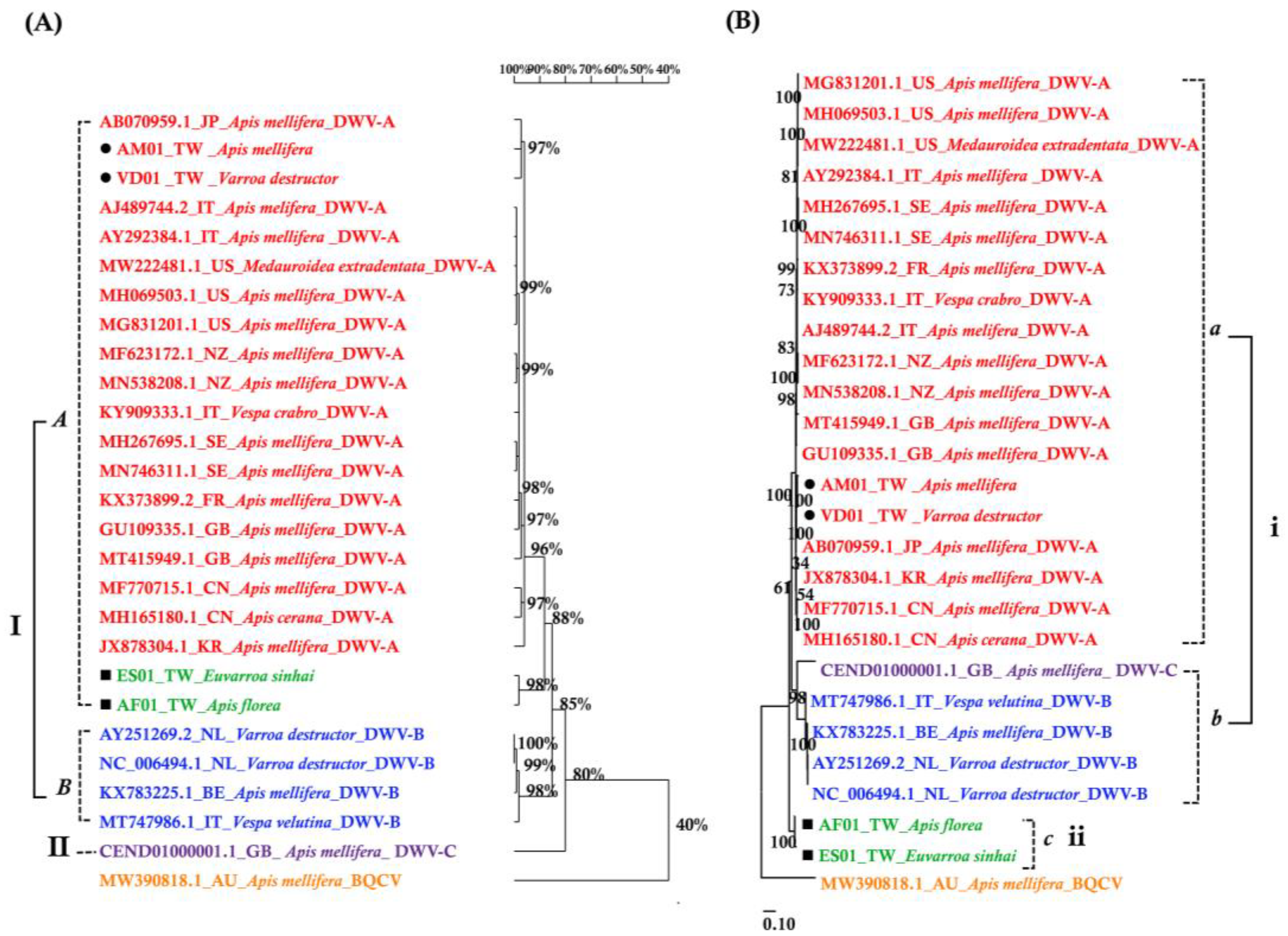
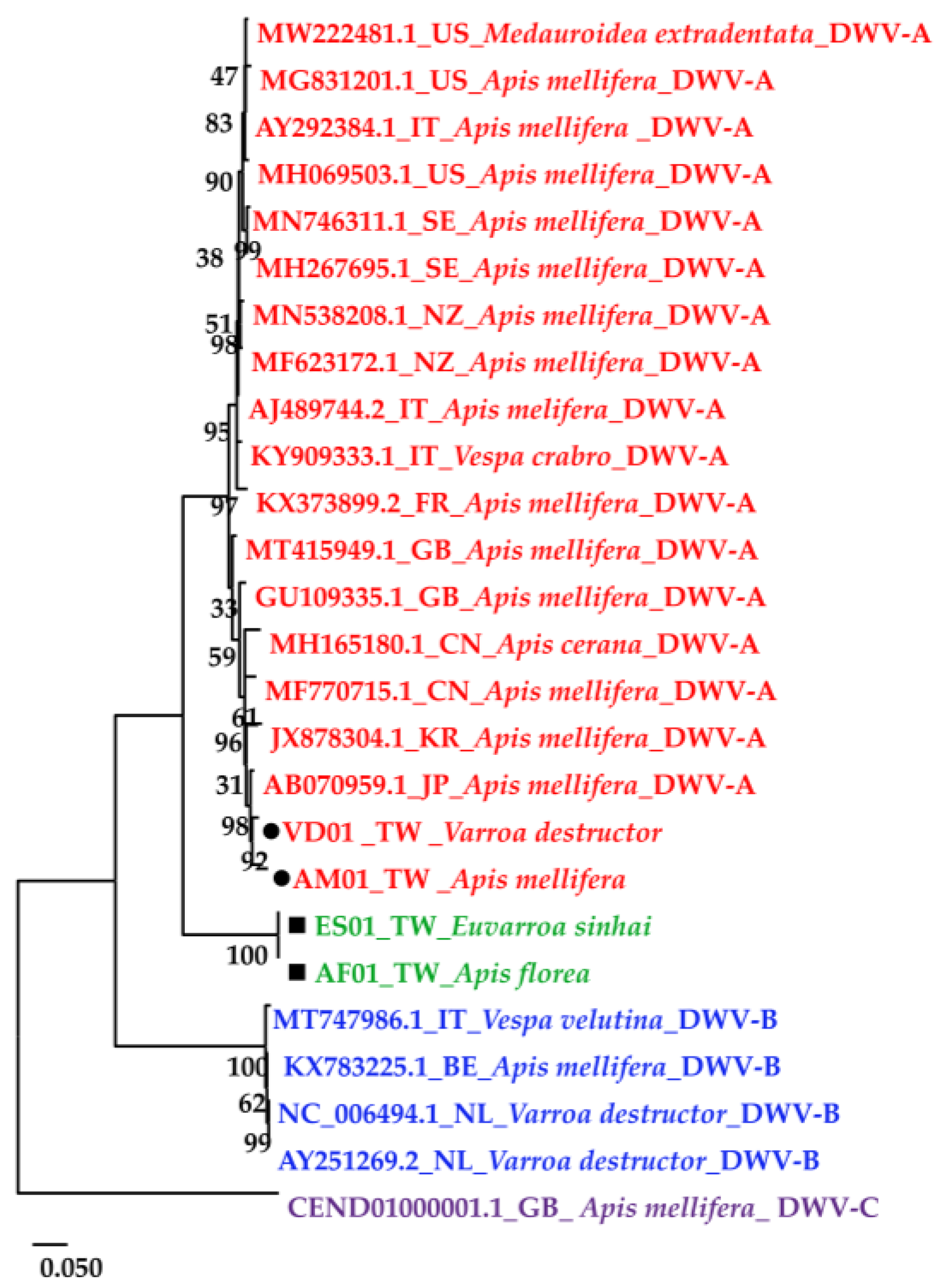
The DWV homology tree and phylogenetic tree were constructed from complete poly protein nt sequences of A. florea (AF01T243 (AF01), accession nos. OP889266), A. mellifera (AM01TGS8 (AM01), accession nos. OP889268), E. sinhai (ES01TFS11 (ES01), accession nos. OP889267), and V. destructor (VD01Tvd1 (VD01), accession nos. OP889269) in this study, along with the previously reported DWV isolates (Table 2) and outgroup Black queen cell virus (BQCV) (MW390818.1) from the GenBank [39]. The DWV isolates in the homology tree were closely related to form a monophyletic subclade, with a relatively large branch of 25 isolates in lineage I and an isolate of DWV-C in II (Figure 2A). Lineage I divided into two subgroups for A and B AF01, ES01, AM01, VD01, and 17 DWV-A isolates in subgroup A, whereas four DWV-B isolates were placed in group B. The two master lineages showed 80% sequence homology, while two subgroups from lineage I showed 85% sequence homology. Within subgroup A, the isolates were further subdivided into two branches: one for AM01, VD01, and 17 DWV-A isolates and the second for AF01 and ES01 isolates. Moreover, the two branches shared 88% sequence identity. With respect to the maximum-likelihood phylogenetic tree (Figure 2B), the 26 DWV isolates (including AF01, ES01, AM01, VD01) were divided into two branches for lineages i and ii. Lineage i contained two subgroups for a and b AM01, VD01, and other 17 DWV-A isolates in subgroup a, compared to four DWV-B isolates and one DWV-C isolate in b. The AF01 and ES01 isolates were found in lineage ii. Furthermore, the phylogenetic tree that was constructed for RdRp nt sequences of A. florea (AF01), A. mellifera (AM01), E. sinhai (ES01), and V. destructor (VD01) in this study, along with the previously reported DWV isolates (Table 2) and DWV-C reference strains as the outgroup sequence, formed the result that AF01 and ES01 isolate formed a subcluster distinct from the clusters of the DWV-A (Figure 3).
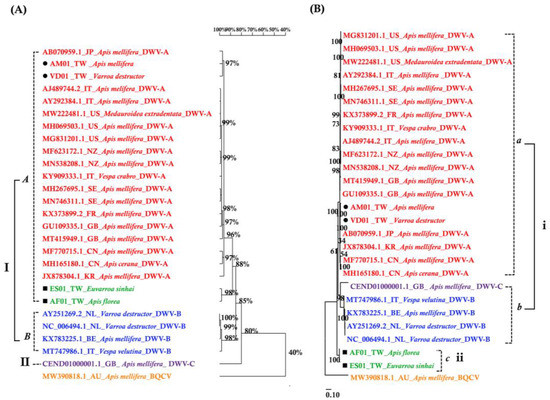
Figure 2.
(A) Homology tree constructed based on the complete polyprotein sequence of four samples in this study; the 22 DWV-associated strains and Black queen cell virus complete genome (MW390818.1) were from the NCBI database. (B) Maximum-likelihood phylogenetic tree (Tamura-Nei model; 1000 replicates) inferred from complete polyprotein sequence.
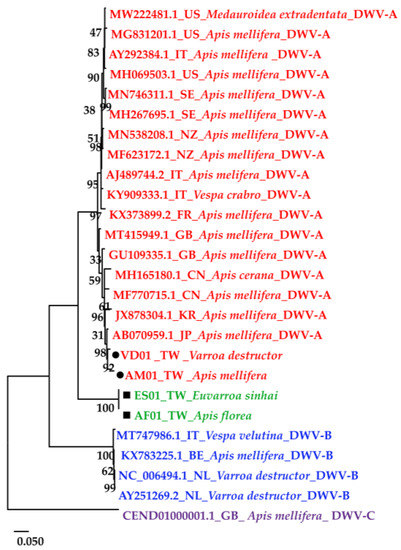
Figure 3.
Maximum-likelihood phylogenetic tree (Tamura-Nei model; 1000 replicates) constructed based on the RdRp region sequence of four isolates in this study; the 22 DWV-associated strains and complete genome were from the NCBI database.
Based on the present results, it can be seen that DWV isolates from A. florea and its ectoparasite E. sinhai formed another distinct clade. Genome regions of alignment need to be studied for further analysis: (1) The ORF is predicted to encode a 2896 aa polyprotein. (2) 950 aa capsid proteins; (3) 713 aa 3C + RdRp; (4) 472 aa helicase; (5) 211 aa leader protein; (6) 320 nt 3′ UTR (without poly-A); and (7) 1135 nt 5′ UTR. Homology analyses performed by nucleotides and amino acids of 3′ UTR, 5′ UTR, the polyprotein, VP1-4 of the capsid protein (structural protein), the leader protein of structural polyprotein, helicase, 3-chymotrypsin-like protease, and the 3-chymotrypsin-like protease (3C + RdRp) region were referred to the complete DWV-A genome sequence (AJ489744.2). Here, we only describe the nucleotide identity in each region of the DWV genome. The findings of the complete nucleotide (nt) and amino acid (aa) sequence identities are in alignment with AF01, and the corresponding regions of the four isolates (ES01, DWV-A (AJ489744.2), DWV-B (AY251269.2) and DWV-C (CEND01000001.1)) was shown (Table 5). For the 3′ UTR and 5′ UTR regions, there was a 99.7–99.9% shared nt identity within the two isolates of AF01 and ES01, which shared 94–95.6% nt similarity with DWV-A, 82.6–89.9% with DWV-B, and 82.8–86.9% with DWV-C. For the polyprotein region, there was 98.2% shared nt identity within AF01 and ES01 (complete sequence in Table S2), while the two isolates shared 89% with DWV-A, 83% with DWV-B, and 79.1% with DWV-C. For the leader protein region, there was a 99.5% shared nt identity within AF01 and ES01, while two isolates shared an 82% similarity with DWV-A, 74.8% with DWV-B, and 65.2% with DWV-C. For the capsid protein region, there was a 99.8% shared nt identity within AF01 and ES01, while two isolates shared 86.9% with DWV-A, 82.6% with DWV-B, and 79.3% with DWV-C. For the helicase region, there was a 93.5% shared nt identity within AF01 and ES01 (Figure S4), while two isolates shared 94.1% with DWV-A, 87.5% with DWV-B, and 82.1% with DWV-C. It is also worth noting that the AF01 helicase sequence was significantly lower than the other regions. This result was highlighted in the disparity between AF01 and ES01. For the 3C + RdRp region, there was a 99.9% shared nt identity within AF01 and ES01, while the two isolates shared an 89.2% similarity with DWV-A, 84.5% with DWV-B, and 82% with DWV-C.

Table 5.
Percentage nucleotide and amino acid sequence’s identity in an alignment between 243 isolate from Apis florea, three genotypes, and other isolates in this study.
4. Discussion
In this study, we emphasized only the prevalence and variants of DWV in the invasive honeybee Apis florea and its ectoparasite Euvarroa sinhai in Taiwan. Four master variants have been described in the DWV of the quasispecies [7,14,17,40]. Unfortunately, the complete viral polyprotein sequence from A. florea and E. sinhai has not been reported to the GenBank database. According to the International Committee on Taxonomy of Viruses (ICTV), species of the Iflaviridae are demarcated by amino acid identity in the sequence of the capsid proteins (CPs) when the strains of a species are above 90% [41]. In light of this, an over 90% sequence homology was displayed between the isolates from A. florea (AF01T243), E. sinhai (ES01TFS11), and the DWV-A strain (AJ489744.2) with regard to the CPs; therefore, we conclude that two virus isolates exist in the new variant of DWV. In addition, the phylogenetic comparison of DWV from various species in this study suggests that there are at least three distinct main groups within the DWV quasispecies. DWV isolates found in Taiwan were clustered within the DWV-A clade. Each one of the isolates from A. florea and E. sinhai in this study formed a monophyletic subgroup with other strains of the DWV-A, and the similarity of the two isolates was only 89% with DWV-A sequences. The 3C-pro and RdRp regions of the DWV genome from A. florea and E. sinhai tend to be highly conserved among the RNA viruses [42]. Therefore, they have usually been used as a reliable protein for the construction of phylogenetic trees and for the classification of subtypes of RNA viruses [42,43,44]. In the present study, the phylogenetic analysis of 3C + RdRp showed that the two novel isolates all harbored a distinct DWV-A-clade and formed a distinct subgroup. They might share a common ancestor and could have evolved independently in secluded geographic regions.
Turning to the experimental result on the sequence homology between the isolate from A. florea and E. sinhai, the sequence similarity in the helicase coding regions was the lowest (93.5%). According to previous studies, helicase is the most highly conserved region and key recombinant hotspot of DWV genomes, but whether there is a recombination between isolates in the helicase region needs to be further explored [14,35]. Specifically, we observed an additional difference in the helicase region between isolates from A. florea and E. sinhai. As a result of all the above results combined, it was found that there was still genetic variation between the two isolates and DWV-A isolates. We have evidence that the isolates from A. florea and E. sinhai are both new variants of DWV. In fact, at least a slight sequence difference in the genome, or the substitution of a few amino acids, is enough to produce different toxicities in certain types of viruses [45]. In the future, artificial infection experiments using infectious clones of variants will be needed to verify these possibilities. In this study, a high prevalence of DWV was observed in A. florea without apparent symptoms and in E. sinhai samples. It can also be observed that the nucleotide and amino acid sequences between the A. florea and E. sinhai DWV isolates were highly identical. From the above result, we can determine the pathogenic correlation between mites and honeybees, but there is not yet strong enough evidence of which is the original virus carrier. Previous studies have indicated that A. florea and A. mellifera have been found to prey on honey sources from hives, and on top of that, A. florea carries the ectoparasitic mite E. sinhai and has also been shown to survive on A. mellifera and A. cerana [22,46]. In Thailand, E. sinhai has been found in the nests of A. mellifera [46]. The results of this study found a novel DWV variant from samples of A. florea and E. sinhai. The V. destructor, which was originally transferred from A. cerana to A. mellifera, contributed to a significant increase in the prevalence of DWV in the honeybee colony, a decrease in the diversity of the deformed wing virus, and the dominance of a single DWV genotype [47].
The presence of different species of bees and their associated parasitic mites in sympatric areas may facilitate the exchange of parasites between them and the simultaneous infestation of multiple mites at the population or individual level [48,49]. The dwarf honeybee A. florea and its acquired mite E. sinhai are invasive species in Taiwan and several other countries [50,51], and it is also worth noting that they carry new DWV variants. However, few studies have evaluated the influences of Euvarroa mites on honeybees. The life cycle and feeding behavior of E. sinhai are ecologically similar to the V. destructor [23]. As far as E. sinhai is concerned, it may be a dominant pathogen transmitter and lead to a chain reaction of cross-infection in the overlapping niches of sympatric honeybee species, which indirectly affects the evolution of the virus [52,53,54], such as the mite Tropilaelaps mercedesae and V. destructor [55,56].To sum up, based on the comparison results of nucleotide and amino acid sequences in each coding gene region and the nucleotide sequences in the non-translated region, it can first be concluded that the virus has high genetic diversity traits when targeting different hosts in A. mellifera and A. florea [57], and secondly, the phenomenon of high homology between the virus isolates of honeybees and their ectoparasitic mites was found.
5. Conclusions
Our data indicate that the new virus variants are suspected to be carried by invasive A. florea and its ectoparasitic mite E. sinhai. Moreover, mites might be a biological vector and open up new avenues of inter-taxa virus transmission. Future research should consider the potential effects of new virus strains more carefully, and it should be investigated whether it has become a new variant of the deformed wing virus so as to establish the invasion risk level of the invasive A. florea and avoid endangering the apiculture of Taiwan and the world.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects14020103/s1: Figure S1: An open nest of Apis florea. Figure S2: Ventral view of an Euvarroa sinhai. Figure S3: Sampling locations of A. florea in Kaohsiung City. Samples collected from the nest and open space are shown in blue and red spots, respectively. Figure S4: Pairwise alignment of DWV helicase encoding domain between ES01TFS11 and AF01T243. Table S1: Detailed information of collection Table S2: sequence of the two novel isolates, AF01T243 and ES01TFS11.
Author Contributions
Designed the research, I.-H.S., W.-S.T., and J.-X.T.; conducted field samplings and experiments, J.-X.T. and I.-H.S.; analyzed data, I.-H.S., W.-S.T., and J.-X.T.; wrote the draft manuscript, J.-X.T. and I.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan, Taiwan. Grant: Scheme of the Strengthening of Plant Pest Management System, BAPHIQ (107-111) Rescue Adjustment-Q-01 & 02 (Z); Undergraduate research fellowship, Ministry of Science and Technology (MOST), Taiwan (MOST 109-2813-C-415-013-B).
Data Availability Statement
The viral polyprotein sequence of DWV isolates in this study were submitted to GenBank with an accession number (OP889266-OP889269).
Acknowledgments
We are grateful to Y.-C. Lin for her collaboration during preliminary investigations and sample collections and grateful to C.-H. Chao for helping provide the photograph, and grateful to Y.-S. Nai for his helpful comments on the first draft of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Brettell, L.E. Deformed wing virus in honeybees and other insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Koziy, R.V.; Wood, S.C.; Kozii, I.V.; van Rensburg, C.J.; Moshynskyy, I.; Dvylyuk, I.; Simko, E. Deformed wing virus infection in honey bees (Apis mellifera L.). Vet. Pathol. 2019, 56, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.I.; Buttstedt, A. pH-dependent stability of honey bee (Apis mellifera) major royal jelly proteins. Sci. Rep. 2019, 9, 9014. [Google Scholar] [CrossRef]
- Lauring, A.S.; Andino, R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010, 6, e1001005. [Google Scholar] [CrossRef]
- Vignuzzi, M.; Stone, J.K.; Arnold, J.J.; Cameron, C.E.; Andino, R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 2006, 439, 344–348. [Google Scholar] [CrossRef]
- Fujiyuki, T.; Takeuchi, H.; Ono, M.; Ohka, S.; Sasaki, T.; Nomoto, A.; Kubo, T. Novel insect Picorna-like virus identified in the brains of aggressive worker honeybees. J. Virol. 2004, 78, 1093–1100. [Google Scholar] [CrossRef]
- Lamp, B.; Url, A.; Seitz, K.; Eichhorn, J.; Riedel, C.; Sinn, L.J.; Indik, S.; Köglberger, H.; Rümenapf, T. Construction and rescue of a molecular clone of Deformed wing virus (DWV). PLoS ONE 2016, 11, e0164639. [Google Scholar] [CrossRef]
- Levin, S.; Sela, N.; Chejanovsky, N. Two novel viruses associated with the Apis mellifera pathogenic mite Varroa destructor. Sci. Rep. 2016, 6, 37710. [Google Scholar] [CrossRef]
- Levin, S.; Sela, N.; Erez, T.; Nestel, D.; Pettis, J.; Neumann, P.; Chejanovsky, N. New viruses from the ectoparasite mite Varroa destructor infesting Apis mellifera and Apis cerana. Viruses 2019, 11, 94. [Google Scholar] [CrossRef]
- Brettell, L.E.; Schroeder, D.C.; Martin, S.J. RNAseq analysis reveals virus diversity within hawaiian apiary insect communities. Viruses 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.S.; Kevill, J.L.; Correia-Oliveira, M.E.; de Carvalho, C.A.L.; Martin, S.J. Occurrence of Deformed wing virus variants in the stingless bee Melipona subnitida and honey bee Apis mellifera populations in Brazil. J. Gen. Virol. 2019, 100, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, M.; Papach, A.; Cristina, E.; Yañez, O.; Williams, G.R.; Neumann, P. Deformed wings of small hive beetle independent of virus infections and mites. J. Invertebr. Pathol. 2020, 172, 107365. [Google Scholar] [CrossRef]
- Lanzi, G.; de Miranda, J.R.; Boniotti, M.B.; Cameron, C.E.; Lavazza, A.; Capucci, L.; Camazine, S.M.; Rossi, C. Molecular and biological characterization of Deformed wing virus of honeybees (Apis mellifera L.). J. Virol. 2006, 80, 4998–5009. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Hammond, J.; Hsu, H.T.; Evans, J.; Feldlaufer, M. Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J. Invertebr. Pathol. 2004, 87, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.; Feldlaufer, M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006, 92, 152–159. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Wilfert, L.; Martin, S.J.; Jones, I.M.; Schroeder, D.C. Diversity in a honey bee pathogen: First report of a third master variant of the deformed wing virus quasispecies. ISME J. 2016, 10, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Zewdu, A.; Desalegn, B.; Amssalu, B.; Tolera, K.; Gebreamlak, B. Assessment of alien honeybee species (Apis florea) in North West and Northern Ethiopia. Greener J. Agric. Sci. 2016, 6, 093–101. [Google Scholar] [CrossRef]
- Silva, D.P.; Castro, A.C.F.; Vilela, B.; Ong, X.R.; Thomas, J.C.; Alqarni, A.S.; Engel, M.S.; Ascher, J.S. Colonizing the east and the west: Distribution and niche properties of a dwarf Asian honey bee invading Africa, the Middle East, the Malay Peninsula, and Taiwan. Apidologie 2020, 51, 75–87. [Google Scholar] [CrossRef]
- Hsu, P.S.; Wu, T.H.; Tian, J.X.; Sung, I.H. Origins and invasion characteristics of the recently introduced dwarf honeybee Apis florea Fabricius, 1787 (Hymenoptera, Apidae) in Taiwan. Bioinvasions Rec. 2022, 11, 124–135. [Google Scholar] [CrossRef]
- Delfinado, M.D.; Baker, E.W. Varroidae, a new family of mites on honey bees (Mesostigmata: Acarina). J. Wash. Acad. Sci. 1974, 64, 4–10. [Google Scholar]
- Koeniger, N.; Koeniger, G.; de Guzman, L.I.; Lekprayoon, C. Survival of Euvarroa sinhai Delfinado and Baker (Acari, Varroidae) on workers of Apis cerana Fabr, Apis florea Fabr and Apis mellifera L. in cages. Apidologie 1993, 24, 403–410. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Delfinado-Baker, M. Observations on mites of the Asian honeybee species (Apis cerana, Apis dorsata, Apis florea). Apidologie 1983, 14, 197–204. [Google Scholar] [CrossRef]
- Mossadegh, M.S. Geographical distribution, levels of infestation and population density of the mite Euvarroa sinhai Delfinado and Baker (Acarina: Mesostigmata) in Apis florea F Colonies in Iran. Apidologie 1991, 22, 127–134. [Google Scholar] [CrossRef]
- Zhang, X.; He, S.Y.; Evans, J.D.; Pettis, J.S.; Yin, G.F.; Chen, Y.P. New evidence that Deformed wing virus and Black queen cell virus are multi-host pathogens. J. Invertebr. Pathol. 2012, 109, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Ken, T.; Berthoud, H.; Neumann, P. The ectoparasitic mite Tropilaelaps mercedesae (Acari, Laelapidae) as a vector of honeybee viruses. Insectes Soc. 2009, 56, 40–43. [Google Scholar] [CrossRef]
- Schöning, C.; Gisder, S.; Geiselhardt, S.; Kretschmann, I.; Bienefeld, K.; Hilker, M.; Genersch, E. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. J. Exp. Biol. 2012, 215, 264–271. [Google Scholar] [CrossRef]
- di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Gómez-moracho, T.; Heeb, P.; Lihoreau, M. Effects of parasites and pathogens on bee cognition. Ecol. Entomol. 2017, 42, 51–64. [Google Scholar] [CrossRef]
- Ngor, L.; Palmer-Young, E.C.; Burciaga Nevarez, R.; Russell, K.A.; Leger, L.; Giacomini, S.J.; Pinilla-Gallego, M.S.; Irwin, R.E.; McFrederick, Q.S. Cross-infectivity of honey and bumble bee-associated parasites across three bee families. Parasitology 2020, 147, 1290–1304. [Google Scholar] [CrossRef]
- Seeley, T.D.; Smith, M.L. Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 2015, 46, 716–727. [Google Scholar] [CrossRef]
- Wu, M.C.; Lu, T.H.; Lu, K.H. PCR-RFLP of mitochondrial DNA reveals two origins of Apis mellifera in Taiwan. Saudi J. Biol. Sci. 2017, 24, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Wilfinger, W.; Kennedy, A.; Rymaszewski, M.; Mackey, K. RNAzol® RT: A new single-step method for isolation of RNA. Nat. Methods 2010, 7, 4–5. [Google Scholar] [CrossRef]
- Chen, Y. The influence of RNA integrity on the detection of honey bee viruses: Molecular assessment of different sample storage methods. J. Apic. Res. 2007, 81–87. [Google Scholar] [CrossRef]
- Zioni, N.; Soroker, V.; Chejanovsky, N. Replication of Varroa destructor virus 1 (VDV-1) and a Varroa destructor virus 1-Deformed wing virus recombinant (VDV-1-DWV) in the head of the honey bee. Virology 2011, 417, 106–112. [Google Scholar] [CrossRef]
- Ye, J.; McGinnis, S.; Madden, T.L. BLAST: Improvements for better sequence analysis. Nucleic Acids Res. 2006, 34, 6–9. [Google Scholar] [CrossRef]
- Al-Abbadi, A.A.; Hassawi, D.S.; Abu-Mallouh, S.A.; Al-Mazra’awi, M.S. Novel detection of Israel acute paralysis virus and Kashmir bee virus from honeybees Apis mellifera L. (Hymenoptera: Apidae) of Jordan using reverse transcriptase PCR technique. Appl. Entomol. Zool. 2010, 45, 183–190. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Reddy, K.E.; Noh, J.H.; Choe, S.E.; Kweon, C.H.; Yoo, M.S.; Doan, H.T.T.; Ramya, M.; Yoon, B.S.; Nguyen, L.T.K.; Nguyen, T.T.D.; et al. Analysis of the complete genome sequence and capsid region of black queen cell viruses from infected honeybees (Apis mellifera) in Korea. Virus Genes 2013, 47, 126–132. [Google Scholar] [CrossRef]
- de Miranda, J.R.; Brettell, L.E.; Chejanovsky, N.; Childers, A.K.; Dalmon, A.; Deboutte, W.; de Graaf, D.C.; Doublet, V.; Gebremedhn, H.; Genersch, E.; et al. Cold case: The disappearance of Egypt bee virus, a fourth distinct master strain of Deformed wing virus linked to honeybee mortality in 1970′s Egypt. Virol. J. 2022, 19, 12. [Google Scholar] [CrossRef]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; de Miranda, J.R.; Ryabov, E. ICTV virus taxonomy profile: Iflaviridae. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.C.; Schroeder, D.C. The use of RNA-dependent RNA polymerase for the taxonomic assignment of Picorna-like viruses (order Picornavirales) infecting Apis mellifera L. populations. Virol. J. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Culley, A.I.; Lang, A.S.; Suttle, C.A. High diversity of unknown Picorna-like viruses in the sea. Nature 2003, 424, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Wolf, Y.I.; Nagasaki, K.; Dolja, V.V. The big bang of Picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat. Rev. Microbiol. 2008, 6, 925–939. [Google Scholar] [CrossRef]
- Fujiyuki, T.; Ohka, S.; Takeuchi, H.; Ono, M.; Nomoto, A.; Kubo, T. Prevalence and phylogeny of Kakugo virus, a novel insect Picorna-like virus that infects the honeybee (Apis mellifera L.), under various colony conditions. J. Virol. 2006, 80, 11528–11538. [Google Scholar] [CrossRef] [PubMed]
- Kavinseksan, B.; Wongsiri, S. Grooming behavior of Apis dorsata Fabricius, Thai commercial, and Primorsky honey bees (Apis mellifera Linnaeus) to the bee mite Euvarroa sinhai Delfinado & Baker. J. Asia Pac. Entomol. 2016, 19, 359–363. [Google Scholar] [CrossRef]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef]
- Chantawannakul, P.; de Guzman, L.I.; Li, J.; Williams, G.R. Parasites, pathogens, and pests of honeybees in Asia. Apidologie 2016, 47, 301–324. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Haddad, N.; Bataieneh, A.; Shalmon, B.; Hefetz, A. Invasion of the dwarf honeybee Apis florea into the Near East. Biol. Invasions 2010, 12, 1093–1099. [Google Scholar] [CrossRef]
- El-Niweiri, M.A.A.; Moritz, R.F.A.; Lattorff, M.G.H. The invasion of the dwarf honeybee, Apis florea, along the River Nile in Sudan. Insects 2019, 10, 405. [Google Scholar] [CrossRef]
- Abou-Shaara, H.; Mahfouz, H.; Owayss, A. Species distribution modeling of potential invasion of dwarf honey bee, Apis florea Fab., to Africa and Europe after occurrence in Egypt in view of climatic changes. J. Plant Prot. Pathol. 2021, 12, 609–614. [Google Scholar] [CrossRef]
- Manley, R.; Temperton, B.; Doyle, T.; Gates, D.; Hedges, S.; Boots, M.; Wilfert, L. Knock-on community impacts of a novel vector: Spillover of emerging DWV-B from Varroa-infested honeybees to wild bumblebees. Ecol. Lett. 2019, 22, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed wing virus: Replication and viral load in mites (Varroa destructor). J. Gen. Virol. 2009, 90, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Möckel, N.; Gisder, S.; Genersch, E. Horizontal transmission of Deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 2011, 92, 370–377. [Google Scholar] [CrossRef]
- Khongphinitbunjong, K.; Neumann, P.; Chantawannakul, P.; Williams, G.R. The ectoparasitic mite Tropilaelaps mercedesae reduces western honey bee, Apis mellifera, longevity and emergence weight, and promotes Deformed wing virus infections. J. Invertebr. Pathol. 2016, 137, 38–42. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A virulent strain of Deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef]
- Reddy, K.E.; Thu, H.T.; Yoo, M.S.; Ramya, M.; Reddy, B.A.; Kim Lien, N.T.; Phuong Trang, N.T.; Thuy Duong, B.T.; Lee, H.J.; Kang, S.W.; et al. Comparative genomic analysis for genetic variation in Sacbrood virus of Apis cerana and Apis mellifera honeybees from different regions of Vietnam. J. Insect Sci. 2017, 17, 101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).