Colour Selection and Olfactory Responses of Papilio demoleus during Foraging and Courtship
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Facility
2.2. Butterflies
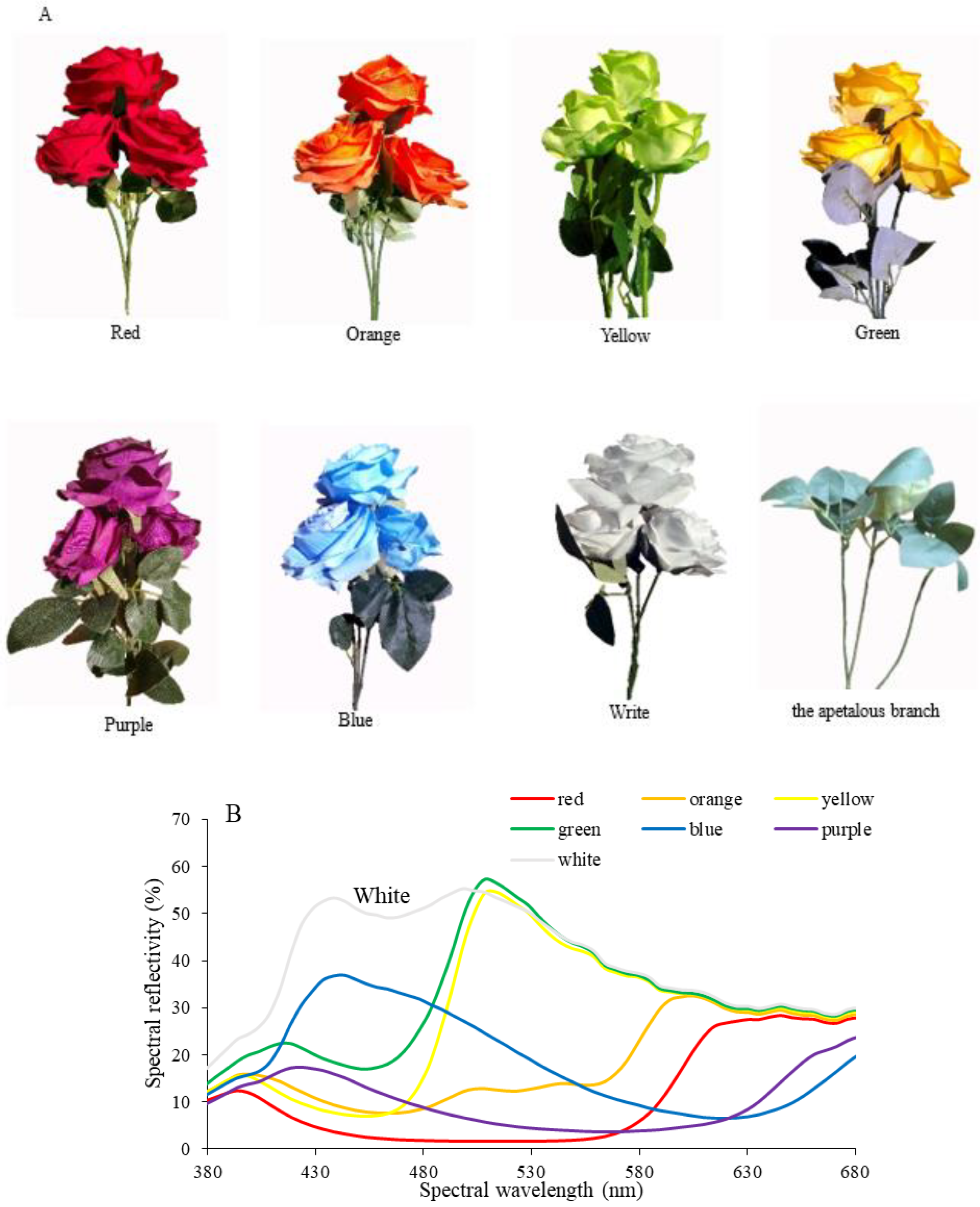
2.3. Artificial Flowers and Apetalous Branch
2.4. Butterfly Mimics
2.5. Observation of the Behaviour of Butterflies Visiting Flowers
2.6. Observation of Courtship with Natural Butterflies and Mimics
2.7. Opsin Cloning
2.8. Data Analysis
3. Results
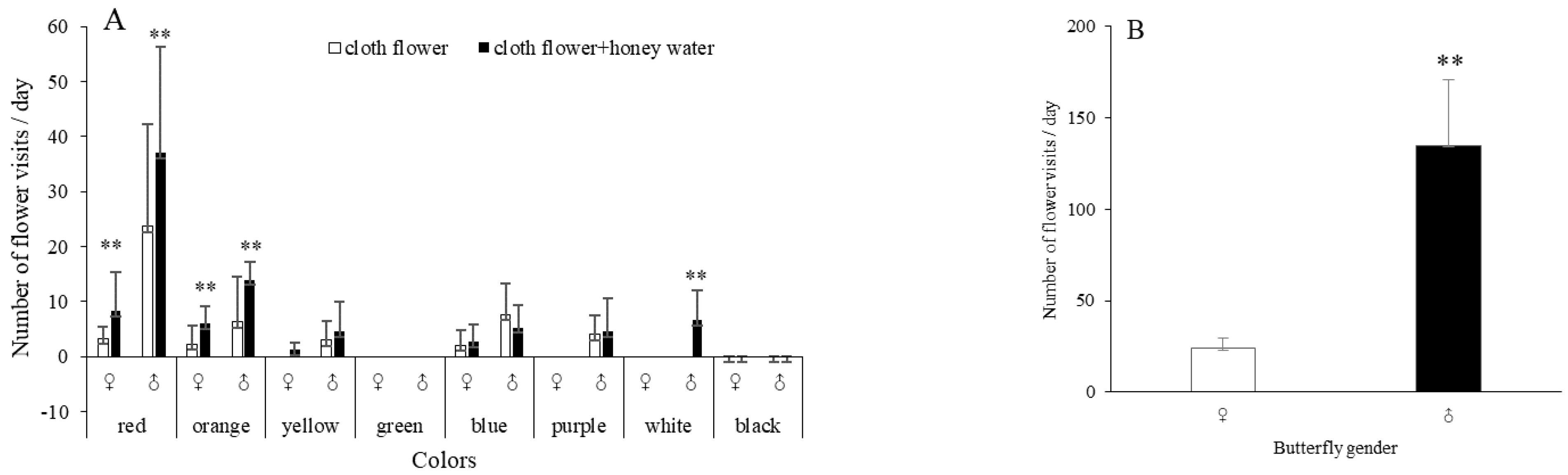
3.1. Olfactory and Visual Responses of P. demoleus during Foraging
3.2. Visual and Olfactory Behaviour of P. demoleus during Courtship
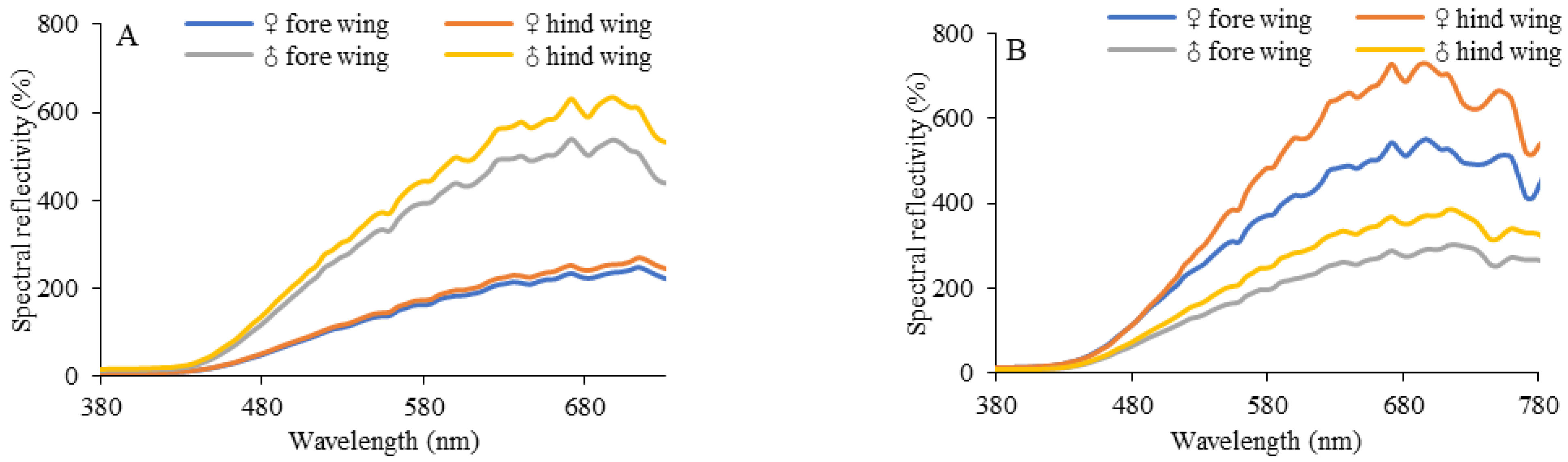
3.3. Colour and Reflectance Spectra of Wings
3.4. Opsin Gene Analysis of P. demoleus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilse, D. Über den farbensinn der Tagfalter. Z. Für Vgl. Physiol. 1928, 8, 658–692. [Google Scholar] [CrossRef]
- Lewis, A.C.; Lipani, G.A. Learning and flower use in butterflies: Hypotheses from honey bees. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, pp. 95–110. [Google Scholar]
- Goulson, D.; Cory, J.S. Flower constancy and learning in foraging preferences of the green-veined white butterfly Pieris napi. Ecol. Entomol. 1993, 18, 315–320. [Google Scholar] [CrossRef]
- Weiss, M.R. Associative colour learning in a nymphalid butterfly. Ecol. Entomol. 1995, 20, 298–301. [Google Scholar] [CrossRef]
- Andersson, S. Foraging responses in the butterflies Inachisio, Aglaisurticae (Nymphalidae), and Gonepteryxrhamni (Pieridae) to floral scents. Chemoecology 2003, 13, 1–11. [Google Scholar] [CrossRef]
- Balkenius, A.; Rosén, W.; Kelber, A. The relative importance of olfaction and vision in a diurnal and anocturnal hawkmoth. J. Comp. Physiol. 2006, 192, 431–437. [Google Scholar] [CrossRef]
- Ômura, H. Foraging behavior of adult butterflies and its semiochemicals as olfactory signals. Comp. Biochem. Phys. C 2006, 23, 134–142. [Google Scholar]
- Kinoshita, M.; Arikawa, K. Colour constancy in the swallowtail butterfly Papilio xuthus. J. Exp. Biol. 2000, 203, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Mizuno, S.; Kinoshita, M.; Stavenga, D.G. Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of the butterfly Papilio xuthus. J. Neurosci. 2003, 23, 4527–4532. [Google Scholar] [CrossRef]
- Borges, R.M.; Gowda, V.; Zacharias, S.M. Butterfly pollination and high-contrast visual signals in a low-density distylous plant. Oecologia 2003, 136, 571–573. [Google Scholar] [CrossRef]
- Ômura, H.; Honda, K. Priority of color over scent during flower visitation by adult Vanessa indica butterflies. Oecologia 2005, 142, 588–598. [Google Scholar] [CrossRef]
- Arikawa, K. The eyes and vision of butterflies. J. Physiol. 2017, 595, 5457–5464. [Google Scholar] [CrossRef]
- Honda, K.; Ômura, H.; Hayashi, N. Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly, Pieris rapae. J. Chem. Ecol. 1998, 24, 2167–2180. [Google Scholar] [CrossRef]
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 147–198. [Google Scholar]
- Corbet, S.A. Butterfly nectaring flowers: Butterfly morphology and flower form. Entomol. Exp. Appl. 2000, 96, 289–298. [Google Scholar] [CrossRef]
- Lewis, A.C. Memory Constraints and Flower Choice in Pieris rapae. Science 1986, 232, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Jennersten, O. Flower visitation and pollination efficiency of some North European butterflies. Oecologia 1984, 63, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Dobson, H.E.M. Behavioral foraging responses by the butterfly Heliconius melpomene to Lantana camara floral scent. J. Chem. Ecol. 2003, 29, 2303–2318. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Zhou, C.L.; Chen, X.M.; Zheng, H. Visual and olfactory responses of eight butterfly species during foraging. J. Insect Behav. 2013, 26, 387–401. [Google Scholar] [CrossRef]
- Scott, J.A. Mating of butterflies. J. Res. Lepid. 1972, 11, 99–127. [Google Scholar] [CrossRef]
- Costanzo, K.; Monteiro, A. The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proc. R. Soc. B 2007, 274, 845–851. [Google Scholar] [CrossRef]
- Obara, Y.; Majerus, M.E.N. Initial mate recognition in the British cabbage butterfly, Pieris rapaerapae. Zool. Sci. 2000, 17, 725–730. [Google Scholar] [CrossRef]
- Kemp, D.J. Heightened phenotypic variation and age-based fading of ultraviolet butterfly wing coloration. Evol. Ecol. Res. 2006, 8, 515–527. [Google Scholar]
- Marini-Filho, O.J.; Benson, W.W. Use of sound and aerial chases in sexual recognition in Neotropical Hamadryas butterflies (Nymphalidae). J. Res. Lepid. 2010, 42, 5–12. [Google Scholar]
- Silberglied, R.E.; Taylor, O.R. Ultraviolet reflection and its behavioral role in the courtship of the sulfur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae). Behav. Ecol. Sociobiol. 1978, 3, 203–243. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, H.; Chen, X.; Yao, J.; Shi, L.; Zhou, C. Role of visual and olfactory cues in sex recognition in butterfly Cethosia cyane cyane. Sci. Rep. 2017, 7, 5033. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Borg-Karlson, A.K.; Wiklund, C. Sexual cooperation and conflict in butterflies: A male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc. R. Soc. B 2000, 267, 1271–1275. [Google Scholar] [CrossRef]
- Tenna, T.; Diana, P.; José, A.; Navarro-Cano, C.W.; Karl, G.; Johan, E. Butterfly-host plant synchrony determines patterns of host use across years and regions. Oikos 2018, 128, 493–502. [Google Scholar] [CrossRef]
- Celorio-Mancera, M.D.L.P.; Wheat, C.W.; Huss, M.; Vezzi, F.; Neethiraj, R.; Reimegård, J.; Nylin, S.; Janz, N. Evolutionary history of host use, rather than plant phylogeny, determines gene expression in a generalist butterfly. BMC Evol. Biol. 2016, 16, 59. [Google Scholar] [CrossRef]
- Thom, M.D.; Daniels, J. Patterns of microhabitat and larval host-plant use by an imperiled butterfly in northern Florida. J. Insect Conserv. 2017, 21, 39–52. [Google Scholar] [CrossRef]
- Vane-Wright, I.; Boppre, M. Visual and chemical signalling in butterflies: Functional and phylogenetic perspectives. Philos. Trans. R. Soc. B 1993, 340, 197–205. [Google Scholar]
- Spaethe, J.; Briscoe, A.D. Molecular characterization and expression of the UV Opsin in bumblebees: Three ommatidial subtypes in the retina and a new photoreceptor organ in the lamina. J. Exp. Biol. 2005, 208, 2347–2361. [Google Scholar] [CrossRef]
- Eguchi, E.; Watanabe, K.; Hariyama, T.; Yamamoto, K. A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. J. Insect Physiol. 1982, 28, 675–682. [Google Scholar] [CrossRef]
- Kitamoto, J.; Sakamoto, K.; Ozaki, K.; Mishina, Y.; Arikawa, K. Two visual pigments in a single photoreceptor cell: Identification and histological localization of three mRNAs encoding visual pigment opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 1998, 201, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Monographia Rhopalocerorum Sinensium; Henan Science and Technology Press: Zhengzhou, China, 1994; p. 154. [Google Scholar]
- Chen, S.A.; Li, M.T.; Liu, J.; Feng, Y.; Yao, J.; Shi, L.; Chen, X.M. Visual and olfactory sensory responses of the butterfly Papilio maackii during foraging and courtship. Entomol. Res. 2021, 51, 518–527. [Google Scholar] [CrossRef]
- Klun, J.A. Insect sex pheromones: Intraspecific pheromonal variability of ostrinia nubilalis in north america and europe. Environ. Entomol. 1975, 4, 891–894. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Urs, K.C.D. Studies On The Sex Pheromone Of The Diamondback Moth Plutella Xylostella (Lepidoptera, Yponomeutidae) In India. Bntomol. Entomol. Res. 1996, 86, 585–590. [Google Scholar] [CrossRef]
- Yang, M.H.; Zhang, J.T.; Liu, J.L.; Jing, X.Y.; Luo, Y.Q.; Zong, S.X.; Cao, C.J.; Li, Y.H. Reproductive behavior and circadian rhythm of sex pheromone production and release of Holcocerus vicarius (Walker) (Lepidoptera: Cossidae). Acta Entomol. Sin. 2010, 53, 1273–1280. [Google Scholar]
- Zheng, H.; Zhang, W.; Xu, F.; Zhang, H.; Chen, X.; Rui, Y. Study on volatile components of butterfly nectar plants and host plants. Asian. J. Chem. 2013, 25, 7861–7863. [Google Scholar] [CrossRef]
- Li, C.; Lin, Y.; Deng, G. Analysis of volatile components in eight kinds of honey. Fine Chem. Eng. 2006, 23, 1082–1088. [Google Scholar]
- Obara, Y. Studies on the mating behavior of the White Cabbage Butterfly, Pieris rapae crucivora Boisduval. J. Comp. Physiol. B 1970, 69, 99–116. [Google Scholar] [CrossRef]
- Hidaka, T.; Yamashita, K. Wing color pattern as the releaser of mating behavior in the swallowtail butterfly, Papilio xuthus L. (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 1975, 10, 263–267. [Google Scholar] [CrossRef]
- Rutowski, R.L. Sexual Discrimination Using Visual Cues in the Checkered White Butterfly (Pieris protodice). Z. Für Tierpsychol. 1981, 55, 325–334. [Google Scholar] [CrossRef]
- Rutowski, R.L.; Rajyaguru, P.K. Male-specific iridescent coloration in the pipevine swallowtail (Battus philenor) is used in mate choice by females but not sexual discrimination by males. J. Insect Behav. 2013, 26, 200–211. [Google Scholar] [CrossRef]
- Sherratt, T.N. The evolution of female-limited polymorphisms in damselflies: A signal detection model. Ecol. Lett. 2001, 4, 22–29. [Google Scholar] [CrossRef]
- Grula, J.W.; Taylor, O.R. A micromorphological and experimental study of the antennae of the Sulphur Butterflies, Colias eurytheme and C. philodice (Lepidoptera: Pieridae). J. Kansas Entomol. Soc. 1980, 53, 476–484. [Google Scholar]




| Reference Opsin Gene Name | GenBank ID | Recognition Wavelength Range of the Spectrum | |
|---|---|---|---|
| 1 | Papilio xuthus PxRh1 mRNA | AB007423.1 | Long wavelength |
| 2 | Papilio xuthus PxRh2 mRNA | AB007424.1 | Long wavelength |
| 3 | Papilio xuthus PxRh3 mRNA | AB007425.1 | Long wavelength |
| 4 | Papilio xuthus PxRh4 mRNA | AB028217.1 | Blue |
| 5 | Papilio xuthus PxRh5 mRNA | AB028218.1 | Ultraviolet |
| Opsin Gene of P. demoleus | Primer Name | Sequence |
|---|---|---|
| Rh1 | Primer 1 | CTTCCTGCCGAGGTAGAA |
| Primer 2 | CTCCGTTGATGCTCATTGG | |
| Rh2 | Primer 1 | CGGCGTCTTAGGCTTCATATC |
| Primer 2 | AGCAATAGTCCAGGCGAGAG | |
| Rh3 | Primer 1 | TGCGGTGGTTCCTTATATGGTA |
| Primer 2 | CGATGATGTAACTGCGGCTAA | |
| Rh4 | Primer 1 | CGTTGTGCCACTACTCAC |
| Primer 2 | CGAGCAAGTTGTCAGGAA | |
| Rh5 | Primer 1 | GAGCAGTCAGTCAGTTGGTG |
| Primer 2 | TACAAGCGTTGGTCAGTCC |
| Opsin Gene of P. demoleus | Similar Genes Name | GenBank ID | Per.Ident |
|---|---|---|---|
| Rh2 | PREDICTED: Papilio polytes opsin-1 (LOC106107962), mRNA | XM_013288994.1 | 92.78% |
| Papilio anactus PaL2 mRNA for long wavelength-sensitive opsin 2, complete cds | AB725229.1 | 90.72% | |
| Rh3 | PREDICTED: Papilio polytes opsin-1-like (LOC106105734), mRNA | XM_013286167.1 | 94.24% |
| Papilio xuthus PxRh3 mRNA for opsin, complete cds | AB007425.1 | 93.01% | |
| Rh4 | Papilio xuthus PxRh4 mRNA for blue opsin, partial cds | AB028217.1 | 88.37% |
| Papilio anactus PaB mRNA for B-sensitive opsin, complete cds | AB725227.1 | 87.37% | |
| Rh5 | PREDICTED: Papilio xuthus opsin, ultraviolet-sensitive-like (LOC106116057) | XM_013309794.1 | 91.59% |
| PREDICTED: Papilio polytes opsin, ultraviolet-sensitive-like (LOC106103556) | XM_013283340.1 | 91.59% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Li, M.; Liu, J.; Feng, Y.; Yao, J.; Shi, L.; Chen, X. Colour Selection and Olfactory Responses of Papilio demoleus during Foraging and Courtship. Insects 2023, 14, 249. https://doi.org/10.3390/insects14030249
Chen S, Li M, Liu J, Feng Y, Yao J, Shi L, Chen X. Colour Selection and Olfactory Responses of Papilio demoleus during Foraging and Courtship. Insects. 2023; 14(3):249. https://doi.org/10.3390/insects14030249
Chicago/Turabian StyleChen, Shunan, Mingtao Li, Ji Liu, Ying Feng, Jun Yao, Lei Shi, and Xiaoming Chen. 2023. "Colour Selection and Olfactory Responses of Papilio demoleus during Foraging and Courtship" Insects 14, no. 3: 249. https://doi.org/10.3390/insects14030249
APA StyleChen, S., Li, M., Liu, J., Feng, Y., Yao, J., Shi, L., & Chen, X. (2023). Colour Selection and Olfactory Responses of Papilio demoleus during Foraging and Courtship. Insects, 14(3), 249. https://doi.org/10.3390/insects14030249






