Cicadomorpha Community (Hemiptera: Auchenorrhyncha) in Portuguese Vineyards with Notes of Potential Vectors of Xylella fastidiosa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Identification of Insects
2.3. Data Analysis
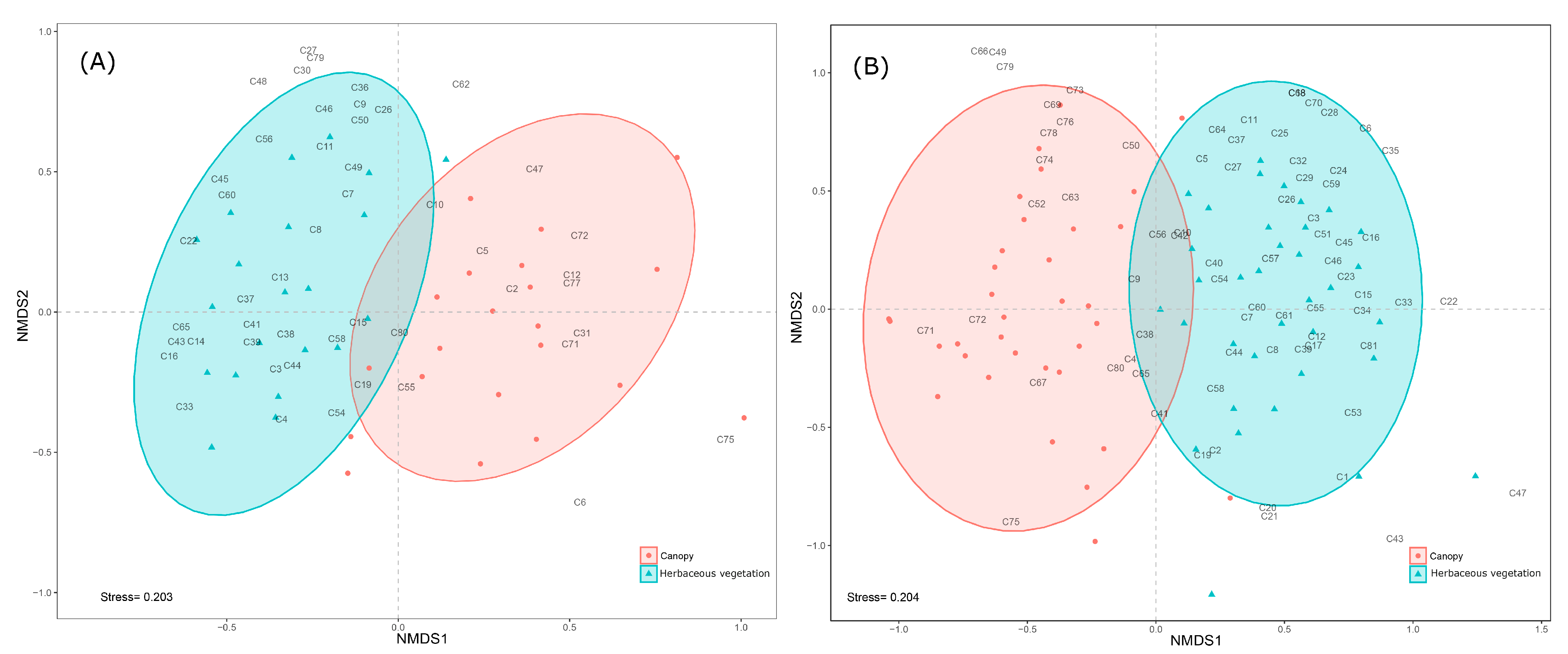
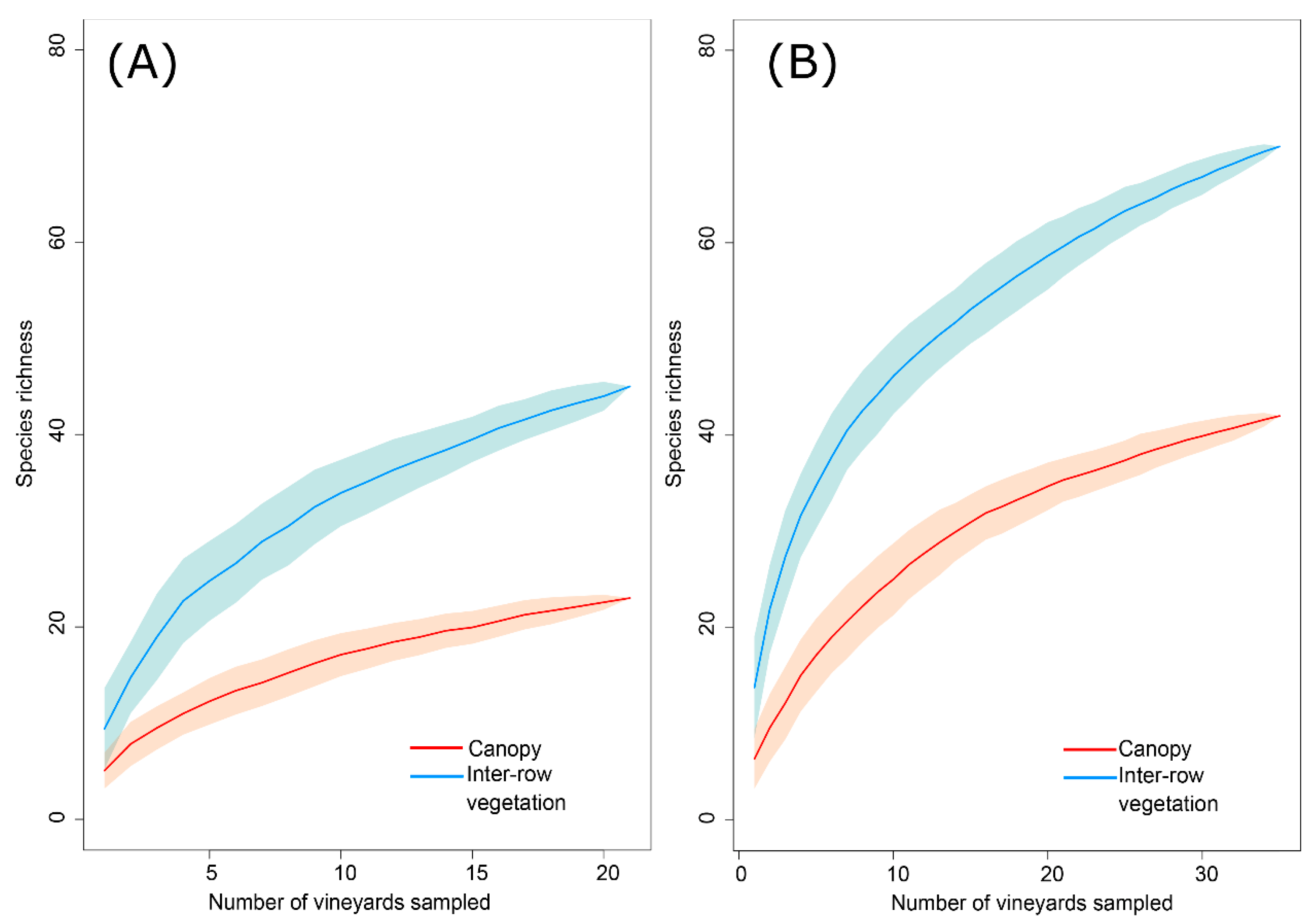
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Viticulture in Portugal: A review of recent trends and climate change projections. OENO One 2017, 51, 61–69. [Google Scholar] [CrossRef]
- Chuche, J.; Thiéry, D. Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Decante, D.; van Helden, M. Population ecology of Empoasca vitis (Göthe) and Scaphoideus titanus (Ball) in Bordeaux vineyards: Influence of migration and landscape. Crop Prot. 2006, 25, 696–704. [Google Scholar] [CrossRef]
- Kyrkou, I.; Pusa, T.; Ellegaard-Jensen, L.; Sagot, M.-F.; Hansen, L.H. Pierce's Disease of Grapevines: A Review of Control Strategies and an Outline of an Epidemiological Model. Front. Microbiol. 2018, 9, 2141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Gonçalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontrol Sci. Technol. 2018, 28, 122–141. [Google Scholar] [CrossRef]
- Dietrich, C.H. Evolution of Cicadomorpha (Insecta, Hemiptera). Denisia 2002, 176, 155–170. [Google Scholar]
- Raven, J.A. Phytophages of xylem and phloem: A comparison of animal and plant sap-feeders. Adv. Ecol. Res. 1983, 13, 135–234. [Google Scholar] [CrossRef]
- Atakan, E. Damage assessment of the leafhopper complex [Asymmetrasca decedens (Paoli) and Empoasca decipiens Paoli] (Homoptera: Cicadellidae) in cotton. J. Pest Sci. 2009, 82, 227–234. [Google Scholar] [CrossRef]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Scott, E.R.; Li, X.; Wei, J.-P.; Kfoury, N.; Morimoto, J.; Guo, M.-M.; Agyei, A.; Robbat, A., Jr.; Ahmed, S.; Cash, S.B.; et al. Changes in Tea Plant Secondary Metabolite Profiles as a Function of Leafhopper Density and Damage. Front. Plant Sci. 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Nielson, W. The Leafhopper Vectors of Phytopathogenic Viruses (Homoptera, Cicadellidae): Taxonomy, Biology, and Virus Transmission; United States Department of Agriculture: Washington, DC, USA, 1968; pp. 1689–1699. [Google Scholar]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell III, R.F.; Andersen, P.C. The biology of xylem fluid–feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, D.; Pavan, F.; Pozzebon, A.; Picotti, P.; Duso, C. Relative infestation level and sensitivity of grapevine cultivars to the leafhopper Empoasca vitis (Hemiptera: Cicadellidae). J. Econ. Entomol. 2016, 109, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Román, C.; Arnó, J.; Planas, S. Map-based zonal dosage strategy to control yellow spider mite (Eotetranychus carpini) and leafhoppers (Empoasca vitis & Jacobiasca lybica) in vineyards. Crop Prot. 2021, 147, 105690. [Google Scholar] [CrossRef]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.-W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Ivanauskas, A.; Valiūnas, D.; Jomantienė, R.; Picciau, L.; Davis, R.E. Possible insect vectors of 'Candidatus Phytoplasma asteris' and 'Ca. Phytoplasma pruni’-related strains in Lithuania. Zemdirbyste 2014, 101, 313–320. [Google Scholar] [CrossRef]
- Swisher, K.D.; Munyaneza, J.E.; Velásquez-Valle, R.; Mena-Covarrubias, J. Detection of Pathogens Associated with Psyllids and Leafhoppers in Capsicum annuum L. in the Mexican States of Durango, Zacatecas, and Michoacán. Plant Dis. 2018, 102, 146–153. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Purcell, A.H. Xylella fastidiosa: Cause of Pierce's Disease of Grapevine and Other Emergent Diseases. Plant Dis. 2002, 86, 1056–1066. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa Subspecies Pauca Sequence Type 53 by Different Insect Species. Insects 2019, 10, 324. [Google Scholar] [CrossRef]
- Almeida, R.P.P.; Nunney, L. How Do Plant Diseases Caused by Xylella fastidiosa Emerge? Plant Disease 2015, 99, 1457–1467. [Google Scholar] [CrossRef]
- Janse, J.D.; Obradovic, A. Xylella fastidiosa: Its biology, diagnosis, control and risks. J. Plant Pathol. 2010, 92, 35–48. [Google Scholar]
- Tumber, K.P.; Alston, J.M.; Fuller, K.B. Pierce's disease costs California $104 million per year. Calif. Agric. 2014, 68, 20–29. [Google Scholar] [CrossRef]
- Berisha, B.; Chen, Y.D.; Zhang, G.Y.; Xu, B.Y.; Chen, T.A. Isolation of Peirce’s disease bacteria from grapevines in Europe. Eur. J. Plant Pathol. 1998, 104, 427–433. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (southern Italy). J. Plant Pathol. 2013, 95, 659–668. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- EPPO. EPPO Global Database. 2022. Available online: https://gd.eppo.int/ (accessed on 2 December 2022).
- DGAV. Direção Geral da Alimentação e Veterinária/Divisão de Inspeção Fitossanitária e de Materiais de Propagação Vegetativa. Plano de Contingência: Xylella fastidiosa e Seus Vetores. 2021. Available online: https://www.dgav.pt/destaques/noticias/plano-de-contingencia-da-xylella-fastidiosa-e-seus-vetores/ (accessed on 20 January 2023).
- DGAV 2022. Xylella fastidiosa: Zonas Demarcadas de Xylella fastidiosa em Portugal. Available online: https://www.dgav.pt/plantas/conteudo/sanidade-vegetal/inspecao-fitossanitaria/informacao-fitossanitaria/xylella-fastidiosa/ (accessed on 2 December 2022).
- Biedermann, R.; Niedringhaus, R. The Plant- and Leafhoppers of Germany: Identification Keys to All Species; Wissenschaftlich Akademischer Buchvertrieb-Frund: Osnabrück, Germany, 2009; 409p. [Google Scholar]
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Dmitry, A.D. The Leafhoppers and Planthoppers of German (Hemiptera, Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects. Ann. Entomol. Soc. Am. 2006, 99, 187–188. [Google Scholar] [CrossRef]
- Le Quesne, W.J.; Payne, K.R. Cicadellidae (Typhlocybinae) with a check list of the British Auchenorhyncha (Hemiptera, Homoptera). Handb. Identif. Br. Insects 1981, 95 Pt 2, 95. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 January 2023).
- Magurran, A.E. Ecological Diversity and Its Measurement, 1st ed.; Springer: Dordrecht, The Netherlands, 1988; 179p. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, 3989. [Google Scholar] [CrossRef]
- Guerreiro, V. Monitorização e Medidas de Gestão de Auchenorrhyncha em Pomares de Prunóideas na Beira Interior: Estudo de Caso de Asymmetrasca decedens. Master’s Thesis, Faculty of Science of Lisbon, Lisbon, Portugal, 2020; p. 65. [Google Scholar]
- Nascimento, P. Auchenorrhyncha Monitoring and Proposal of Management Measures for Potential Pests on Peach Orchards in Beira Interior Region. Master’s Thesis, Faculty of Science of Lisbon, Lisbon, Portugal, 2020; p. 80. [Google Scholar]
- Neto, A. Potential Vectors of Xylella fastidiosa in Portuguese Olive Orchards: Survey in Alentejo Region and Control Measures. Master’s Thesis, Faculty of Science of Lisbon, Lisbon, Portugal, 2017; p. 130. [Google Scholar]
- Popova, G. Identification of Potential Vectors of Xylella fastidiosa in Portuguese Olive Orchards and Weeds. Master’s Thesis, Faculty of Science of Lisbon, Lisbon, Portugal, 2020; p. 151. [Google Scholar]
- Beal, D.J.; Cooper, M.; Daugherty, M.P.; Purcell, A.H.; Almeida, R.P.P. Seasonal Abundance and Infectivity of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa in California Vineyards. Environ. Entomol. 2021, 50, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Sicard, A.; Zeilinger, A.R.; Porcelli, F.; Purcell, A.H.; Almeida, R.P.P. Transmission of Xylella fastidiosa to Grapevine by the Meadow Spittlebug. Phytopathology 2016, 106, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Severin, H.H.P. Spittle-insect vectors of Pierce's disease virus: II. Life history and virus transmission. Hilgardia 1950, 19, 357–382. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovann, C.; Altamura, G.; Saladini, M.; Saponari, M.; Bosco, D. Transmission of Xylella fastidiosa subsp. pauca ST53 by the sharpshooter Cicadella viridis from different source plants and artificial diets. J. Econ. Entomol. 2022, 115, 1852–1858. [Google Scholar] [CrossRef]
- Bodino, N.; Demichelis, S.; Simonetto, A.; Volani, S.; Saladini, M.A.; Gilioli, G.; Bosco, D. Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy. Insects 2021, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- López-Mercadal, J.; Delgado, S.; Mercadal, P.; Seguí, G.; Lalucat, J.; Busquets, A.; Gomila, M.; Lester, K.; Kenyon, D.M.; Ruiz-Pérez, M.; et al. Collection of data and information in Balearic Islands on biology of vectors and potential vectors of Xylella fastidiosa (GP/EFSA/ALPHA/017/01). EFSA Support. Publ. 2021, 18, 6925E. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in olive orchards of Greece with special reference to Xylella fastidiosa vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Carpio, A.J.; Solana, M.; Tortosa, F.S.; Castro, J. Effect of cover crops in olive groves on Cicadomorpha communities. Span. J. Agric. Res. 2020, 18, e0303. [Google Scholar] [CrossRef]
- Elbeadaino, T.; Yaseen, T.; Valentini, F.; ben Moussa, I.; Mazzoni, V.; D’Onghia, A.M. Identification of three potential insect vectors of Xylella fastidiosa in southern Italy. Phytopathol. Mediterr. 2014, 53, 328–332. [Google Scholar] [CrossRef]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and Relative Abundance of Insect Vectors of Xylella fastidiosa in Olive Groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Tzobanoglou, D.K.; Goumas, D.; Trantas, E.; Zarboutis, I.; et al. Identification and Seasonal Abundance of Auchenorrhyncha with a Focus on Potential Insect Vectors of Xylella fastidiosa in Olive Orchards in Three Regions of Greece. J. Econ. Entomol. 2018, 111, 2536–2545. [Google Scholar] [CrossRef]
- Villa, M.; Rodrigues, I.; Baptista, P.; Fereres, A.; Pereira, J.A. Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects 2020, 11, 720. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Ben Moussa, I.E.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D'Onghia, A.M. Seasonal fluctuations of sap-feeding insect species infected by Xylella fastidiosa in Apulian olive groves of southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed xylem and phloem sap ingestion in sheath-feeders as normal dietary behavior: Evidence from the leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef]
- Purcell, A.H. Almond Leaf Scorch: Leafhopper and Spittlebug Vectors. J. Econ. Entomol. 1980, 73, 834–838. [Google Scholar] [CrossRef]
- Picciau, L.; Orrù, B.; Mandrioli, M.; Gonella, E.; Alma, A. Ability of Euscelidius variegatus to Transmit Flavescence Dorée Phytoplasma with a Short Latency Period. Insects 2020, 11, 603. [Google Scholar] [CrossRef]
- Laviña, A.; Sabaté, J.; Batlle, A. Spread and transmission of Bois noir phytoplasma in two regions of Spain. In Proceedings of the 15th Meeting ICVG, Stellenbosch, South Africa, 3–7 April 2006. Abstracts Extended. [Google Scholar]
- Quaglino, F.; Sanna, F.; Moussa, A.; Faccincani, M.; Passera, A.; Casati, P.; Bianco, P.A.; Mori, N. Identification and ecology of alternative insect vectors of ‘Candidatus Phytoplasma solani’ to grapevine. Sci. Rep. 2019, 9, 19522. [Google Scholar] [CrossRef]
- Batlle, A.; Martínez, M.A.; Laviña, A. Occurrence, distribution and epidemiology of Grapevine Yellows in Spain. Eur. J. Plant Pathol. 2000, 106, 811–816. [Google Scholar] [CrossRef]
- Riolo, P.; Landi, L.; Nardi, S.; Isidoro, N. Relationships among Hyalesthes obsoletus, its herbaceous host plants and "bois noir" phytoplasma strains in vineyard ecosystems in the Marche region (central-eastern Italy). Bull. Insectol 2007, 60, 353–354. [Google Scholar]
- Minuz, R.L.; Isidoro, N.; Casavecchia, S.; Burgio, G.; Riolo, P. Sex-Dispersal Differences of Four Phloem-Feeding Vectors and Their Relationship to Wild-Plant Abundance in Vineyard Agroecosystems. J. Econ. Entomol. 2013, 106, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A.; Wilson, R.C.; Schmidt, L.L. The effects of living mulches and weed cover on the dynamics of foliage- and soil-arthropod communities in three crop systems. Crop Prot. 1985, 4, 201–213. [Google Scholar] [CrossRef]
- Geppert, C.; La Bella, G.; Boscutti, F.; Sanna, F.; Marangoni, F.; Marini, L. Effects of temperature and plant diversity on orthopterans and leafhoppers in calcareous dry grasslands. J. Insect Conserv. 2021, 25, 287–296. [Google Scholar] [CrossRef]
- Kőrösi, À.; Batáry, P.; Orosz, A.; Rédei, D.; Báldi, A. Effects of grazing, vegetation structure and landscape complexity on grassland leafhoppers (Hemiptera: Auchenorrhyncha) and true bugs (Hemiptera: Heteroptera) in Hungary. Insect Conserv. Divers. 2012, 5, 57–66. [Google Scholar] [CrossRef]
- Masters, G.J.; Brown, V.K.; Clarke, I.P.; Whittaker, J.B.; Hollier, J.A. Direct and indirect effects of climate change on insect herbivores: Auchenorrhyncha (Homoptera). Ecol. Entomol. 1998, 23, 45–52. [Google Scholar] [CrossRef]
- McClure, M.S. Factors Affecting Colonization of an Orchard by Leafhopper (Homoptera: Cicadellidae) Vectors of Peach X-Disease. Environ. Entomol. 1982, 11, 695–700. [Google Scholar] [CrossRef]
- Abad, J.; Hermoso de Mendoza, I.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover crops in viticulture. A systematic review (1): Implications on soil characteristics and biodiversity in vineyard. OENO One 2021, 55, 295–312. [Google Scholar] [CrossRef]

| Family | Subfamily | Species | 2018 | 2019 | Total | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canopy | Inter-Row Vegetation | Canopy | Inter-Row Vegetation | ||||||||||||||||||||
| ME | ± | SE | N | ME | ± | SE | N | ME | ± | SE | N | ME | ± | SE | N | ME | ± | SE | N | ||||
| Aphrophoridae | C1 | Lepyronia coleoptrata (Linnaeus. 1758) | 0.0 | ± | 0.0 | 0 | 0.03 | ± | 0.03 | 1 | 0.0 | ± | 0.0 | 0 | 0.17 | ± | 0.10 | 6 | 0.13 | ± | 0.07 | 7 | |
| C2 | Neophilaenus campestris (Fallén. 1805) | 0.1 | ± | 0.1 | 2 | 0.10 | ± | 0.07 | 2 | 0.23 | ± | 0.11 | 8 | 1.43 | ± | 0.50 | 50 | 1.11 | ± | 0.34 | 62 | ||
| C3 | Neophilaenus lineatus (Linnaeus. 1758) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.04 | 2 | 0.05 | ± | 0.03 | 3 | ||
| C4 | Philaenus spumarius (Linnaeus. 1758) | 0.2 | ± | 0.1 | 5 | 1.29 | ± | 0.34 | 27 | 0.46 | ± | 0.16 | 16 | 1.83 | ± | 0.67 | 64 | 2.00 | ± | 0.45 | 112 | ||
| Cicadellidae | Agalliinae | C5 | Agallia consobrina Curtis. 1833 | 0.1 | ± | 0.1 | 3 | 0.05 | ± | 0.05 | 1 | 0.34 | ± | 0.20 | 12 | 0.26 | ± | 0.10 | 9 | 0.45 | ± | 0.15 | 25 |
| C6 | Agallia sp.1 | 0.0 | ± | 0.0 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.09 | ± | 0.06 | 3 | 0.07 | ± | 0.04 | 4 | ||
| C7 | Anaceratagallia glabra Dmitriev, 2020 (A. laevis) | 0.1 | ± | 0.1 | 3 | 1.57 | ± | 0.80 | 33 | 0.46 | ± | 0.17 | 16 | 7.51 | ± | 1.67 | 263 | 5.63 | ± | 1.21 | 315 | ||
| C8 | Anaceratagallia sp.1 | 0.1 | ± | 0.1 | 2 | 1.19 | ± | 0.31 | 25 | 0.14 | ± | 0.07 | 5 | 0.97 | ± | 0.40 | 34 | 1.18 | ± | 0.28 | 66 | ||
| C9 | Anaceratagallia venosa (de Fourcroy. 1785) | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.10 | 2 | 0.23 | ± | 0.23 | 8 | 0.14 | ± | 0.08 | 5 | 0.27 | ± | 0.15 | 15 | ||
| C10 | Austroagallia sinuata (Mulsant & Rey. 1855) | 0.2 | ± | 0.2 | 5 | 0.86 | ± | 0.42 | 18 | 0.34 | ± | 0.14 | 12 | 2.40 | ± | 0.72 | 84 | 2.13 | ± | 0.55 | 119 | ||
| C11 | Dryodurgades antoniae (Melichar. 1907) | 0.0 | ± | 0.0 | 1 | 1.05 | ± | 0.61 | 22 | 0.09 | ± | 0.06 | 3 | 0.46 | ± | 0.22 | 16 | 0.75 | ± | 0.28 | 42 | ||
| C12 | Dryodurgades sp.1 | 0.0 | ± | 0.0 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.14 | ± | 0.06 | 5 | 0.11 | ± | 0.04 | 6 | ||
| Aphrodinae | C13 | Anoscopus albifrons (Linnaeus. 1758) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.02 | 1 | |
| C14 | Aphrodes bicinctus (Schrank. 1776) | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.07 | 2 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.04 | ± | 0.03 | 2 | ||
| C15 | Aphrodes makarovi Zachvatkin. 1948 | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.10 | 2 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.05 | ± | 0.04 | 3 | ||
| C16 | Aphrodes sp.1 | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.07 | 2 | 0.00 | ± | 0.00 | 0 | 0.14 | ± | 0.06 | 5 | 0.13 | ± | 0.04 | 7 | ||
| C17 | Aphrodes sp.2 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.46 | ± | 0.25 | 16 | 0.29 | ± | 0.16 | 16 | ||
| C18 | Stroggylocephalus sp. | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | ||
| Cicadellinae | C19 | Cicadella viridis (Linnaeus. 1758) | 0.2 | ± | 0.1 | 4 | 0.43 | ± | 0.18 | 9 | 0.06 | ± | 0.04 | 2 | 8.34 | ± | 4.95 | 292 | 5.48 | ± | 3.12 | 307 | |
| Deltocephalinae | C20 | Arocephalus punctum (Flor. 1861) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | |
| C21 | Arocephalus sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | ||
| C22 | Artianus manderstjernii (Kirschbaum. 1868) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.51 | ± | 0.30 | 18 | 0.34 | ± | 0.19 | 19 | ||
| C23 | Artianus sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.11 | ± | 0.09 | 4 | 0.07 | ± | 0.06 | 4 | ||
| C24 | Athysanus argentarius Metcalf. 1955 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.31 | ± | 0.13 | 11 | 0.20 | ± | 0.08 | 11 | ||
| C25 | Balclutha frontalis (Ferrari. 1882) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.11 | ± | 0.07 | 4 | 0.07 | ± | 0.04 | 4 | ||
| C26 | Balclutha punctata (Fabricius. 1775) | 0.0 | ± | 0.0 | 0 | 0.48 | ± | 0.27 | 10 | 0.00 | ± | 0.00 | 0 | 1.57 | ± | 0.53 | 55 | 1.16 | ± | 0.35 | 65 | ||
| C27 | Balclutha sp.1 | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.09 | ± | 0.05 | 3 | 1.11 | ± | 0.55 | 39 | 0.77 | ± | 0.37 | 43 | ||
| C28 | Cicadula sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | ||
| C29 | Circulifer sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.04 | 2 | 0.04 | ± | 0.03 | 2 | ||
| C30 | Circulifer tenellus (Baker. 1896) | 0.0 | ± | 0.0 | 0 | 0.48 | ± | 0.31 | 10 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.18 | ± | 0.12 | 10 | ||
| C31 | Cosmotettix panzeri (Flor. 1861) | 0.0 | ± | 0.0 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.02 | 1 | ||
| C32 | Doliotettix lunulatus (Zetterstedt. 1838) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.11 | ± | 0.07 | 4 | 4.94 | ± | 2.89 | 173 | 3.16 | ± | 1.84 | 177 | ||
| C33 | Doratura homophyla (Flor. 1861) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.49 | ± | 0.38 | 17 | 0.32 | ± | 0.24 | 18 | ||
| C34 | Doratura stylata (Boheman. 1847) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.06 | 2 | 0.04 | ± | 0.04 | 2 | ||
| C35 | Enantiocephalus sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | ||
| C36 | Euscelidius schenckii (Kirschbaum. 1868) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.38 | 1 | ||
| C37 | Euscelidius variegatus (Kirschbaum. 1858) | 0.0 | ± | 0.0 | 0 | 0.38 | ± | 0.10 | 8 | 0.06 | ± | 0.04 | 2 | 1.14 | ± | 0.57 | 40 | 0.89 | ± | 0.02 | 50 | ||
| C38 | Euscelidius sp.1 | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.07 | 2 | 0.06 | ± | 0.06 | 2 | 0.06 | ± | 0.06 | 2 | 0.11 | ± | 0.91 | 6 | ||
| C39 | Euscelis incisus (Kirschbaum. 1858) | 0.0 | ± | 0.0 | 1 | 3.29 | ± | 1.76 | 69 | 0.03 | ± | 0.03 | 1 | 3.57 | ± | 0.99 | 125 | 3.50 | ± | 0.04 | 196 | ||
| C40 | Euscelis lineolatus Brullé. 1832 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.06 | 2 | 0.04 | ± | 0.08 | 2 | ||
| C41 | Euscelis ohausi W.Wagner. 1939 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.23 | ± | 0.11 | 8 | 0.16 | ± | 0.61 | 9 | ||
| C42 | Euscelis sp.1 | 0.0 | ± | 0.0 | 1 | 1.57 | ± | 0.82 | 33 | 0.20 | ± | 0.09 | 7 | 2.09 | ± | 0.80 | 73 | 2.04 | ± | 4.42 | 114 | ||
| C43 | Exitianus capicola (Stål. 1855) | 0.9 | ± | 0.7 | 18 | 17.48 | ± | 9.46 | 367 | 0.06 | ± | 0.06 | 2 | 9.46 | ± | 3.70 | 331 | 12.82 | ± | 0.41 | 718 | ||
| C44 | Exitianus sp.1 | 0.0 | ± | 0.0 | 0 | 1.62 | ± | 0.99 | 34 | 0.00 | ± | 0.00 | 0 | 0.40 | ± | 0.26 | 14 | 0.86 | ± | 0.21 | 48 | ||
| C45 | Goniagnathus brevis (Herrich-Schäffer. 1835) | 0.0 | ± | 0.0 | 0 | 0.29 | ± | 0.17 | 6 | 0.00 | ± | 0.00 | 0 | 0.63 | ± | 0.32 | 22 | 0.50 | ± | 0.45 | 28 | ||
| C46 | Goniagnathus guttulinervis (Kirschbaum. 1868) | 0.0 | ± | 0.0 | 0 | 0.57 | ± | 0.31 | 12 | 0.03 | ± | 0.03 | 1 | 2.17 | ± | 0.68 | 76 | 1.59 | ± | 0.10 | 89 | ||
| C47 | Goniagnathus sp.1 | 0.0 | ± | 0.0 | 1 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.29 | ± | 0.16 | 10 | 0.21 | ± | 0.02 | 12 | ||
| C48 | Hardya sp.1 | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.03 | 1 | ||
| C49 | Hardya tenuis (Germar. 1821) | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.03 | ± | 0.03 | 1 | 0.00 | ± | 0.00 | 0 | 0.04 | ± | 0.07 | 2 | ||
| C50 | Macrosteles alpinus (Zetterstedt. 1828) | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.10 | 2 | 0.03 | ± | 0.03 | 1 | 0.11 | ± | 0.09 | 4 | 0.13 | ± | 0.08 | 7 | ||
| C51 | Macrosteles sexnotatus (Fallén. 1806) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.17 | ± | 0.12 | 6 | 0.11 | ± | 0.05 | 6 | ||
| C52 | Macrosteles sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.09 | ± | 0.06 | 3 | 0.06 | ± | 0.06 | 2 | 0.09 | ± | 0.06 | 5 | ||
| C53 | Deltocephalinae sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.77 | ± | 0.77 | 27 | 0.48 | ± | 0.48 | 27 | ||
| C54 | Neoaliturus fenestratus (Herrich-Schäffer. 1834) | 0.2 | ± | 0.1 | 5 | 0.62 | ± | 0.23 | 13 | 0.37 | ± | 0.14 | 13 | 3.34 | ± | 0.70 | 117 | 2.64 | ± | 0.52 | 148 | ||
| C55 | Phlepsius ornatus (Perris. 1857) | 0.0 | ± | 0.0 | 1 | 0.10 | ± | 0.07 | 2 | 0.00 | ± | 0.00 | 0 | 0.71 | ± | 0.20 | 25 | 0.50 | ± | 0.13 | 28 | ||
| C56 | Phlepsius sp.1 | 0.0 | ± | 0.0 | 0 | 0.24 | ± | 0.14 | 5 | 0.03 | ± | 0.03 | 1 | 0.06 | ± | 0.06 | 2 | 0.14 | ± | 0.06 | 8 | ||
| C57 | Platymetopius major (Kirschbaum. 1868) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.06 | 2 | 0.04 | ± | 0.04 | 2 | ||
| C58 | Psammotettix sp.1 | 1.9 | ± | 0.5 | 39 | 37.81 | ± | 11.44 | 794 | 3.09 | ± | 0.76 | 108 | 67.80 | ± | 15.43 | 2373 | 59.18 | ± | 10.79 | 3314 | ||
| C59 | Rhopalopyx vitripennis (Flor. 1861) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.63 | ± | 0.23 | 22 | 0.39 | ± | 0.15 | 22 | ||
| C60 | Sardius argus (Marshall. 1866) | 0.0 | ± | 0.0 | 0 | 0.38 | ± | 0.13 | 8 | 0.09 | ± | 0.05 | 3 | 1.26 | ± | 0.39 | 44 | 0.98 | ± | 0.25 | 55 | ||
| C61 | Scaphoideus titanus Ball. 1932 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.09 | ± | 0.06 | 3 | 2.34 | ± | 2.03 | 82 | 1.52 | ± | 1.27 | 85 | ||
| C62 | Selenocephalus sacarroi Rodrigues. 1968 | 0.0 | ± | 0.0 | 0 | 0.05 | ± | 0.05 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.02 | 1 | ||
| C63 | Sonronius binotatus (Sahlberg. 1871) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.03 | ± | 0.03 | 1 | 0.04 | ± | 0.04 | 2 | ||
| C64 | Stegelytra putoni Mulsant & Rey. 1875 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.11 | ± | 0.06 | 4 | 0.09 | ± | 0.05 | 5 | ||
| C65 | Eupelix cuspidata (Fabricius. 1775) | 0.0 | ± | 0.0 | 0 | 0.19 | ± | 0.09 | 4 | 0.09 | ± | 0.05 | 3 | 0.57 | ± | 0.21 | 20 | 0.48 | ± | 0.06 | 27 | ||
| Idiocerinae | C66 | Idiocerus lituratus (Fallén. 1806) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.02 | 1 | |
| C67 | Idiocerus sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.14 | ± | 0.12 | 5 | 0.03 | ± | 0.03 | 1 | 0.11 | ± | 0.05 | 6 | ||
| Megophthalminae | |||||||||||||||||||||||
| C68 | Megophthalmus scabripennis Edwards. 1915 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | ||
| Typhlocybinae | C69 | Alebra coryli Le Quesne. 1977 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.63 | ± | 0.63 | 22 | 0.06 | ± | 0.06 | 2 | 0.43 | ± | 0.39 | 24 | |
| C70 | Arboridia sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.06 | ± | 0.04 | 2 | 0.04 | ± | 0.03 | 2 | ||
| C71 | Empoasca sp.1 | 8.2 | ± | 3.9 | 172 | 2.05 | ± | 1.25 | 43 | 7.83 | ± | 1.68 | 274 | 0.86 | ± | 0.33 | 30 | 9.27 | ± | 1.82 | 519 | ||
| C72 | Empoasca vitis (Göthe. 1875) | 18.4 | ± | 6.1 | 386 | 6.29 | ± | 2.50 | 132 | 59.97 | ± | 2099 | 17.18 | 7.11 | ± | 1.94 | 249 | 51.18 | ± | 11.87 | 2866 | ||
| C73 | Eupteryx sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.14 | ± | 0.07 | 5 | 0.14 | ± | 0.12 | 5 | 0.18 | ± | 0.10 | 10 | ||
| C74 | Fruticidia bisignata (Mulsant & Rey. 1855) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.11 | ± | 0.05 | 4 | 0.00 | ± | 0.00 | 0 | 0.07 | ± | 0.03 | 4 | ||
| C75 | Jacobiasca lybica (de Bergevin & Zanon. 1922) | 13.8 | ± | 7.4 | 289 | 0.00 | ± | 0.00 | 0 | 13.29 | ± | 12.12 | 465 | 0.26 | ± | 0.19 | 9 | 13.63 | ± | 8.06 | 763 | ||
| C76 | Ribautiana tenerrima (Herrich-Schäffer. 1834) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.46 | ± | 0.21 | 16 | 0.06 | ± | 0.06 | 2 | 0.32 | ± | 0.15 | 18 | ||
| C77 | Zygina lunaris (Mulsant & Rey. 1855) | 0.0 | ± | 0.0 | 1 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.02 | ± | 0.02 | 1 | ||
| C78 | Zygina ordinaria (Ribaut. 1936) | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.14 | ± | 0.06 | 5 | 0.03 | ± | 0.03 | 1 | 0.11 | ± | 0.05 | 6 | ||
| C79 | Zygina sp.1 | 0.0 | ± | 0.0 | 0 | 0.10 | ± | 0.10 | 2 | 1.43 | ± | 1.40 | 50 | 0.00 | ± | 0.00 | 0 | 0.93 | ± | 0.87 | 52 | ||
| C80 | Zyginidia scutellaris (Herrich-Schäffer. 1838) | 2.6 | ± | 0.6 | 55 | 14.52 | ± | 5.13 | 305 | 2.43 | ± | 0.47 | 85 | 18.11 | ± | 3.75 | 634 | 19.27 | ± | 3.11 | 1079 | ||
| Ulopinae | C81 | Uteca sp.1 | 0.0 | ± | 0.0 | 0 | 0.00 | ± | 0.00 | 0 | 0.00 | ± | 0.00 | 0 | 0.03 | ± | 0.03 | 1 | 0.02 | ± | 0.02 | 1 | |
| Total | 12.01 | ± | 6.12 | 987 | 24.30 | ± | 11.11 | 2016 | 39.46 | ± | 25.97 | 2099 | 66.94 | ± | 29.92 | 5556 | 146.10 | ± | 65.13 | 11834 | |||
| 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|
| Richness | Canopy | 4.95 | ± | 0.5 | 6.43 | ± | 0.68 |
| Inter-row vegetation | 9.62 | ± | 0.9 | 13.86 | ± | 0.10 | |
| p-value | <0.001 | <0.001 | |||||
| H’ | Canopy | 1.00 | ± | 0.10 | 1.01 | ± | 0.10 |
| Inter-row vegetation | 1.44 | ± | 0.09 | 1.75 | ± | 0.11 | |
| p-value | 0.002 | <0.001 | |||||
| J’ | Canopy | 1.48 | ± | 0.60 | 1.42 | ± | 0.41 |
| Inter-row vegetation | 1.55 | ± | 0.40 | 1.60 | ± | 0.43 | |
| p-value | 0.64 | 0.14 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, I.; Rebelo, M.T.; Baptista, P.; Pereira, J.A. Cicadomorpha Community (Hemiptera: Auchenorrhyncha) in Portuguese Vineyards with Notes of Potential Vectors of Xylella fastidiosa. Insects 2023, 14, 251. https://doi.org/10.3390/insects14030251
Rodrigues I, Rebelo MT, Baptista P, Pereira JA. Cicadomorpha Community (Hemiptera: Auchenorrhyncha) in Portuguese Vineyards with Notes of Potential Vectors of Xylella fastidiosa. Insects. 2023; 14(3):251. https://doi.org/10.3390/insects14030251
Chicago/Turabian StyleRodrigues, Isabel, Maria Teresa Rebelo, Paula Baptista, and José Alberto Pereira. 2023. "Cicadomorpha Community (Hemiptera: Auchenorrhyncha) in Portuguese Vineyards with Notes of Potential Vectors of Xylella fastidiosa" Insects 14, no. 3: 251. https://doi.org/10.3390/insects14030251
APA StyleRodrigues, I., Rebelo, M. T., Baptista, P., & Pereira, J. A. (2023). Cicadomorpha Community (Hemiptera: Auchenorrhyncha) in Portuguese Vineyards with Notes of Potential Vectors of Xylella fastidiosa. Insects, 14(3), 251. https://doi.org/10.3390/insects14030251









