Effects of Species Invasion and Inundation on the Collembola Community in Coastal Mudflat Wetland from the Perspective of Functional Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Collection and Treatment of the Samples
2.3. Statistical Analyses
3. Results
3.1. Physicochemical Properties of the Soil in the Different Plots
3.2. Community Composition of Collembola
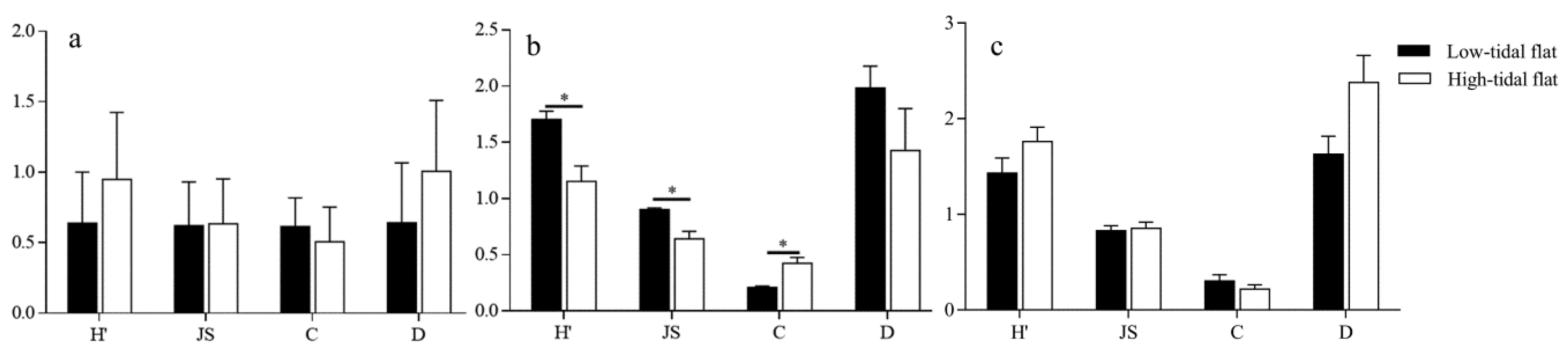
3.3. Response of Collembola Species Diversity to the Environment
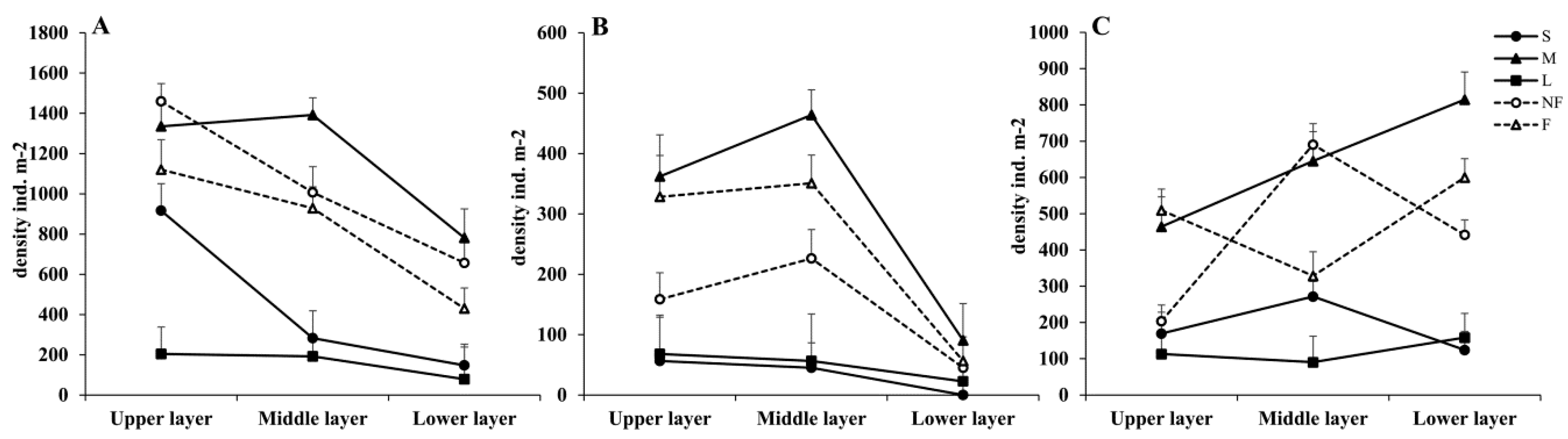
3.4. Spatial and Temporal Distribution of the Functional Traits of Collembola
3.4.1. Body Length and Furcula Length
3.4.2. Sense-Related Organs: PAO and Antennae
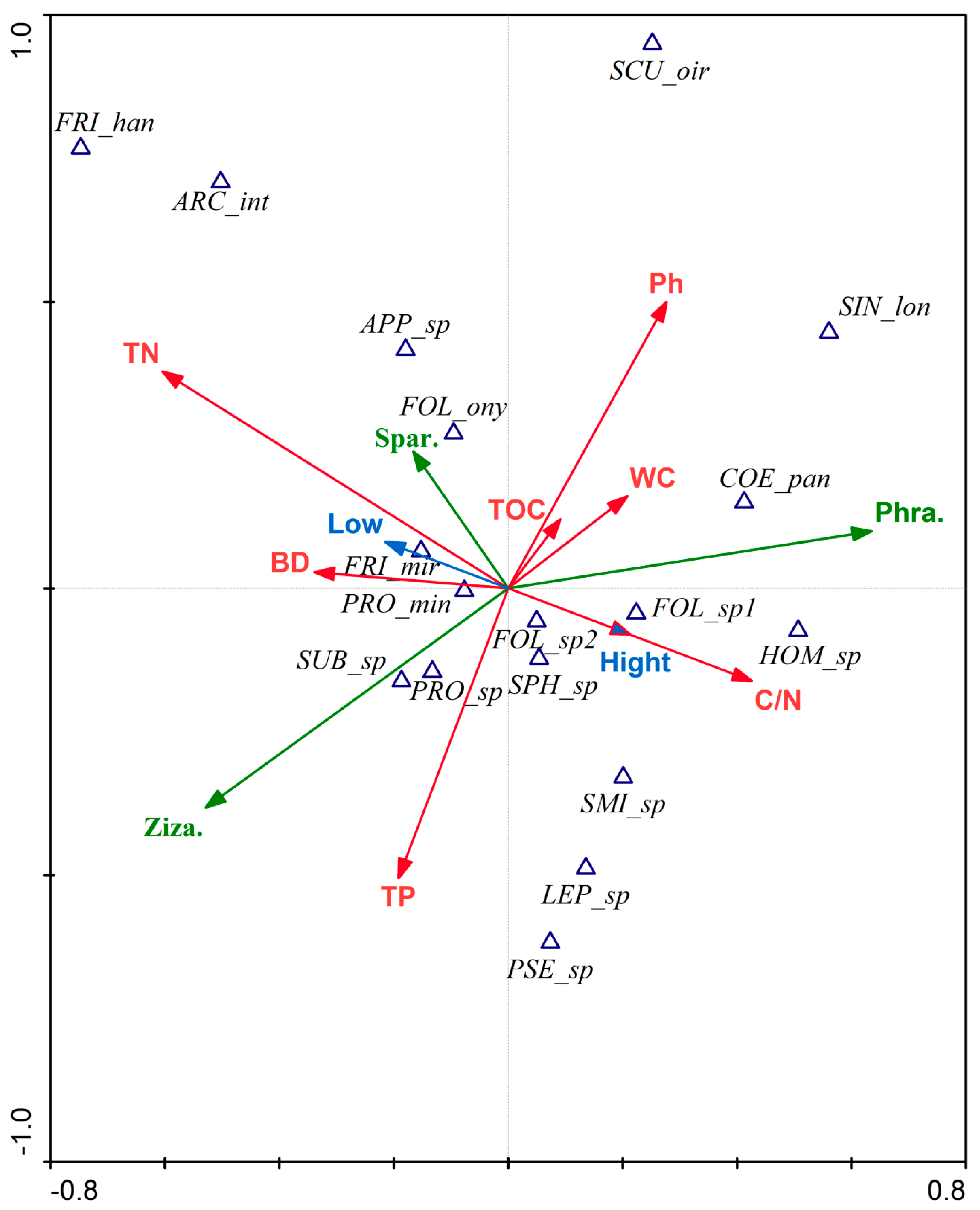
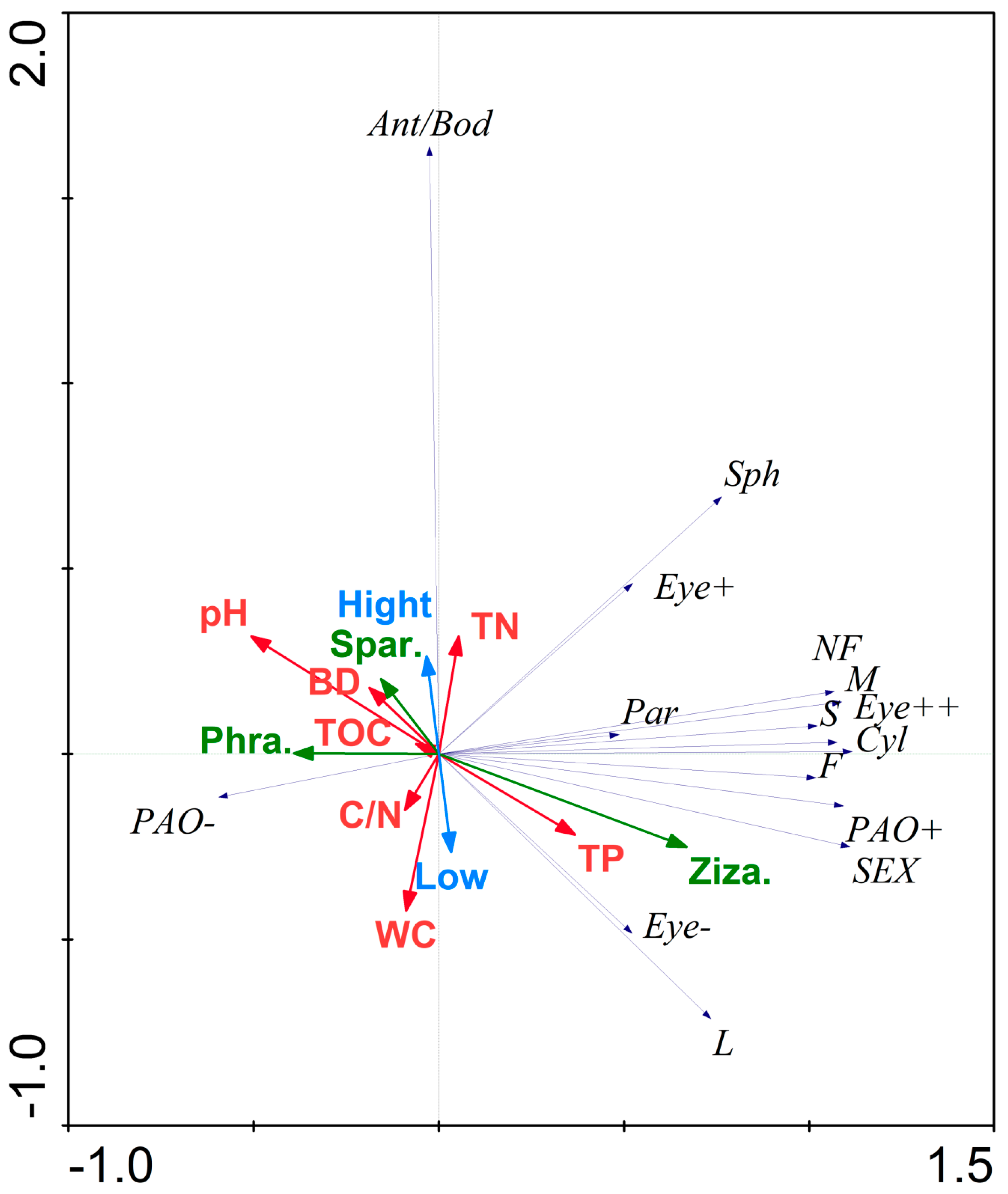
3.5. Response Model of the Functional Traits of Collembola Diversity to the Environment
4. Discussion
4.1. Analysis of the Physicochemical Properties of the Soil
4.2. Response Model of Springtail Species Diversity to the Environment
4.3. Response Model of the Functional Traits Diversity of Springtails to the Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, L.; Fox, A.D. Birds and people both depend on China’s wetlands. Nature 2009, 460, 173. [Google Scholar] [CrossRef]
- Mao, D.; Wang, Z.; Wu, J.; Wu, B.; Zeng, Y.; Song, K.; Yi, K.; Luo, L. China’s wetlands loss to urban expansion. Land Degrad. Dev. 2018, 29, 2644–2657. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Zhang, L.Y.; Xiao, Y.; Zheng, H.; Oouyang, Z.Y. Spatial variation analysis of biodiversity in the Bohai region coastal wetland. Acta Ecol. Sin. 2018, 38, 909–916. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, L.Y.; Cui, L.J.; Oouyang, Z.Y. Progress in ecosystem services value valuation of coastal wetlands. Acta Ecol. Sin. 2016, 36, 7509–7518. [Google Scholar] [CrossRef]
- Zhang, H.B.; Liu, H.Y.; Hao, J.F.; Li, Y.F. Spatiotemporal characteristics of landscape change in the coastal wetlands of Yancheng caused by natural processes and human activities. Acta Ecol. Sin. 2012, 32, 101–110. [Google Scholar] [CrossRef]
- Potapov, A.; Bellini, B.C.; Chown, S.L.; Deharveng, L.; Janssensa, F.; Kovac, L.; Kuznetsova, N.; Ponge, J.F.; Potapov, M.; Querner, P.; et al. Towards a global synthesis of Collembola knowledge-challenges and potential solutions. Soil Org. 2020, 92, 161–188. [Google Scholar] [CrossRef]
- Chernova, N.; Kuznetsova, N. Collembolan community organization and its temporal predictability. Pedobiologia 2000, 44, 451–466. [Google Scholar] [CrossRef]
- Powell, J.R.; Craven, D.; Eisenhauer, N. Recent trends and future strategies in soil ecological research-integrative approaches at pedobiologia. Pedobiologia 2014, 57, 1–3. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Hooper, J.N.A.; Van Soest, R.W.M.; Pisera, A.; Crowther, A.L.; Tyler, S.; Schilling, S.; Eschmeyer, W.N.; Fong, J.D.; Blackburn, D.C.; et al. Animal biodiversity: An outline of higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–237. [Google Scholar] [CrossRef]
- Ramel, G.; Baquero, E.; Jordana, R. Biodiversity of the Collembola fauna of wetland Kerkini (N. Greece), with description of the sexual dimorphism of Entomobrya atrocincta Schott 1896 (Collembola: Entomobryomorpha). Ann. Soc. Entomol. Fr. 2008, 44, 113–128. [Google Scholar] [CrossRef]
- Hansen, J.; Castelle, A. Insect diversity in soils of tidal and non-tidal wetlands of Spencer Island, Washington. J. Kans. Entomol. Soc. 1999, 72, 262–272. [Google Scholar] [CrossRef]
- Sterzyńska, M.; Nicia, P.; Zadrożny, P.; Fiera, C.; Shrubovych, J.; Ulrich, W. Urban springtail species richness decreases with increasing air pollution. Ecol. Indic. 2018, 94, 328–335. [Google Scholar] [CrossRef]
- Kardol, P.; Wardle, D.A. How understanding aboveground–belowground linkages can assist restoration ecology. Trends Ecol. Evol. 2010, 25, 670–679. [Google Scholar] [CrossRef]
- Barbercheck, M.E.; Neher, D.A.; Anas, O.; El-Allaf, S.M.; Weicht, T.R. Response of soil invertebrates to disturbance across three resource regions in North Carolina. Environ. Monit. Assess. 2009, 152, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Giller, P.S. The diversity of soil communities, the “poor man’s tropical rainforest”. Biodivers. Conserv. 1996, 5, 135–168. [Google Scholar] [CrossRef]
- Ivask, M.; Kuu, A.; Meriste, M.; Kutti, S.; Palo, A.; Raamets, J.; Kilki, S. Springtails of flooded meadows along Matsalu Bay and the Kasari River, Estonia. Pedobiologia 2018, 66, 1–10. [Google Scholar] [CrossRef]
- Shao, Y.; Cao, S.; Cao, W.; Liu, C. Effects of degradation and management of Nanniwan wetland on soil fauna diversity. J. Ecol. Rural Environ. 2019, 35, 634–643. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Zheng, R.Q.; Chen, P.; Li, T.; Lu, J.J.; Zhang, J.Y. Effects of the invasion by Solidago canadensis L. on the community structure of soil animals. Acta Ecol. Sin. 2012, 32, 7072–7081. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Yan, X.; Chang, L.; Wu, D. Euedaphic and Hemiedaphic Collembola Suffer Larger Damages than Epedaphic Species to Nitrogen Input. Environ. Pollut. 2016, 208, 413–415. [Google Scholar] [CrossRef]
- Salmon, S.; Ponge, J.F. Species traits and habitats in springtail communities: A regional scale study. Pedobiologia 2012, 55, 295–301. [Google Scholar] [CrossRef]
- McKirdy, S.J.; O’Connor, S.; Thomas, M.L.; Horton, K.L.; Williams, A.; Hardie, D.; Coupland, G.T.; van der Merwe, J. Biosecurity risks posed by a large sea-going passenger vessel: Challenges of terrestrial arthropod species detection and eradication. Sci. Rep. 2019, 9, 19339. [Google Scholar] [CrossRef]
- Ponge, J.F.; Peres, G.; Guernion, M.; Ruiz-Camacho, N.; Cortet, J.; Pernin, C.; Villenave, C.; Chaussod, R.; Martin-Laurent, F.; Bispo, A.; et al. The impact of agricultural practices on soil biota: A regional study. Soil Biol. Biochem. 2013, 67, 271–284. [Google Scholar] [CrossRef]
- Vincent, Q.; Leyval, C.; Beguiristain, T.; Auclerc, A. Functional structure and composition of Collembola and soil macrofauna communities depend on abiotic parameters in derelict soils. Appl. Soil Ecol. 2018, 130, 259–270. [Google Scholar] [CrossRef]
- Birkhofer, K.; Gossner, M.M.; Diekoetter, T.; Drees, C.; Ferlian, O.; Maraun, M.; Scheu, S.; Weisser, W.W.; Wolters, V.; Wurst, S.; et al. Land-use type and intensity differentially filter traits in above- and below-ground arthropod communities. J. Anim. Ecol. 2017, 86, 511–520. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Jia, J.; Fu, X.; Le, Y.; Chen, X.; Sun, Y. Response of Soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil Biol. Biochem. 2011, 43, 638–646. [Google Scholar] [CrossRef]
- Sui, H.C.; Yan, J.G.; Cui, B.S.; Zhou, Y.F. The co-effects of seagrass and spartina on macroinvertebrate communities in salt marshes. Environ. Ecol. 2019, 1, 21–27. [Google Scholar]
- Shen, N.; Liang, Z.; Liu, Q.; Tu, C.; Dong, K.; Wang, C.; Chen, M. Antifungal secondary metabolites isolated from mangrove rhizosphere soil-derived penicillium fungi. J. Ocean Univ. China 2020, 19, 717–721. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, B.; Barter, M.; Piersma, T.; Zhou, C.F.; Li, F.S.; Zhang, Z.W. Impacts of tidal land reclamation in Bohai Bay, China: Ongoing losses of critical Yellow Sea waterbird staging and wintering sites. Bird Conserv. Int. 2011, 21, 241–259. [Google Scholar] [CrossRef]
- Chen, L.R.; Gong, X.L.; Zhu, M.; Wang, D.H.; Sun, Y. Spatial and temporal pattern of fish composition in tidal gully of Jiuduansha wetland. J. Shanghai Ocean. Univ. 2015, 24, 915–925. [Google Scholar]
- Wu, P.F.; Sun, Y.; Wang, L.; Yan, J.F.; Hu, Y. Distribution patterns and dynamics of typical pollutants in the waters of Jiuduansha wetland. Wetl. Sci. Manag. 2018, 14, 54–57. [Google Scholar] [CrossRef]
- Macfadyen, A. Improved funnel-type extractors for soil arthropods. J. Anim. Ecol. 1961, 30, 171–184. [Google Scholar] [CrossRef]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark. I. Poduromorpha and Onychiuridae; Brill: Leiden, The Netherlands, 1998; p. 35. [Google Scholar]
- Fjellberg, A. The Collembola of Fennoscandia and Denmark, Part II: Entomobryomorpha and Symphypleona; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Potapov, M.B. Synopses on Palaearctic Collembola. Volume 3. Isotomidae; Staatliches Museum Für Naturkunde Görlitz: Görlitz, Germany, 2001; p. 603. [Google Scholar]
- Thibaud, J.M. Contribution to the Knowledge of Interstitial Collembola from Littoral Sands; Revue Francaise d’Entomologie (Nouvelle Serie): Paris, France, 2002; pp. 201–209. [Google Scholar]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998; pp. 1–618. [Google Scholar]
- Krab, E.J.; Oorsprong, H.; Berg, M.P.; Cornelissen, J.H.C. Turning northern peatlands upside down: Disentangling microclimate and substrate quality effects on vertical distribution of Collembola. Funct. Ecol. 2010, 24, 1362–1369. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; van Hal, J.R.; Callaghan, T.V.; Press, M.C.; Aerts, R. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol. Biochem. 2011, 43, 377–384. [Google Scholar] [CrossRef]
- Bokhorst, S.; Phoenix, G.K.; Bjerke, J.W.; Callaghan, T.V.; Huyer-Brugman, F.; Berg, M.P. Extreme winter warming events more negatively impact small rather than large soil fauna: Shift in community composition explained by traits not taxa. Glob. Chang. Biol. 2012, 18, 1152–1162. [Google Scholar] [CrossRef]
- Martins, C.; Natal-da-Luz, T.; Sousa, J.P.; Goncalves, M.J.; Salgueiro, L.; Canhoto, C. Effects of essential oils from Eucalyptus Globulus leaves on soil organisms involved in leaf degradation. PLoS ONE 2013, 8, e61233. [Google Scholar] [CrossRef]
- van Dooremalen, C.; Suring, W.; Ellers, J. Fatty acid composition and extreme temperature tolerance following exposure to fluctuating temperatures in a soil arthropod. J. Insect Physiol. 2011, 57, 1267–1273. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Bengtsson, J.; Berggren, A.; Zwiggelaar, K.; Spijkman, E.; Huyer-Brugman, F.; Berg, M.P. Spatially structured environmental filtering of collembolan traits in late successional salt marsh vegetation. Oecologia 2015, 179, 537–549. [Google Scholar] [CrossRef]
- Wolfson, A.W.C.; Allee, A.E.; Emerson, O.P.; Thomas, P.; Karl, P.S. Principles of Animal Ecology. Physiol. Zool. 1951, 24, 269–271. [Google Scholar] [CrossRef]
- Ter Braak, C. CANOCO—A FORTRAN Program for Canonical Community Ordination by [Partial] [Detrended] [Canonical] Correspondence Analysis, Principal Components Analysis and Redundancy Analysis (Version 2.1); Agricultural Mathematics Group: Wageningen, The Netherlands, 1988; p. 95. [Google Scholar]
- Kaiser, O.; Pühler, A.; Selbitschka, W. Phylogenetic analysis of microbial diversity in the Rhizoplane of Oilseed Rape (Brassica Napus cv. Westar) employing cultivation-dependent and cultivation-independent approaches. Microb. Ecol. 2001, 42, 136–149. [Google Scholar] [CrossRef]
- Ju, Z. Review and prospects on methodology and affecting factors of soil microbial diversity. Biodivers. Sci. 2007, 15, 306. [Google Scholar] [CrossRef]
- Ai, J.W.; Jian, C.; Dong, Y.L.; Zhi, Q.Z. Spatial variations of carbon and nitrogen in coastal wetland sediments of Quanzhou Bay in China. Huan Jing Ke Xue 2007, 28, 2361–2368. [Google Scholar]
- Zhang, P.; Nie, M.; Li, B.; Wu, J.H. The transfer and allocation of newly fixed c by invasive Spartina alterniflora and native Phragmites australis to soil microbiota. Soil Biol. Biochem. 2017, 113, 231–239. [Google Scholar] [CrossRef]
- Pei, J.; Li, J.; Mia, S.; Singh, B.; Wu, J.; Dijkstra, F.A. Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma 2021, 382, 114710. [Google Scholar] [CrossRef]
- Ni, Z.; Yan, X.; Chang, L.; Sun, X.; Wu, D.; Zhang, B. Habitat preferences rather than morphological traits affect the recovery process of Collembola (Arthropoda, Hexapoda) on a bare saline–alkaline land. PeerJ 2020, 8, e9519. [Google Scholar] [CrossRef]
- Saifutdinov, R.A.; Gongalsky, K.B.; Zaitsev, A.S. Evidence of a trait-specific response to burning in springtails (Hexapoda: Collembola) in the boreal forests of European Russia. Geoderma 2018, 332, 173–179. [Google Scholar] [CrossRef]
- Tian, Y.; Miao, X.; Song, S.; Zhang, Z.; Hu, S.; Wei, D.; Wang, M. Complete mitochondrial genome of Proisotoma Minuta (Collembola: Proisotominae). Mitochondrial DNA Part B 2022, 7, 727–728. [Google Scholar] [CrossRef]
- Gao, M.X.; Sun, X.; Wu, D.H.; Zhang, X.P. Spatial autocorrelation at multi-scale of soil collembolan community in farmland of the Sanjiang Plain, Northeast China. Acta Ecol. Sin. 2014, 34, 4980–4990. [Google Scholar] [CrossRef]
- Zhang, P.; Neher, D.A.; Li, B.; Wu, J. The Impacts of Above- and Belowground Plant Input on soil microbiota: Invasive Spartina alterniflora versus native Phragmites australis. Ecosystems 2018, 21, 469–481. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Malmstrom, A.; Berg, M.P.; Bengtsson, J. Small-scale collembola community composition in a pine forest soil—Overdispersion in functional traits indicates the importance of species interactions. Soil Biol. Biochem. 2016, 103, 52–62. [Google Scholar] [CrossRef]
- Kooch, Y.; Parsapour, M.K.; Egli, M.; Moghimian, N. Forest floor and soil properties in different development stages of oriental beech forests. Appl. Soil Ecol. 2021, 161, 103823. [Google Scholar] [CrossRef]
- Salmon, S.; Ponge, J.F.; Gachet, S.; Deharveng, L.; Lefebvre, N.; Delabrosse, F. Linking species, traits and habitat characteristics of Collembola at European scale. Soil Biol. Biochem. 2014, 75, 73–85. [Google Scholar] [CrossRef]
- Chauvat, M.; Forey, E. Temperature Modifies the Magnitude of a Plant Response to Collembola Presence. Appl. Soil Ecol. 2021, 158, 103814. [Google Scholar] [CrossRef]
- Mebes, K.H.; Filser, J. Method for Estimating the Significance of Surface Dispersal for Population Fluctuations of Collembola in Arable Land; IX International Colloquium on Apterygota: Dublin, Ireland, 1997; pp. 115–122. [Google Scholar]
- Bauer, T.; Christian, E. Habitat dependent differences in the flight behaviour of Collembola. Pedobiologia 1987, 30, 233–239. [Google Scholar]
- Altner, H.; Thies, G. The postantennal organ: A specialized unicellular sensory input to the protocerebrum in apterygotan insects (Collembola). Cell Tissue Res. 1976, 167, 97–110. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: London, UK, 1997; pp. 1–330. [Google Scholar]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef]



| Number | Shoal | Plants | Tidal Flat | Coordinate | Code |
|---|---|---|---|---|---|
| 1 | Jiangyanansha | Zizania latifolia | Low tidal flat | 31°13.7833′ N 121°54.2047′ E | ZL-L |
| 2 | Shangsha | Phragmites australis | Low tidal flat | 31°13.7833′ N 121°54.2047′ E | PA-L |
| 3 | Shangsha | Phragmites australis | High tidal flat | 31°13.7828′ N 121°54.2033′ E | PA-H |
| 4 | Zhongxiasha | Spartina alterniflora | Low tidal flat | 31°13.7833′ N 121°54.2047′ E | SA-L |
| 5 | Zhongxiasha | Spartina alterniflora | High tidal flat | 31°10.6453′ N 121°57.8279′ E | SA-H |
| Traits | Functional Traits | Types of Data for RLQ and Models | Abbreviation |
|---|---|---|---|
| Habitus | Body shape | 1: Cylindrical body | Cyl |
| 2: Spherical body | Sph | ||
| Body length | 1: <0.5 mm | S | |
| 2: 0.5–1 mm | M | ||
| 3: >1 mm | L | ||
| Locomotory organs | Furcula length | 1: >0.1 mm | F |
| 0: <0.1 mm | NF | ||
| Sensory or sense-related organs | Ocelli number | 2: >5 1: 1–5 | Eye++ Eye+ |
| 0: Absent | Eye− | ||
| PAO | 1: Present | PAO+ | |
| 0: Absent | PAO− | ||
| Antenna length | 1: Long, length ratio of antenna/body ≥ 0.25 | Ant/Bod ≥ 0.25 | |
| 2: Short, length ratio of antenna/body < 0.25 | Ant/Bod < 0.25 | ||
| Reproduction | Reproduction mode | 1: Sexual reproduction | Sex |
| 2: Parthenogenesis | Par |
| Code | Water Content (WC) (%) | Bulk Density (BD) (g/cm3) | pH | Total Nitrogen (TN) (g/kg) | Total Phosphorus (TP) (g/kg) | Total Organic Carbon (TOC) (g/kg) | Carbon-Nitrogen Ratio (C/N) |
|---|---|---|---|---|---|---|---|
| ZL-L | 26.408 a (0.211) | 1.017 ab (0.146) | 7.94 b (0.968) | 0.924 ab (0.028) | 0.560 a (0.038) | 12.150 a (0.911) | 13.119 a (0.583) |
| PA-L | 24.866 a (0.622) | 0.974 b (0.237) | 8.34 ab (0.977) | 1.036 a (0.143) | 0.523 ab (0.029) | 15.431 a (2.706) | 15.609 a (3.674) |
| PA-H | 26.441 a (0.766) | 1.083 a (0.226) | 8.43 a (0.145) | 0.816 ab (0.018) | 0.521 ab (0.019) | 26.168 a (14.487) | 32.395 a (6.182) |
| SA-L | 25.719 b (0.304) | 1.098 a (0.409) | 8.33 ab (0.110) | 1.074 a (0.179) | 0.494 ab (0.011) | 13.593 a (0.907) | 13.339 a (2.287) |
| SA-H | 22.368 b (1.657) | 1.050 ab (0.247) | 8.61 a (0.393) | 1.087 a (0.269) | 0.483 b (0.013) | 9.998 a (0.842) | 10.592 a (2.812) |
| Family | Taxon | Abbreviation | ZL-L | PA-L | PA-H | SA-L | SA-H | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimens | Percentage (%) | Specimens | Percentage (%) | Specimens | Percentage (%) | Specimens | Percentage (%) | Specimens | Percentage (%) | Specimens | Percentage (%) | |||
| Isotomidae | Proisotoma minuta (Tullberg, 1871) | PRO_min | 255 | 56.67 | 14 | 23.73 | 31 | 34.07 | 32 | 44.44 | 90 | 50.28 | 422 | 49.59 |
| Proisotoma sp. | PRO_sp | 141 | 31.33 | 5 | 8.47 | 16 | 17.58 | 9 | 12.50 | 41 | 22.91 | 212 | 24.91 | |
| Subisotoma sp. | SUB_sp | 43 | 9.56 | 6 | 10.17 | 5 | 5.49 | 3 | 4.17 | 8 | 4.47 | 65 | 7.64 | |
| Folsomides sp. 1 | FOL_sp1 | 2 | 0.44 | 11 | 18.64 | 6 | 6.59 | 12 | 16.67 | 19 | 10.61 | 50 | 5.88 | |
| Folsomides sp. 2 | FOL_sp2 | 0 | 0 | 0 | 0 | 2 | 2.20 | 0 | 0 | 3 | 1.68 | 5 | 0.59 | |
| Appendisotoma sp. | APP_sp | 0 | 0 | 1 | 1.69 | 1 | 1.10 | 2 | 2.78 | 3 | 1.68 | 7 | 0.82 | |
| Archisotoma interstitialis Delamare-debouteville, 1953 | ARC_int | 1 | 0.22 | 0 | 0 | 0 | 0 | 2 | 2.78 | 1 | 0.56 | 4 | 0.47 | |
| Folsomina onychiurina Denis, 1931 | FOL_ony | 1 | 0.22 | 1 | 1.69 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.24 | |
| Scutisotoma oirota (Vilkamaa, 1988) | SCU_oir | 0 | 0 | 1 | 1.69 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.12 | |
| Entomobryidae | Coecobrya pani Xu, Yu & Zhang, 2012 | COE_pan | 0 | 0 | 10 | 16.95 | 12 | 13.19 | 8 | 11.11 | 7 | 3.91 | 37 | 4.35 |
| Sinella longiantenna Zhang & Deharveng, 2011 | SIN_lon | 0 | 0 | 2 | 3.39 | 6 | 6.59 | 0 | 0 | 0 | 0 | 8 | 0.94 | |
| Homidia sp. | HOM_sp | 0 | 0 | 2 | 3.39 | 2 | 2.20 | 1 | 1.39 | 0 | 0 | 5 | 0.59 | |
| Lepidocyrtus sp. | LEP_sp | 0 | 0 | 0 | 0 | 1 | 1.10 | 1 | 1.39 | 0 | 0 | 2 | 0.24 | |
| Pseudosinella sp. | PSE_sp | 0 | 0 | 0 | 0 | 1 | 1.10 | 0 | 0 | 0 | 0 | 1 | 0.12 | |
| Neanuridae | Friesea mirabilis (Tullberg, 1871) | FRI_mir | 0 | 0 | 2 | 3.39 | 2 | 2.20 | 2 | 2.78 | 0 | 0 | 6 | 0.71 |
| Friesea handschini Kseneman, 1938 | FRI_han | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.56 | 1 | 0.12 | |
| Sminthurididae | Sphaeridia sp. | SPH_sp | 7 | 1.56 | 4 | 6.78 | 6 | 6.59 | 0 | 0 | 4 | 2.23 | 21 | 2.47 |
| Sminthurides sp. | SMI_sp | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.12 | 2 | 0.24 | |
| total | 450 | 59 | 91 | 72 | 179 | 851 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-Y.; Gao, Y.-X.; Li, C.-Y.; Jin, Y.-L.; Yang, S.-Q.; Xia, J.-H.; Zhang, Y.-F.; Bu, Y.; Li, K. Effects of Species Invasion and Inundation on the Collembola Community in Coastal Mudflat Wetland from the Perspective of Functional Traits. Insects 2023, 14, 210. https://doi.org/10.3390/insects14020210
Li J-Y, Gao Y-X, Li C-Y, Jin Y-L, Yang S-Q, Xia J-H, Zhang Y-F, Bu Y, Li K. Effects of Species Invasion and Inundation on the Collembola Community in Coastal Mudflat Wetland from the Perspective of Functional Traits. Insects. 2023; 14(2):210. https://doi.org/10.3390/insects14020210
Chicago/Turabian StyleLi, Jing-Yang, Yun-Xia Gao, Chun-Yang Li, Ya-Li Jin, Si-Qi Yang, Jian-Hong Xia, Yun-Fei Zhang, Yun Bu, and Kai Li. 2023. "Effects of Species Invasion and Inundation on the Collembola Community in Coastal Mudflat Wetland from the Perspective of Functional Traits" Insects 14, no. 2: 210. https://doi.org/10.3390/insects14020210
APA StyleLi, J.-Y., Gao, Y.-X., Li, C.-Y., Jin, Y.-L., Yang, S.-Q., Xia, J.-H., Zhang, Y.-F., Bu, Y., & Li, K. (2023). Effects of Species Invasion and Inundation on the Collembola Community in Coastal Mudflat Wetland from the Perspective of Functional Traits. Insects, 14(2), 210. https://doi.org/10.3390/insects14020210







