Cut to Disarm Plant Defence: A Unique Oviposition Behaviour in Rhynchites foveipennis (Coleoptera: Attelabidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
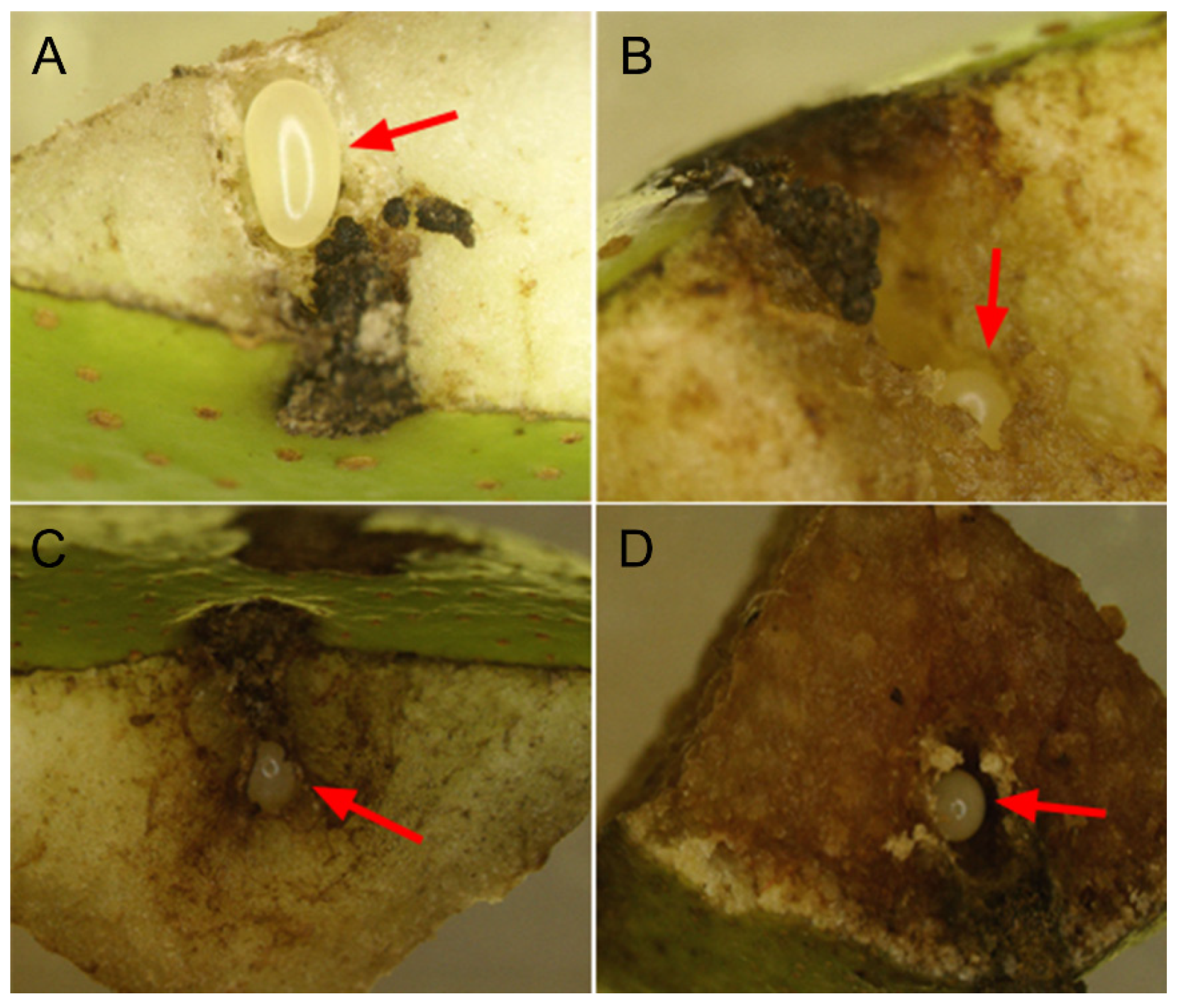
2.1. Study Site and Focal Species
2.2. Experimental Treatments
2.3. Measuring Defensive Substances
2.3.1. Tannin Content
2.3.2. Flavonoid Content
2.4. Data Analysis
3. Results
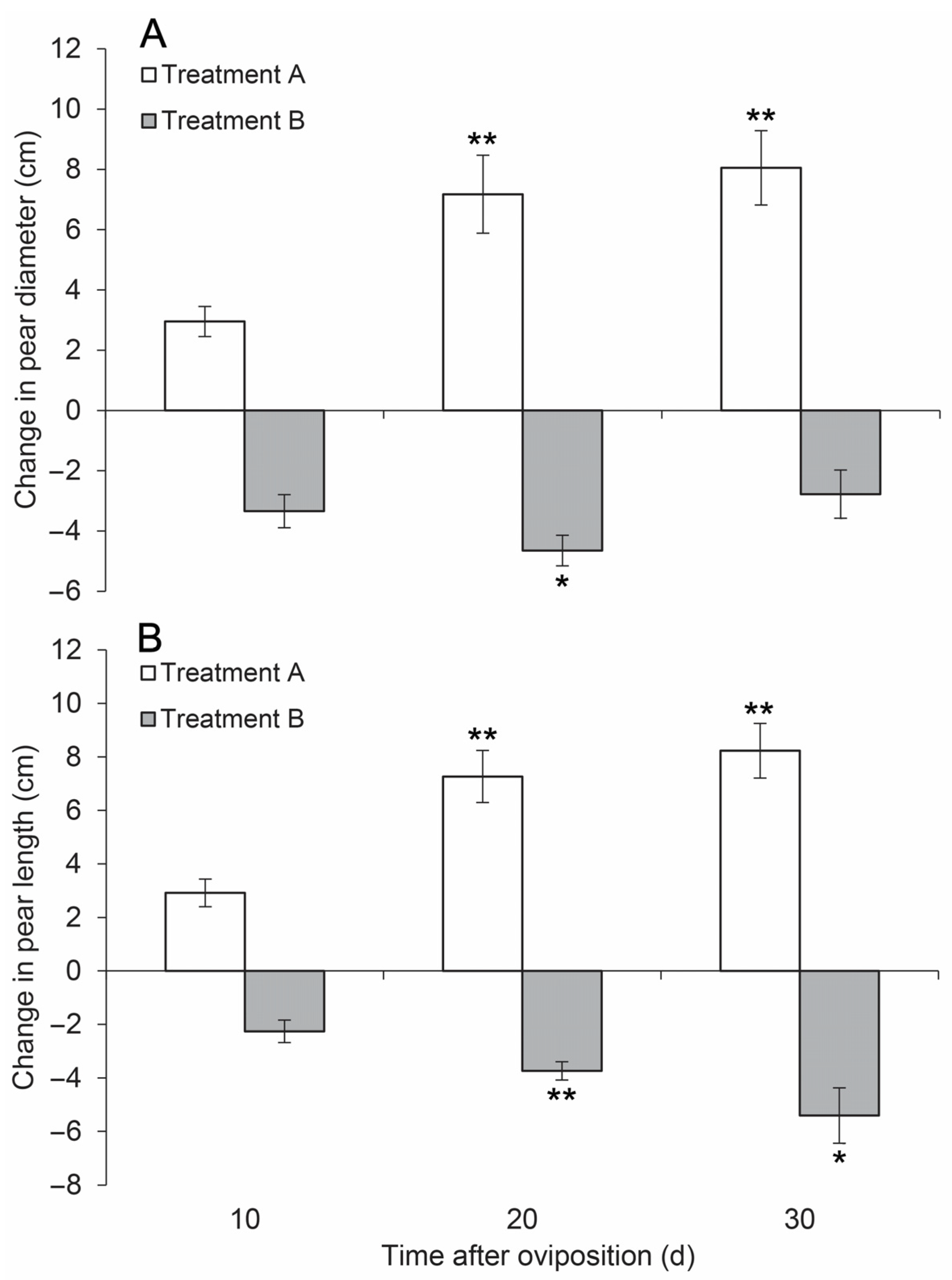
3.1. Effects on Fruit Growth
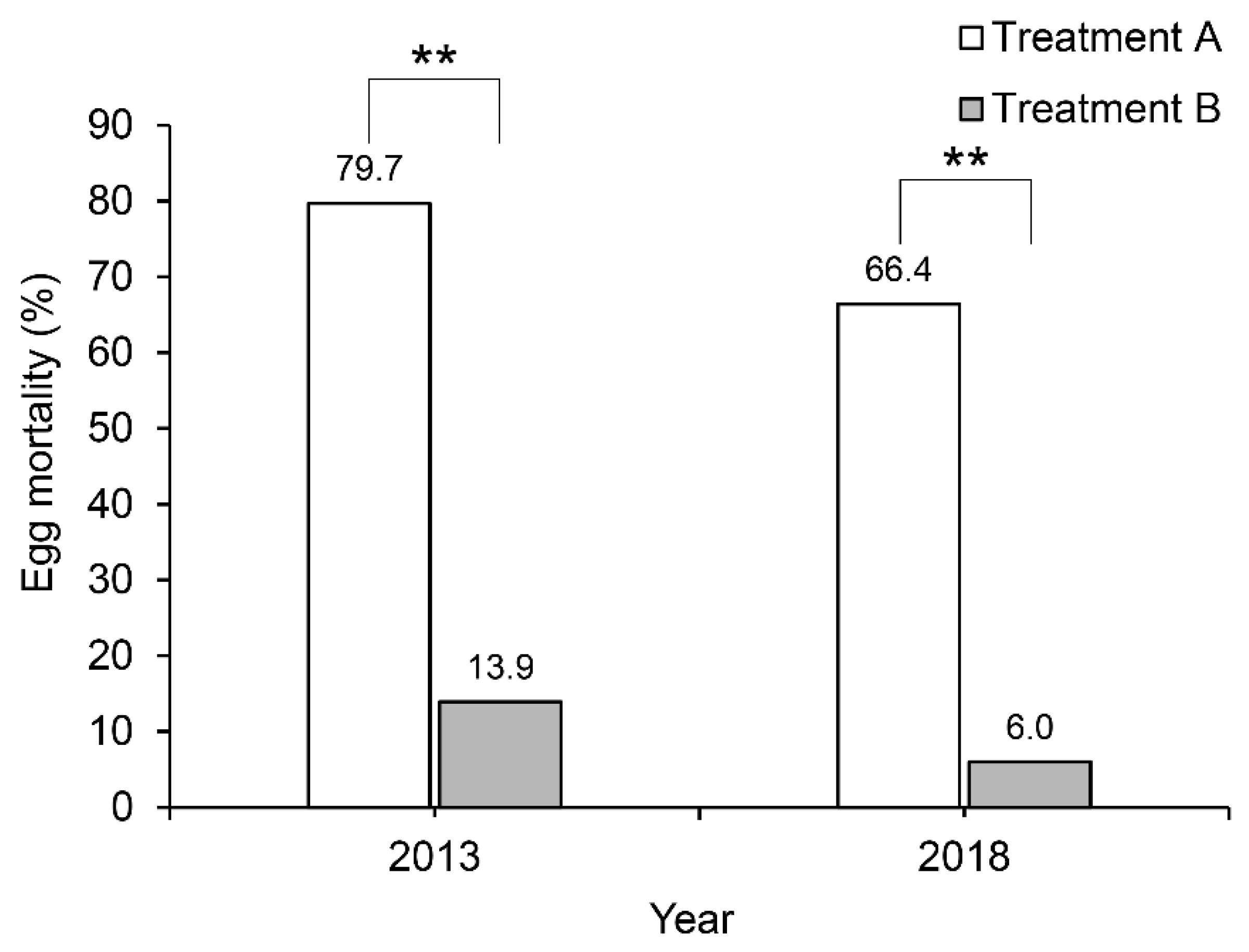
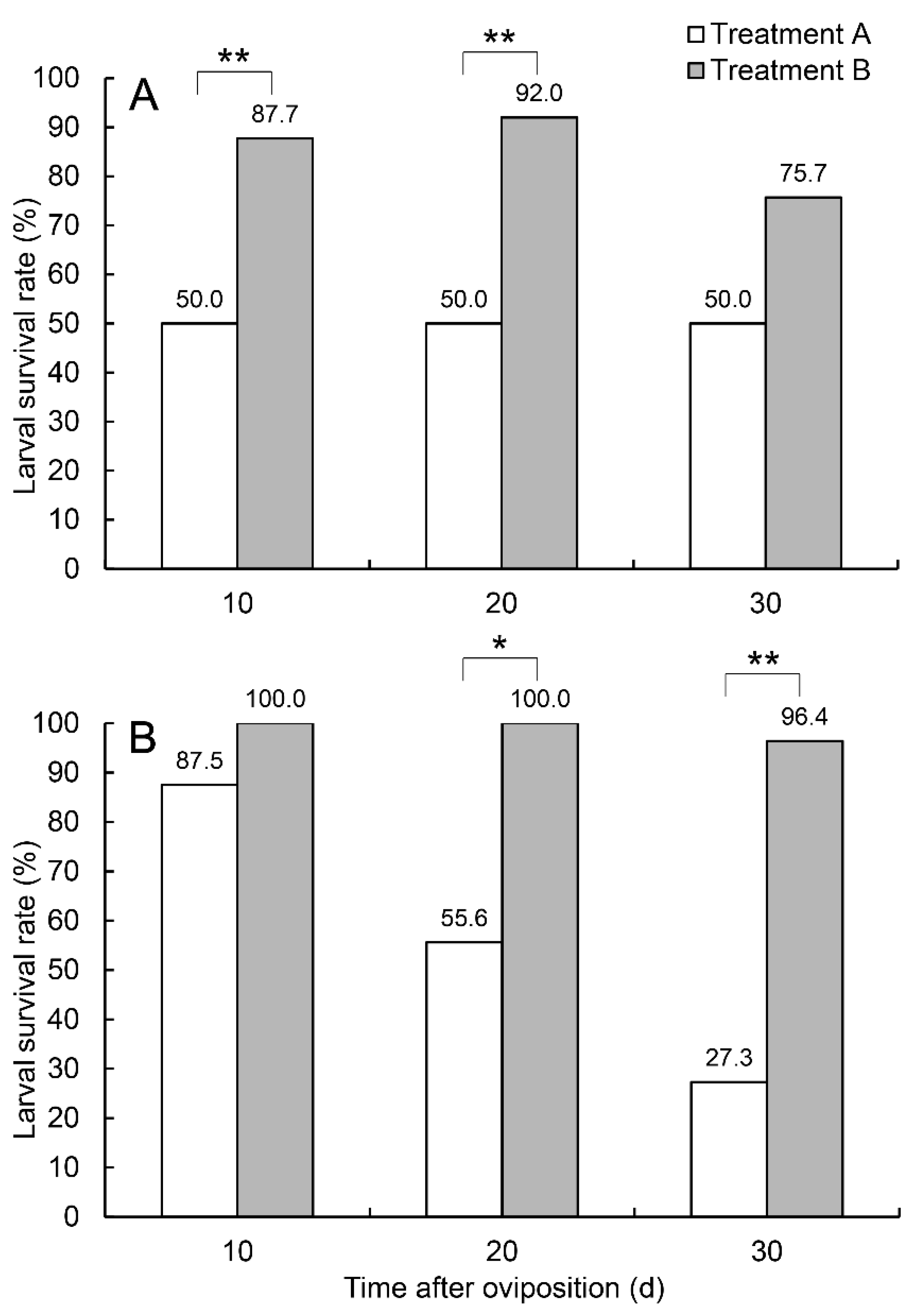
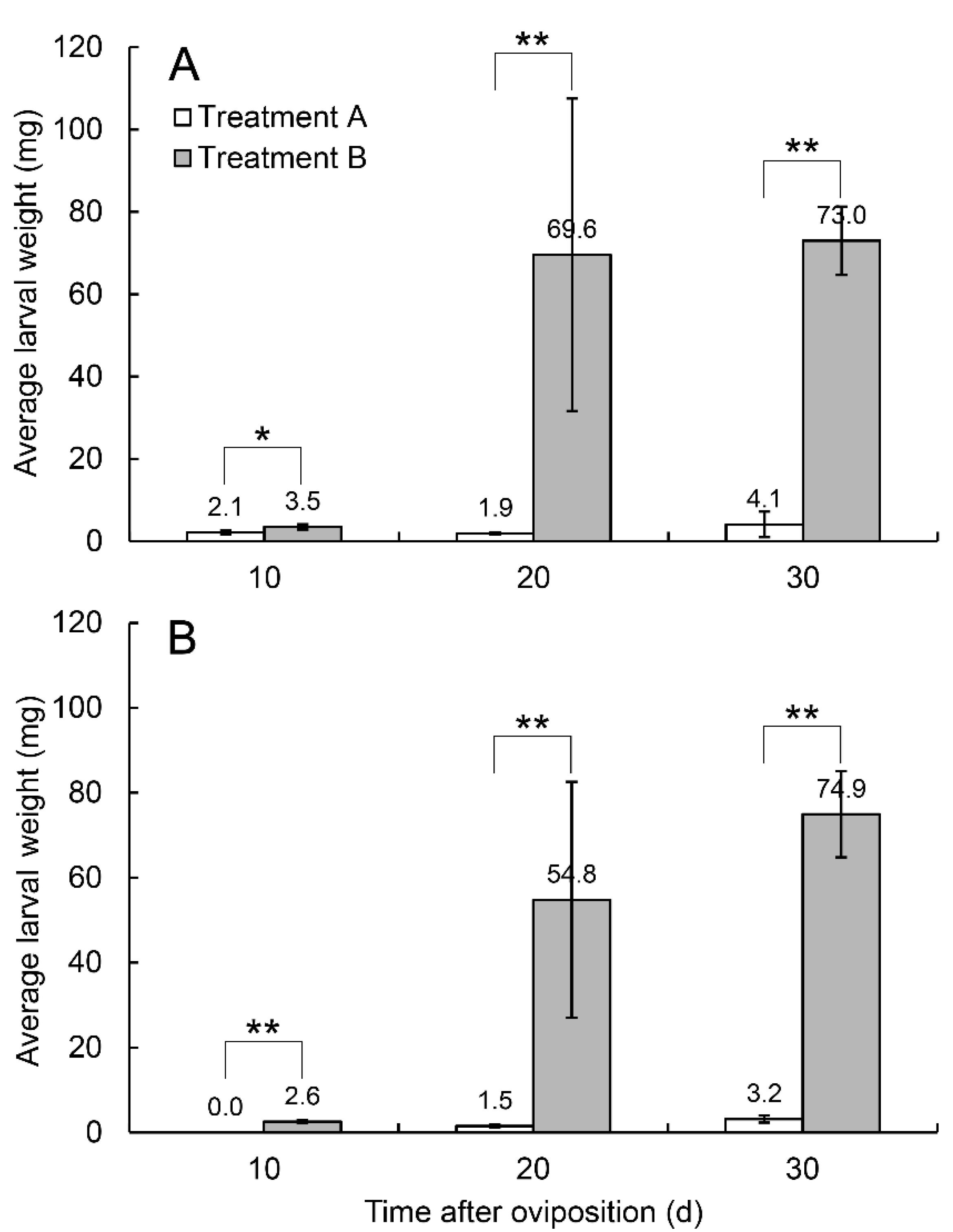
3.2. Effects on Offspring Survival Rate and Development
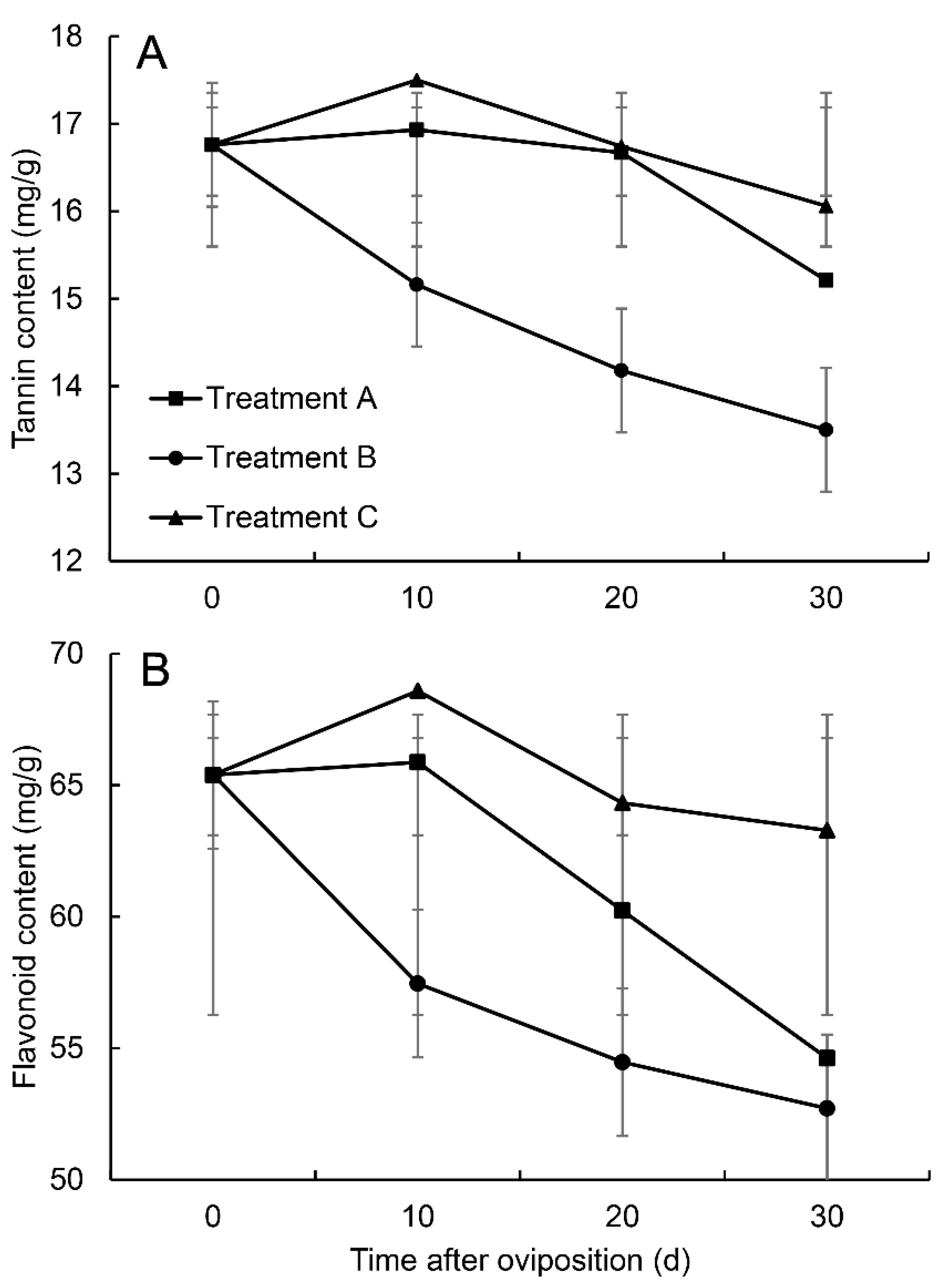
3.3. Changes in Defence Substances
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whalley, P.E.S.; Jarzembowski, E.A. A new assessment of Rhyniella, the earliest known insect, from the Devonian of Rhynie, Scotland. Nature 1981, 291, 317. [Google Scholar] [CrossRef]
- Agrawal, A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2006, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [PubMed]
- Karban, R. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Howe, G.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Bernays, E.A.; Chapman, R.E. Host-Plant Selection by Phytophagous Insects; Chapman & Hall: New York, NY, USA, 1994; p. 312. [Google Scholar]
- Dussourd, D.E. Behavioral sabotage of plant defenses by insect folivores. Annu. Rev. Entomol. 2017, 62, 15–34. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Jongsma, M.; Bolter, C. The adaptation of insects to plant protease inhibitors. J. Insect Physiol. 1997, 43, 885–895. [Google Scholar] [CrossRef]
- Jongsma, M.A.; Bakker, P.L.; Peters, J.; Bosch, D.; Stiekema, W.J. Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 8041–8045. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Koiwa, H.; Salzman, R.A.; Shade, R.E.; Ahn, J.E. Cowpea bruchid Callosobruchus maculatus uses a three-component strategy to overcome a plant defensive cysteine protease inhibitor. Insect Mol. Biol. 2003, 12, 135–145. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Bi, J.L.; Liu, T.X. Molecular strategies of plant defense and insect counter-defense. Insect Sci. 2005, 12, 3–15. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R.S. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Carriere, Y.; Roitberg, D. Evolution of host-selection behaviour in insect herbivores: Genetic variation and covariation in host acceptance within and between populations of Choristoneura rosaceana (Family: Tortricidae), the obliquebanded leadfoller. Heredity 1995, 74, 357–368. [Google Scholar] [CrossRef]
- Bernays, E.A. Evolution of feeding behavior in insect berbivores: Success seen as different ways to eat without being eaten. BioScience 1998, 48, 35–44. [Google Scholar] [CrossRef]
- Gønget, H. The Nemonychidae, Anthribidae and Attelabidae (Coleoptera) of Northern Europe; Brill: New York, NY, USA, 2003; p. 132. [Google Scholar]
- Hamilton, R.W. New life cycle data for two western North American weevils (Coleoptera: Rhynchitidae), with a summary of North American rhynchitid biology. Coleopt. Bull. 1994, 48, 331–343. [Google Scholar]
- Vogt, G.B. Leaf-rolling weevils (Coleoptera: Attelabidae), their host plants, and associated Rhynchitid weevils in North America (Canada through the Republic of Panama): Summary of a long-term field study. In Insects of Panama and Mesoamerica; Quintero, D., Aiello, A., Eds.; Oxford University Press: New York, NY, USA, 1992; pp. 392–420. [Google Scholar]
- Koo, J.W.; Roh, H.C.; Choe, J.C. Oviposition preference and offspring performance in Mechoris ursulus Roelofs (Coleoptera: Attelabidae). J. Ethol. 2003, 21, 37–43. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.J.; Zhang, Z.Y.; Geng, Y.P.; Bai, X. Oviposition preference and offspring performance of Mechoris ursulus (Coleoptera: Attelabidae) in Cyclobalanopsis glaucoides (Fagales: Fagaceae) and Quercus franchetii (Fagales: Fagaceae) in Central Yunnan, China. J. Insect Sci. 2015, 15, 19. [Google Scholar] [CrossRef]
- Kobayashi, C.; Okuyama, Y.; Kawazoe, K.; Kato, M. The evolutionary history of maternal plant-manipulation and larval feeding behaviours in attelabid weevils (Coleoptera; Curculionidae). Mol. Phylogenerics Evol. 2012, 64, 318–330. [Google Scholar] [CrossRef]
- McKenna, D.D.; Clarke, D.J.; Anderson, R.; Astrin, J.J.; Brown, S.; Chamorro, L.; Davis, S.R.; De Medeiros, B.; Del Rio, M.G.; Haran, J.; et al. Morphological and molecular perspectives on the phylogeny, evolution, and classification of weevils (Coleoptera: Curculionoidea): Proceedings from the 2016 International Weevil Meeting. Diversity 2018, 10, 64. [Google Scholar] [CrossRef]
- Chen, Y.Q. Identification of important genera and species of Attelabidae in China. For. Pest Dis. 1990, 2, 39–45. [Google Scholar]
- Guo, R.H.; Yang, M.F.; Wu, W.X. Damage caused by the pear curculio (Rhynchites foveipennis Fairmare) and its control in Liangshan Prefecture. J. Southwest Agric. Univ. 1991, 13, 582–584. [Google Scholar]
- Kim, D.S.; Lee, J.H. Egg and larval survivorship of Carposina sasakii (Lepidoptera: Carposinidae) in apple and peach and their effects on adult population dynamics in orchards. Environ. Entomol. 2002, 31, 686–692. [Google Scholar] [CrossRef]
- Julkunen-Titto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–221. [Google Scholar] [CrossRef]
- Ukoha, P.O.; Cemaluk, E.A.C.; Nnamdi, O.L.; Madus, E.P. Tannins and other phytochemical of the Samanaea saman pods and their antimicrobial activities. Afr. J. Pure Appl. Chem. 2011, 5, 237–244. [Google Scholar]
- Jia, Z.H.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Legalov, A.A. Reconstruction of the phylogeny of the rhynchitids and leaf-rolling weevils (Coleoptera, Rhynchitidae, Attelabidae) using the synap method: Communication 2. Entomol. Rev. 2005, 85, 131–136. [Google Scholar]
- Morimoto, K. Characteristics and evolution of oviposition behavior of Attelabidae. Insect J. 1964, 1, 15–21. [Google Scholar]
- Leschen, R.A.B.; Beutel, R.G. Coleoptera, Beetles. Vol. 3, Morphology and systematics (Phytophaga); De Gruyter: Berlin, Germany, 2014; p. 329. [Google Scholar]
- Becerra, J.X. Synchronous coadaptation in an ancient case of herbivory. Proc. Natl. Acad. Sci. USA 2003, 100, 12804–12807. [Google Scholar] [CrossRef]
- Tallamy, D.W. Squash beetle feeding behavior: An adaptation against induced cucurbit defenses. Ecology 1985, 66, 1574–1579. [Google Scholar] [CrossRef]
- Anderson, R.S. An evolutionary perspective on diversity in Curculionoidea. Mem. Entomol. Soc. Wash. 1995, 14, e114. [Google Scholar]
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.; Wong, S.; Arellano, C.; Gould, F. Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa. Ecol. Entomol. 2011, 36, 700–708. [Google Scholar] [CrossRef]
- Ishiguri, Y.; Toyoshima, S. Larval survival and development of the peach fruit moth, Carposina sasakii (Lepidoptera: Carposinidae), in picked and unpicked apple fruits. Appl. Etomol. Zool. 2006, 41, 685–690. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, P.C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Li, L.S.; Zhao, J.F.; Chen, M. Effect of tannic acid on nutrition and activities of detoxification enzymes and acetylcholinesterase of the fall webworm (Lepidoptera: Arctiidae). J. Insect Sci. 2020, 20, ieaa001. [Google Scholar] [CrossRef]
- Guo, S.H.; Yi, X.F. Gut bacterial composition of two Curculio species and their adaptation to high-tannin food. Acta Microbiol. Sin. 2019, 59, 657–667. [Google Scholar] [CrossRef]
| Data Pair | Substance | Day 10 | Day 20 | Day 30 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | F | P | Sum of Squares | F | P | Sum of Squares | F | P | ||
| A-B | tannin | 0.0006 | 612.3235 | <0.001 ** | 0.4673 | 312.381 | <0.001 ** | 0.1902 | 85.9985 | <0.001 ** |
| flavonoid | 0.3000 | 162.7493 | <0.001 ** | 0.0002 | 95.5981 | <0.001 ** | <0.0001 | 9.6131 | 0.0048 ** | |
| B-C | tannin | 0.0011 | 60.1820 | <0.001 ** | 0.4916 | 207.4074 | <0.001 ** | 0.4261 | 30.9729 | <0.001 ** |
| flavonoid | 0.5207 | 30.4147 | <0.001 ** | 0.0007 | 66.2448 | <0.001 ** | 0.0007 | 27.8848 | <0.001 ** | |
| C-A | tannin | 0.0001 | 3.4896 | 0.0699 | 0.0003 | 0.1184 | 0.7332 | 0.0468 | 3.3359 | 0.0802 |
| flavonoid | 0.0302 | 1.7141 | 0.1987 | 0.0001 | 13.5022 | 0.0010 ** | 0.0005 | 17.5212 | <0.001 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-Y.; Li, W.; Huang, Q.-C.; Yang, L.; Chen, X.-L.; Xiao, R.-D.; Tang, C.Q.; Hu, S.-J. Cut to Disarm Plant Defence: A Unique Oviposition Behaviour in Rhynchites foveipennis (Coleoptera: Attelabidae). Insects 2023, 14, 200. https://doi.org/10.3390/insects14020200
Zhang Z-Y, Li W, Huang Q-C, Yang L, Chen X-L, Xiao R-D, Tang CQ, Hu S-J. Cut to Disarm Plant Defence: A Unique Oviposition Behaviour in Rhynchites foveipennis (Coleoptera: Attelabidae). Insects. 2023; 14(2):200. https://doi.org/10.3390/insects14020200
Chicago/Turabian StyleZhang, Zhi-Ying, Wei Li, Qi-Chao Huang, Liu Yang, Xiao-Lan Chen, Ru-Di Xiao, Cindy Q. Tang, and Shao-Ji Hu. 2023. "Cut to Disarm Plant Defence: A Unique Oviposition Behaviour in Rhynchites foveipennis (Coleoptera: Attelabidae)" Insects 14, no. 2: 200. https://doi.org/10.3390/insects14020200
APA StyleZhang, Z.-Y., Li, W., Huang, Q.-C., Yang, L., Chen, X.-L., Xiao, R.-D., Tang, C. Q., & Hu, S.-J. (2023). Cut to Disarm Plant Defence: A Unique Oviposition Behaviour in Rhynchites foveipennis (Coleoptera: Attelabidae). Insects, 14(2), 200. https://doi.org/10.3390/insects14020200







