Differential Production of Nitric Oxide and Hydrogen Peroxide among Drosophila melanogaster, Apis mellifera, and Mamestra brassicae Immune-Activated Hemocytes after Exposure to Imidacloprid and Amitraz
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Hemocyte Extraction
2.3. Culture Medium and Maintenance
2.4. Exposures
2.5. Viability Assay
2.6. Nitric Oxide Quantification
2.7. Hydrogen Peroxide Assay
2.8. Statistical Analysis
3. Results
3.1. Immune Activation Alleviates the Effect of Pesticides on Hemocyte Viability
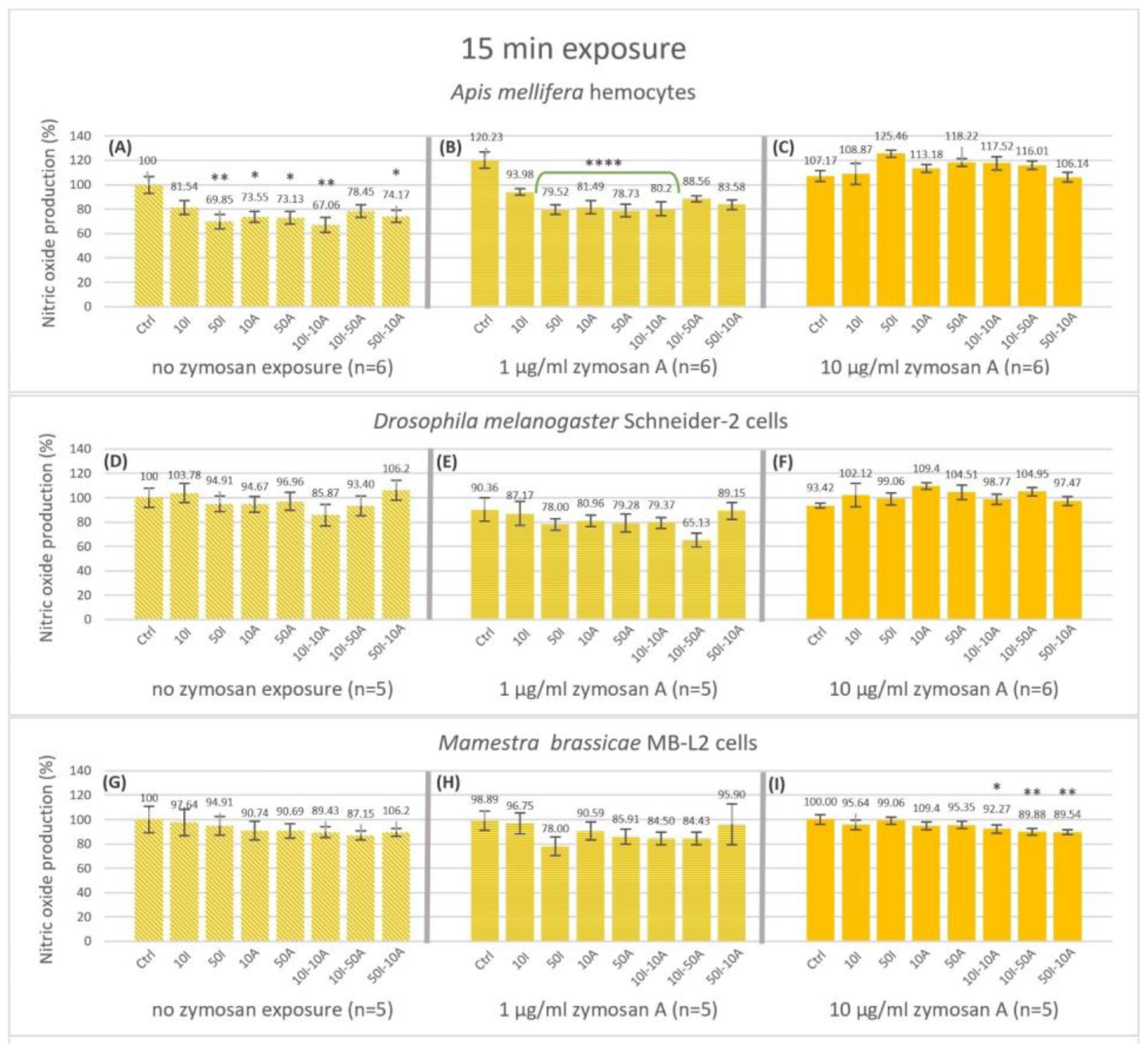
3.2. Nitric Oxide Endpoint Production
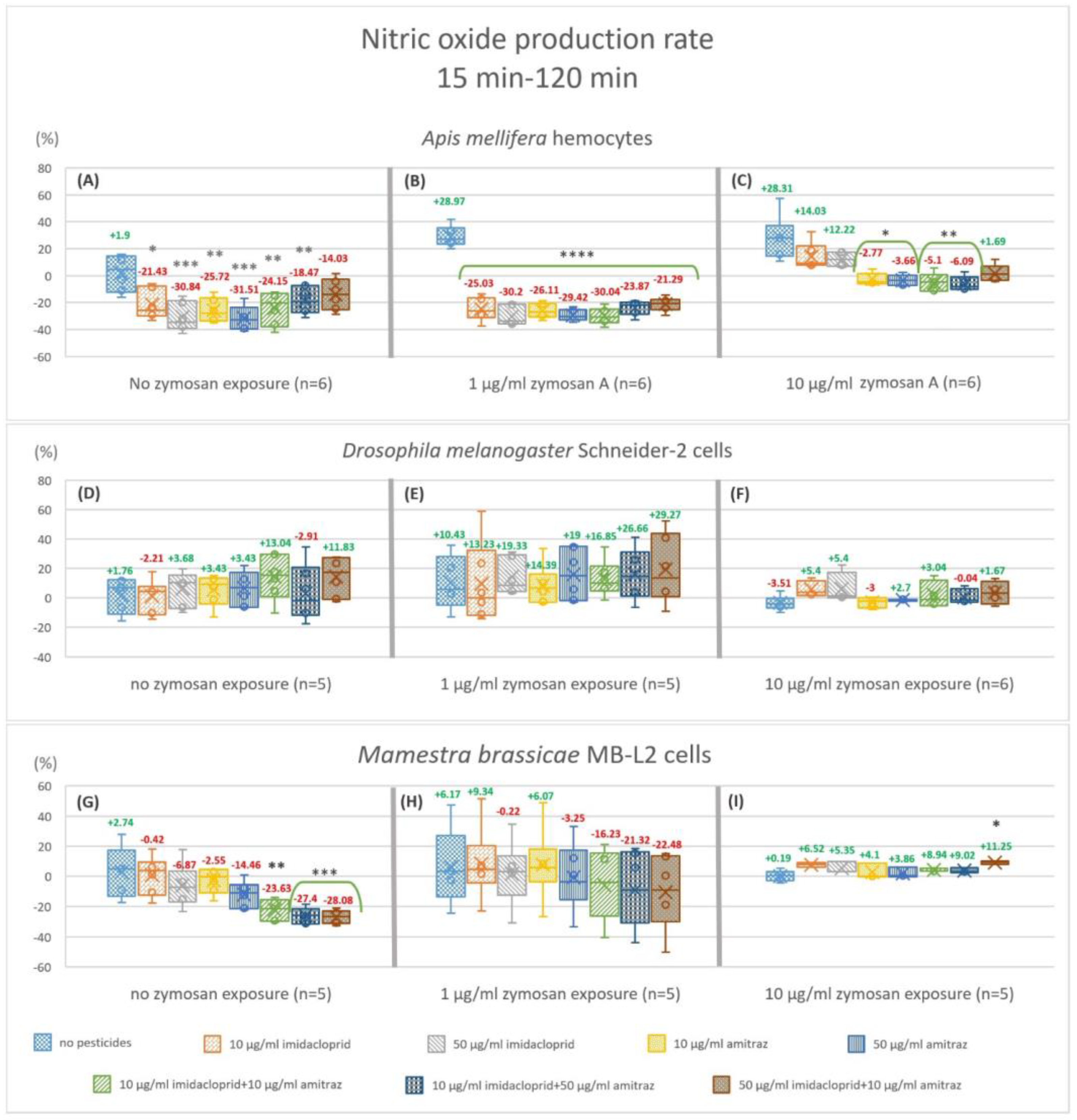
3.3. Nitric Oxide Production Rate
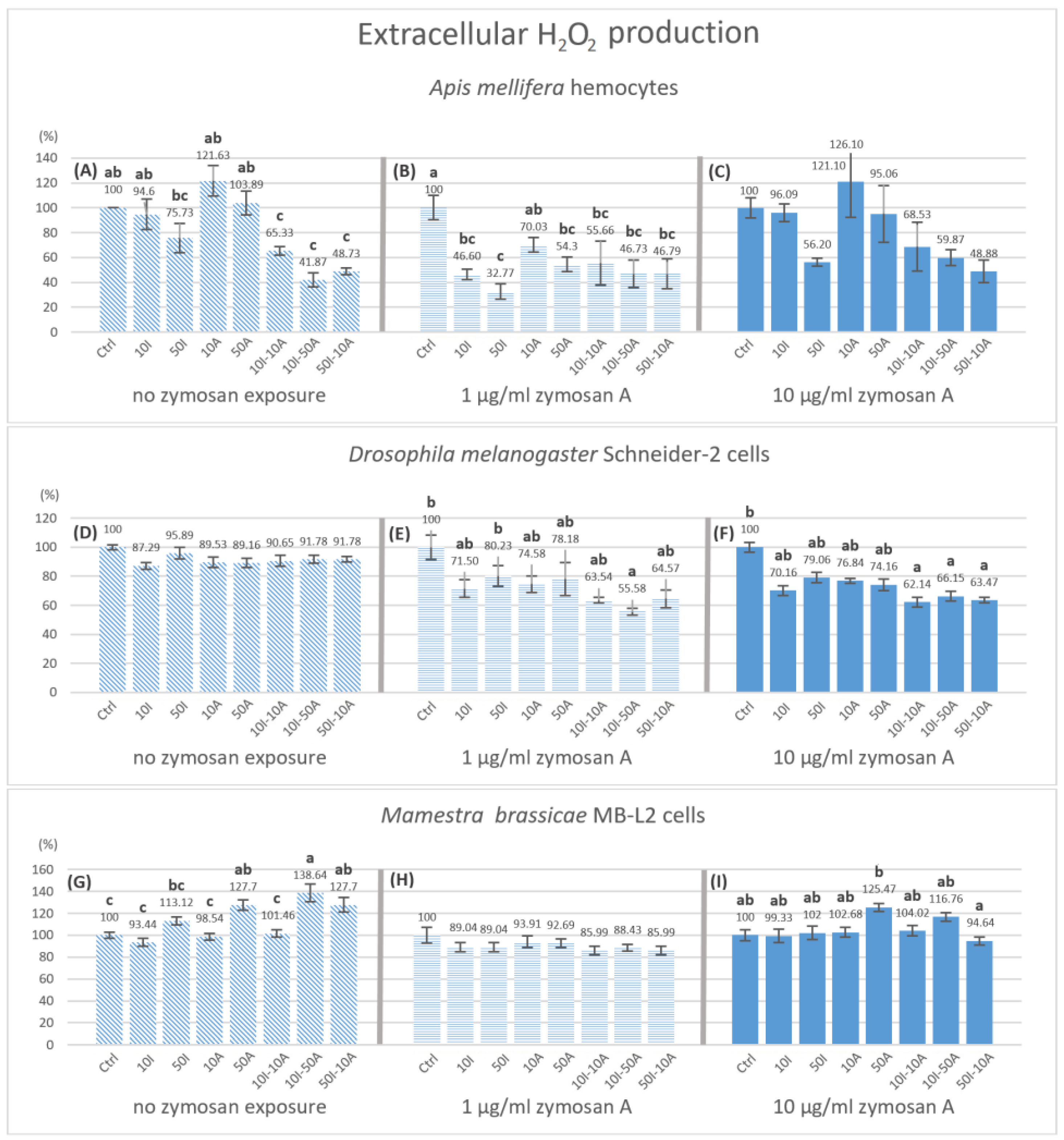
3.4. Extracellular Hydrogen Peroxide Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Harwood, G.P.; Dolezal, A.G. Pesticide–Virus Interactions in Honey Bees: Challenges and Opportunities for Understanding Drivers of Bee Declines. Viruses 2020, 12, 566. [Google Scholar] [CrossRef]
- James, R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.T.; Brewster, C.C.; Bloomquist, J.R.; Anderson, T.D. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J. Invertebr. Pathol. 2017, 149, 119–126. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A. A Short Guide to the Sixth Mass Extinction—Is the Anthropocene an Extended Suicide? Rev. Educ. 2022, 395, 27–41. Available online: https://revistaeducacion.org/EDU/journals/published/1628853580_j47Wb.pdf (accessed on 14 December 2022).
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Allen-Wardell, G.; Bernhardt, P.; Bitner, R.; Burquez, A.; Buchmann, S.; Cane, J.; Cox, P.A.; Dalton, V.; Feinsinger, P.; Ingram, M.; et al. The Potential Consequences of Pollinator Declines on the Conservation of Biodiversity and Stability of Food Crop Yields. Conserv. Biol. 1998, 12, 8–17. [Google Scholar]
- Wei, N.; Kaczorowski, R.L.; Arceo-Gómez, G.; O’Neill, E.M.; Hayes, R.A.; Ashman, T.-L. Pollinators contribute to the maintenance of flowering plant diversity. Nature 2021, 597, 688–692. [Google Scholar] [CrossRef]
- Allsopp, M.H.; De Lange, W.J.; Veldtman, R. Valuing Insect Pollination Services with Cost of Replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef]
- Oldroyd, B.P. What’s Killing American Honey Bees? PLoS Biol. 2007, 5, e168. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Münchener Bienenzeitung 1909, 31, 196–204. [Google Scholar]
- Fries, I. Nosema Apis—A Parasite in the Honey Bee Colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 2013, 52, 1–17. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ong’Amo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu. Rev. Èntomol. 2018, 63, 125–144. [Google Scholar] [CrossRef]
- Macaulay, S.J.; Hageman, K.J.; Piggott, J.J.; Matthaei, C.D. Time-cumulative effects of neonicotinoid exposure, heatwaves and food limitation on stream mayfly nymphs: A multiple-stressor experiment. Sci. Total Environ. 2021, 754, 141941. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects. Annu. Rev. Èntomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [Green Version]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Arbi, M.; Pouliliou, S.; Lampropoulou, M.; Marmaras, V.J.; Tsakas, S. Hydrogen peroxide is produced by E. coli challenged haemocytes and regulates phagocytosis, in the medfly Ceratitis capitata. The active role of superoxide dismutase. Dev. Comp. Immunol. 2011, 35, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Quintana, S.; Maggi, M.; Szawarski, N.; LaMattina, L.; Eguaras, M. Apis mellifera hemocytes generate increased amounts of nitric oxide in response to wounding/encapsulation. Apidologie 2014, 45, 10–22. [Google Scholar] [CrossRef]
- Bartling, M.T.; Thümecke, S.; Russert, J.H.; Vilcinskas, A.; Lee, K.-Z. Exposure to low doses of pesticides induces an immune response and the production of nitric oxide in honeybees. Sci. Rep. 2021, 11, 6819. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.; Correa-Aragunde, N.; Brasesco, C.; Eguaras, M.; LaMattina, L. Nitric oxide participates at the first steps of Apis mellifera cellular immune activation in response to non-self recognition. Apidologie 2013, 44, 575–585. [Google Scholar] [CrossRef]
- Rivero, A. Nitric oxide: An antiparasitic molecule of invertebrates. Trends Parasitol. 2006, 22, 219–225. [Google Scholar] [CrossRef]
- Clifford, D.P.; Repine, J.E. Hydrogen peroxide mediated killing of bacteria. Mol. Cell. Biochem. 1982, 49, 143–149. [Google Scholar] [CrossRef]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef]
- Herrera-Ortiz, A.; Martínez-Barnetche, J.; Smit, N.; Rodriguez, M.H.; Lanz-Mendoza, H. The effect of nitric oxide and hydrogen peroxide in the activation of the systemic immune response of Anopheles albimanus infected with Plasmodium berghei. Dev. Comp. Immunol. 2011, 35, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Wang, D.; Li, H.; Xia, P.; Ran, Y.; You, J. Toxicogenomics provides insights to toxicity pathways of neonicotinoids to aquatic insect, Chironomus dilutus. Environ. Pollut. 2020, 260, 114011. [Google Scholar] [CrossRef]
- Walderdorff, L.; Laval-Gilly, P.; Bonnefoy, A.; Falla-Angel, J. Imidacloprid intensifies its impact on honeybee and bumblebee cellular immune response when challenged with LPS (lippopolysacharide) of Escherichia coli. J. Insect Physiol. 2018, 108, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.A.; Daisley, B.A.; Burton, J.P.; Reid, G. Deleterious Effects of Neonicotinoid Pesticides on Drosophila melanogaster Immune Pathways. mBio 2019, 10, e01395-19. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, B.; Kurtz, J. Immune memory in invertebrates. Semin. Immunol. 2016, 28, 328–342. [Google Scholar] [CrossRef]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the Effects of Sublethal Pesticide Exposure on Honey Bees: A Role for Probiotics as Mediators of Environmental Stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Kuraishi, T.; Hori, A.; Kurata, S. Host-microbe interactions in the gut of Drosophila melanogaster. Front. Physiol. 2013, 4, 375. [Google Scholar] [CrossRef]
- Kounatidis, I.; Ligoxygakis, P. Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biol. 2012, 2, 120075. [Google Scholar] [CrossRef] [PubMed]
- Walderdorff, L.; Laval-Gilly, P.; Wechtler, L.; Bonnefoy, A.; Falla-Angel, J. Phagocytic activity of human macrophages and Drosophila hemocytes after exposure to the neonicotinoid imidacloprid. Pestic. Biochem. Physiol. 2019, 160, 95–101. [Google Scholar] [CrossRef]
- Stothers, C.L.; Burelbach, K.R.; Owen, A.M.; Patil, N.K.; McBride, M.A.; Bohannon, J.K.; Luan, L.; Hernandez, A.; Patil, T.K.; Williams, D.L.; et al. β-Glucan Induces Distinct and Protective Innate Immune Memory in Differentiated Macrophages. J. Immunol. 2021, 207, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Harshbarger, J.C.; Heimpel, A.M. Effect of zymosan on phagocytosis in larvae of the greater wax moth, Galleria mellonella. J. Invertebr. Pathol. 1968, 10, 176–179. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Silva, M.C.L.N.; Sorgine, M.H.F. Validation of Aedes aegypti Aag-2 cells as a model for insect immune studies. Parasites Vectors 2012, 5, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Martínez, S.; Lanz-Mendoza, H.; Martínez-Barnetche, J.; Rodríguez, M.H. Antimicrobial properties of Anopheles albimanus pericardial cells. Cell Tissue Res. 2013, 351, 127–137. [Google Scholar] [CrossRef]
- Hunter, W.B. Medium for development of bee cell cultures (Apis mellifera: Hymenoptera: Apidae). Vitr. Cell. Dev. Biol. Anim. 2010, 46, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Ellis, J.D. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci. Rep. 2018, 8, 5635. [Google Scholar] [CrossRef]
- Young, F.M.; Menadue, M.F.; Lavranos, T.C. Effects of the insecticide amitraz, an α2-adrenergic receptor agonist, on human luteinized granulosa cells. Hum. Reprod. 2005, 20, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Tolnai, S. A method for viable cell count. Methods Cell Sci. 1975, 1, 37–38. [Google Scholar] [CrossRef]
- Yu, S.J. The Toxicology and Biochemistry of Insecticides; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Guo, L.; Fan, X.Y.; Qiao, X.; Montell, C.; Huang, J. An octopamine receptor confers selective toxicity of amitraz on honeybees and Varroa mites. eLife 2021, 10, e68268. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.; Ruiz, M.; García, J.M.; Frejo, M.T.; Baselga, M.J.A.; Lobo, M.; García, J.; Del Pino, J. Oxidative stress and cell death induction by amitraz and its metabolite BTS-27271 mediated through cytochrome P450 and NRF2 pathway alteration in primary hippocampal cell. Food Chem. Toxicol. 2019, 129, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Rubanov, A.; Russell, K.A.; Rothman, J.A.; Nieh, J.C.; McFrederick, Q.S. Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci. Rep. 2019, 9, 3820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Evans, J.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 2017, 12, e0187505. [Google Scholar] [CrossRef]
- Cristino, L.; Guglielmotti, V.; Cotugno, A.; Musio, C.; Santillo, S. Nitric oxide signaling pathways at neural level in invertebrates: Functional implications in cnidarians. Brain Res. 2008, 1225, 17–25. [Google Scholar] [CrossRef]
- Butolo, N.; Azevedo, P.; Alencar, L.; Malaspina, O.; Nocelli, R. Impact of low temperatures on the immune system of honeybees. J. Therm. Biol. 2021, 101, 103082. [Google Scholar] [CrossRef]
- Borsuk, G.; Ptaszyńska, A.A.; Olszewski, K.; Domaciuk, M.; Krutmuang, P.; Paleolog, J. A New Method for Quick and Easy Hemolymph Collection from Apidae Adults. PLoS ONE 2017, 12, e0170487. [Google Scholar] [CrossRef]
- Bruckner, S.; Straub, L.; Neumann, P.; Williams, G.R. Synergistic and Antagonistic Interactions Between Varroa destructor Mites and Neonicotinoid Insecticides in Male Apis mellifera Honey Bees. Front. Ecol. Evol. 2021, 9, 735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukkar, D.; Laval-Gilly, P.; Bonnefoy, A.; Malladi, S.; Azoury, S.; Kanso, A.; Falla-Angel, J. Differential Production of Nitric Oxide and Hydrogen Peroxide among Drosophila melanogaster, Apis mellifera, and Mamestra brassicae Immune-Activated Hemocytes after Exposure to Imidacloprid and Amitraz. Insects 2023, 14, 174. https://doi.org/10.3390/insects14020174
Sukkar D, Laval-Gilly P, Bonnefoy A, Malladi S, Azoury S, Kanso A, Falla-Angel J. Differential Production of Nitric Oxide and Hydrogen Peroxide among Drosophila melanogaster, Apis mellifera, and Mamestra brassicae Immune-Activated Hemocytes after Exposure to Imidacloprid and Amitraz. Insects. 2023; 14(2):174. https://doi.org/10.3390/insects14020174
Chicago/Turabian StyleSukkar, Dani, Philippe Laval-Gilly, Antoine Bonnefoy, Sandhya Malladi, Sabine Azoury, Ali Kanso, and Jairo Falla-Angel. 2023. "Differential Production of Nitric Oxide and Hydrogen Peroxide among Drosophila melanogaster, Apis mellifera, and Mamestra brassicae Immune-Activated Hemocytes after Exposure to Imidacloprid and Amitraz" Insects 14, no. 2: 174. https://doi.org/10.3390/insects14020174
APA StyleSukkar, D., Laval-Gilly, P., Bonnefoy, A., Malladi, S., Azoury, S., Kanso, A., & Falla-Angel, J. (2023). Differential Production of Nitric Oxide and Hydrogen Peroxide among Drosophila melanogaster, Apis mellifera, and Mamestra brassicae Immune-Activated Hemocytes after Exposure to Imidacloprid and Amitraz. Insects, 14(2), 174. https://doi.org/10.3390/insects14020174






