Niche and Range Shifts of the Fall Webworm (Hyphantria cunea Dury) in Europe Imply Its Huge Invasion Potential in the Future
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
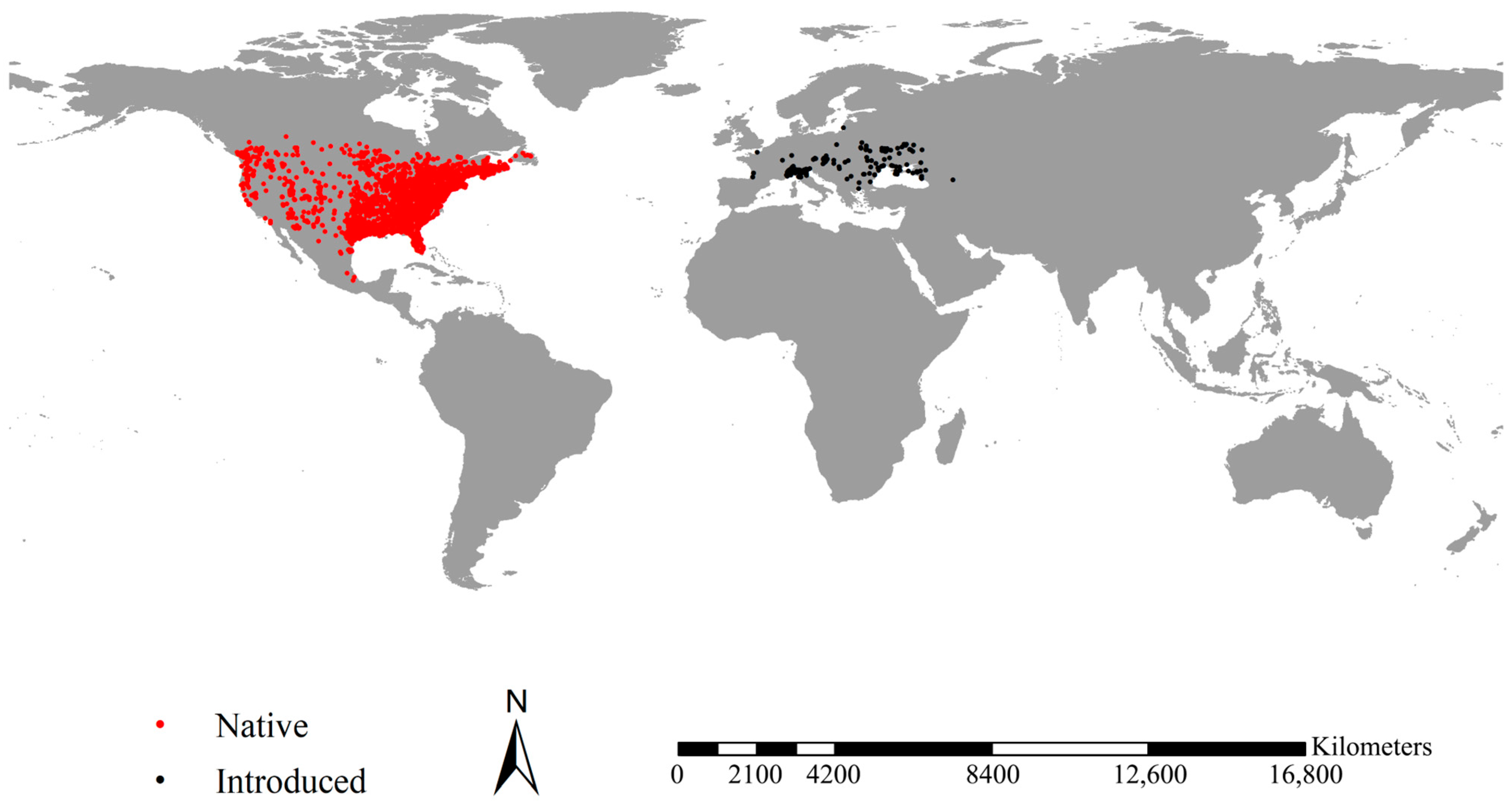
2.1. Occurrence of Fall Webworm
2.2. Climatic Predictors
2.3. Niche Shifts between the Fall Webworm in Europe and North America
2.4. Projecting Potential Ranges of the Fall Webworm in Europe and Those in North America
2.5. Range Shifts between the Fall Webworm in Europe and Those in North America
3. Results
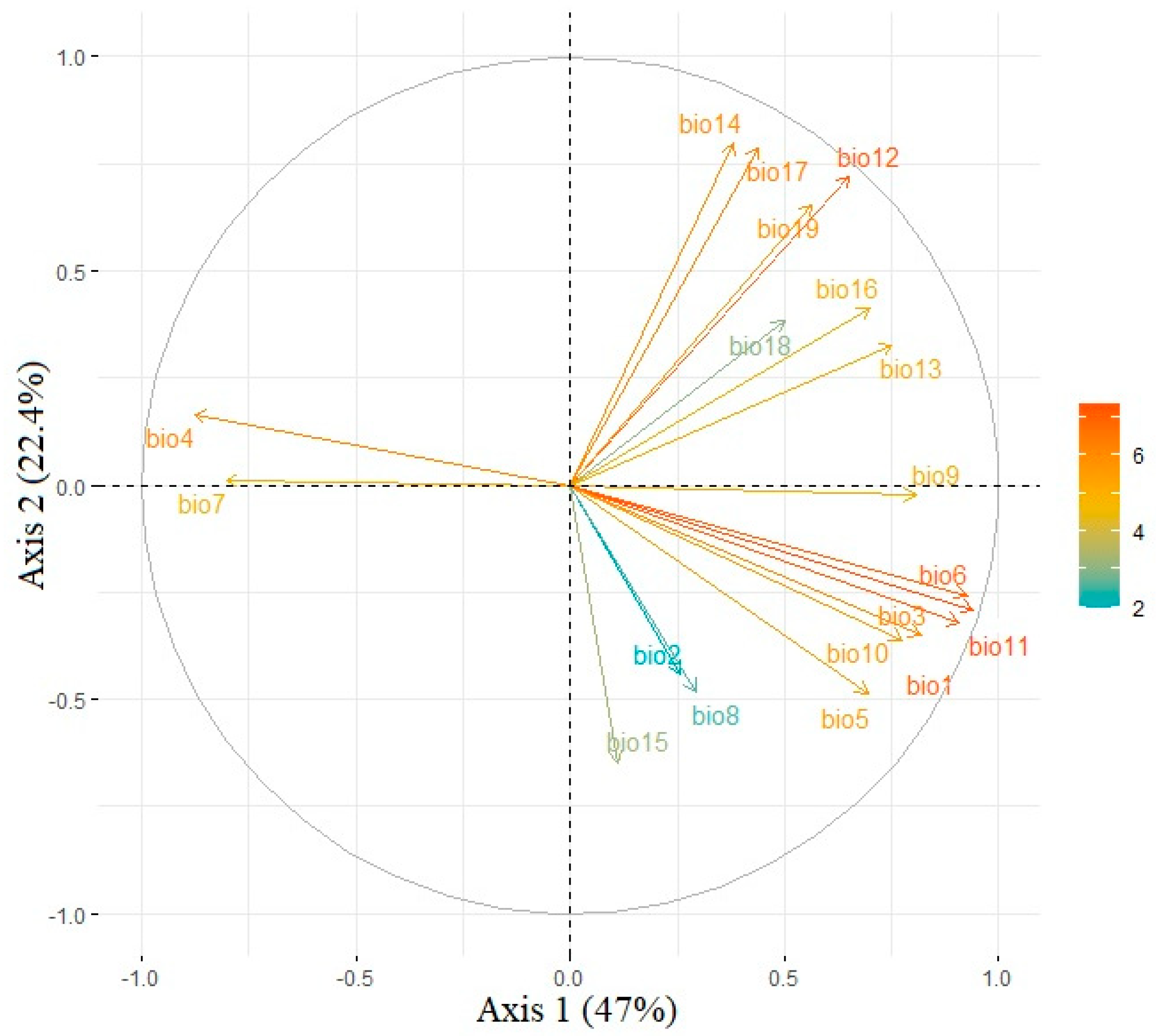
3.1. Climatic Predictors Responsible for the Niche Dynamics
3.2. Niche Dynamics of the Fall Webworm in Europe and Those in North America
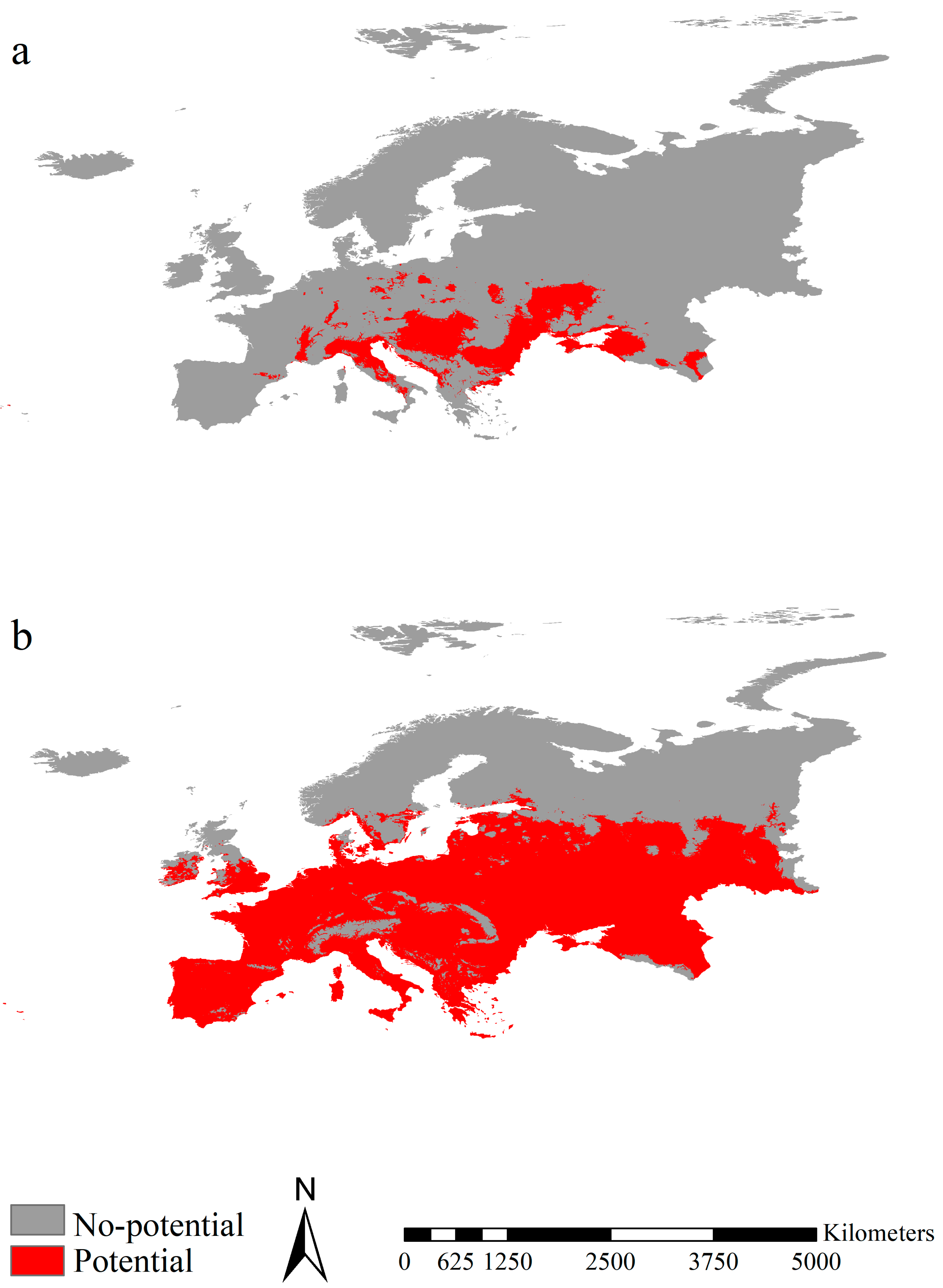
3.3. Potential Ranges of the Fall Webworm in Europe and Those in North America
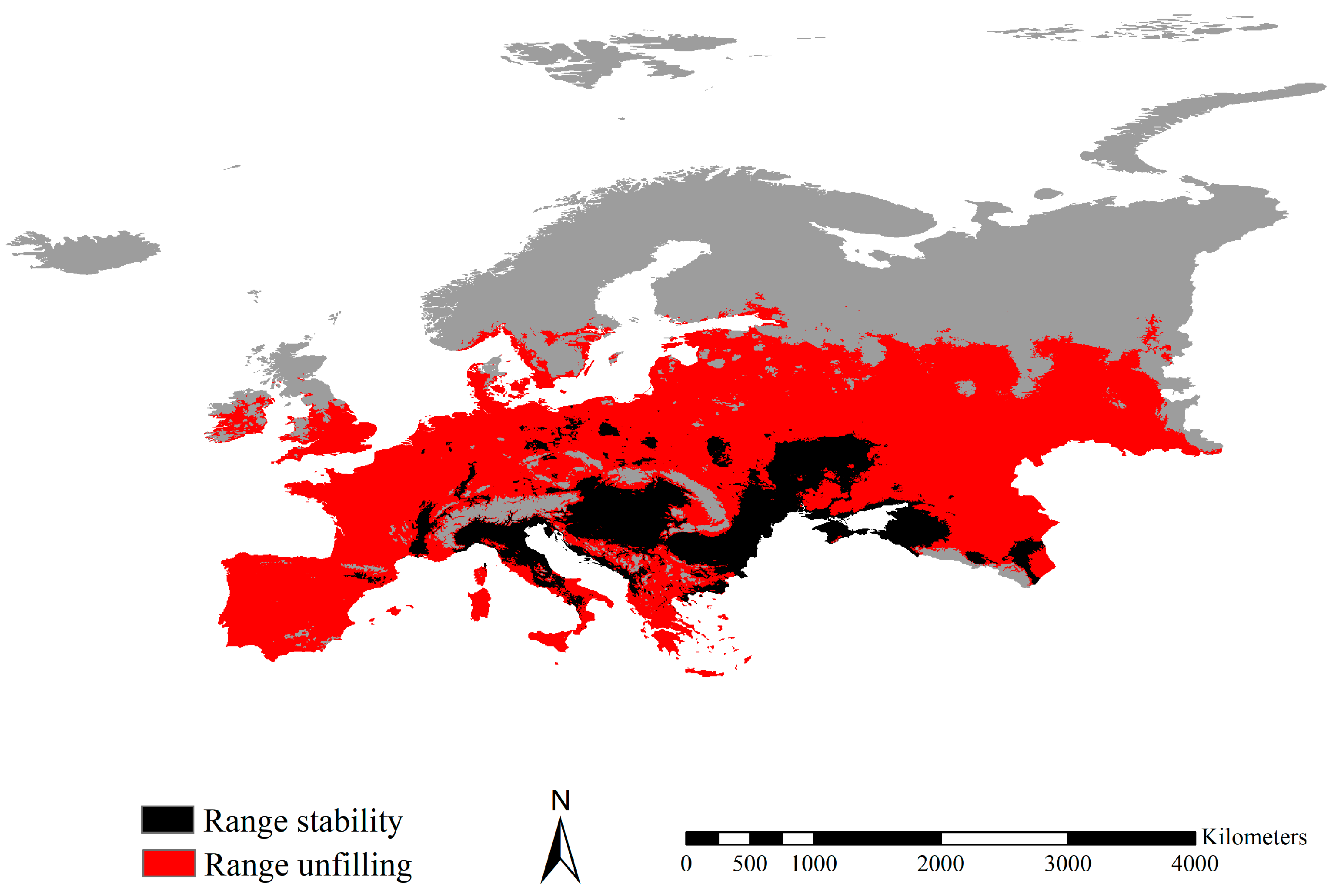
3.4. Range Dynamics between the Fall Webworm in Europe and North America
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Diagne, C.; Catford, J.A.; Essl, F.; Nunez, M.A.; Courchamp, F. What are the economic costs of biological invasions? A complex topic requiring international and interdisciplinary expertise. Neobiota 2020, 63, 25–37. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Madrinan, M.J.; Turell, M. Factors of Concern Regarding Zika and Other Aedes aegypti-Transmitted Viruses in the United States. J. Med. Entomol. 2017, 54, 251–257. [Google Scholar]
- Herath, J.M.M.K.; Abeyasundara, H.T.K.; De Silva, W.A.P.P.; Weeraratne, T.C.; Karunaratne, S.H.P.P. Weather-Based Prediction Models for the Prevalence of Dengue Vectors Aedes aegypti and Ae. albopictus. J. Trop. Med. 2022, 2022, 4494660. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanco, M.C. Risk of spread of tomato yellow leaf curl virus (TYLCV) in tomato crops under various climate change scenarios. Agric. Syst. 2019, 173, 524–535. [Google Scholar] [CrossRef]
- Thomas, S.M.; Verhoeven, M.R.; Walsh, J.R.; Larkin, D.J.; Hansen, G.J.A. Species distribution models for invasive Eurasian watermilfoil highlight the importance of data quality and limitations of discrimination accuracy metrics. Ecol. Evol. 2021, 11, 12567–12582. [Google Scholar] [CrossRef]
- Baer, K.C.; Gray, A.N. Biotic predictors improve species distribution models for invasive plants in Western US Forests at high but not low spatial resolutions. For. Ecol. Manag. 2022, 518, 120249. [Google Scholar] [CrossRef]
- Ma, L.; Cao, L.J.; Hoffmann, A.A.; Gong, Y.J.; Chen, J.C.; Chen, H.S.; Wang, X.B.; Zeng, A.P.; Wei, S.J.; Zhou, Z.S. Rapid and strong population genetic differentiation and genomic signatures of climatic adaptation in an invasive mealybug. Divers. Distrib. 2020, 26, 610–622. [Google Scholar] [CrossRef]
- Jardeleza, M.K.G.; Koch, J.B.; Pearse, I.S.; Ghalambor, C.K.; Hufbauer, R.A. The roles of phenotypic plasticity and adaptation in morphology and performance of an invasive species in a novel environment. Ecol. Entomol. 2022, 47, 25–37. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks of Cold Spring Harbor Symposium. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Cadotte, M.W.; McMahon, S.M.; Fukami, T. Exploring the relationship between niche breadth and invasion success. In Springer Series in Invasion Ecology; Vazquez, D.P., Ed.; Springer Nature One: New York, NY, USA, 2006; pp. 307–322. [Google Scholar]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araujo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Cornuault, J.; Khimoun, A.; Cuneo, P.; Besnard, G. Spatial segregation and realized niche shift during the parallel invasion of twoolive subspecies in south-eastern Australia. J. Biogeogr. 2015, 42, 1930–1941. [Google Scholar] [CrossRef]
- Pili, A.N.; Tingley, R.; Sy, E.Y.; Diesmos, M.L.L.; Diesmos, A.C. Niche shifts and environmental non-equilibrium undermine the usefulness of ecological niche models for invasion risk assessments. Sci. Rep. 2020, 10, 7972. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the Global Distribution of Solenopsis geminata (Hymenoptera: Formicidae) under Climate Change Using the MaxEnt Model. Insects 2021, 12, 229. [Google Scholar] [CrossRef]
- Kambach, S.; Lenoir, J.; Decocq, G.; Welk, E.; Seidler, G.; Dullinger, S.; Gegout, J.C.; Guisan, A.; Pauli, H.; Svenning, J.C. Of niches and distributions: Range size increases with niche breadth both globally and regionally but regional estimates poorly relate to global estimates. Ecography 2019, 42, 467–477. [Google Scholar] [CrossRef]
- Bernard, J.; Wall, C.B.; Costantini, M.S.; Rollins, R.L.; Atkins, M.L.; Cabrera, F.P.; Cetraro, N.D.; Feliciano, C.K.J.; Greene, A.L.; Kitamura, P.K.; et al. Plant part and a steep environmental gradient predict plant microbial composition in a tropical watershed. ISME J. 2021, 15, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Yarmand, H.; Sadeghi, S.; Mohammadi, M.; Mehrabi, A.; Zamani, S.; Ajamhasani, M.; Angeli, S. The fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae): A new emerging pest insect for forests and agricultural crops of Iran. In Review of Forests, Wood Products and Wood Biotechnology of Iran and Germany; Kharazipour, A.R., Schopper, C., Muller, C., Euring, M., Eds.; Gottingen University: Gottingen, Germany, 2009; pp. 120–134. [Google Scholar]
- Varjas, L.; Sehnal, F. Use of a juvenile hormone analogue against the fall webworm, Hyphantria cunea. Entomol. Exp. Appl. 1973, 16, 115–122. [Google Scholar] [CrossRef]
- Li, X.Y.; Cowles, R.S.; Cowles, E.A.; Gaugler, R.; Cox-Foster, D.L. Relationship between the successful infection by entomopathogenic nematodes and the host immune response. Int. J. Parasitol. 2007, 37, 365–374. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Ring, D.R. Biology and Management of the FallWebworm, Hyphantria cunea (Lepidoptera: Erebidae). J. Integr. Pest Manag. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Nakonechna, Y.O.; Stankevych, S.V.; Zabrodina, I.V.; Lezhenina, I.P.; Filatov, M.O.; Yushchuk, D.D.; Lutytska, N.V.; Molchanova, O.A.; Melenti, V.O.; Poliakh, V.M.; et al. Distribution area of Hyphantria cunea Drury: The analysis of Ukrainian and world data. Ukr. J. Ecol. 2019, 9, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Lee, H.S.; Lee, S.G.; Park, J.D.; Ahn, Y.J. Antifeeding activity of isoquinoline alkaloids identified in Coptis japonica roots against Hyphantria cunea (Lepidoptera: Arctiidae) and Agelastica coerulea (Coleoptera: Galerucinae). J. Econ. Entomol. 2000, 93, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, R.; Arakawa, M.; Yamakawa, R.; Yasmin, A.; Ando, T. Biosynthetic pathways of the sex pheromone components and substrate selectivity of the oxidation enzymes working in pheromone glands of the fall webworm, Hyphantria cunea. Insect Biochem. Mol. Biol. 2011, 41, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zhu, M.M.; Li, L.L.; Zhang, G.C.; Zheng, Y.; Li, T.; Sun, S.H. Cold hardiness characteristic of the overwintering pupae of fall webworm Hyphantria cunea (Drury) (Lepidoptera: Arctiidae) in the northeast of China. J. Asia-Pac. Entomol. 2015, 18, 39–45. [Google Scholar] [CrossRef]
- Fields, P.G.; Fleurat-Lessard, F.; Lavenseau, L.; Febvay, G.; Peypelut, L.; Bonnot, G. The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 1998, 44, 955–965. [Google Scholar] [CrossRef]
- Warren, L.O.; Tadic, M. The fall webworm, Hyphantria cunea, its distribution and natural enemies: A world list (Lepidoptera: Arctiidae). J. Kans. Entomol. Soc. 1967, 40, 194–202. [Google Scholar]
- Oliver, A.D. A behavioral study of two races of the fallwebworm, Hyphantria cunea (Lepidoptera: Arctiidae) in Louisiana. Ann. Entomol. Soc. Am. 1964, 57, 192–194. [Google Scholar] [CrossRef]
- Masaki, S. Biology of Hyphantria cunea Drury (Lepidoptera: Arctiidae) in Japan: A review. J. Rev. Plant Prot. Res. 1975, 8, 14–28. [Google Scholar]
- Zhang, X.X.; Wang, Z.J. Research progress on the Hyphantria cunea (Drury) of alien invasive species. J. Anhui Agric. Sci. 2009, 37, 215–219. [Google Scholar]
- Tang, X.G.; Yuan, Y.D.; Liu, X.F.; Zhang, J.C. Potential range expansion and niche shift of the invasive Hyphantria cunea between native and invasive countries. Ecol. Entomol. 2021, 46, 910–925. [Google Scholar] [CrossRef]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, A.S.; Essl, F.; Hojsgaard, D.; Kirchheimer, B.; Klatt, S.; Dawson, W.; Pergl, J.; Pysek, P.; van Kleunen, M.; Weber, E.; et al. Niche dynamics of alien species do not differ among sexual and apomictic flowering plants. New Phytol. 2016, 209, 1313–1323. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Yang, R.G.; Cao, R.Y.; Gong, X.; Feng, J.M. Large shifts of niche and range in the golden apple snail (Pomacea canaliculata), an aquatic invasive species. Ecosphere 2023, 14, e4391. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Marquez, J.R.G.; Jaime, R.; Gruber, B.; Lafourcade, B.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.J.; Wang, T.; Jiang, X.F.; Hu, X.K.; Feng, J.M. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Gong, X.; Feng, J.M.; Yang, R.J. Niche and range dynamics of Tasmanian blue gum (Eucalyptus globulus Labill.), a globally cultivated invasive tree. Ecol. Evol. 2022, 12, e9305. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Gallien, L.; Douzet, R.; Pratte, S.; Zimmermann, N.E.; Thuiller, W. Invasive species distribution models—How violating the equilibrium assumption can create new insights. Glob. Ecol. Biogeogr. 2012, 21, 1126–1136. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence–only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Zobel, M. Eltonian niche width determines range expansion success in ectomycorrhizal conifers. New Phytol. 2018, 220, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.X.; Feng, J.M. Global niche and range shifts of Batrachochytrium dendrobatidis, a highly virulent amphibian-killing fungus. Fungal Biol. 2022, 126, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.O.; Tadic, M. Fall Webworm, Hyphantria cunea (Drury). Ark. Agric. Exp. Sta. Bull. 1970, 759, 1–106. [Google Scholar]
- Szalay-Marzso, L. Biology and control of the Fall webworm (Hyphantria cunea Drury) in the Middle- and East European Countries. Organ. Eur. Mediterr. Pour La Prot. Des Plantes 1972, 3, 25–35. [Google Scholar] [CrossRef]
- Baird, A.B. An Historical Account of the Forest Tent Caterpillar and of the Fall Webworm in North America. In Proceedings of the 47th Annual Report of the Entomological Society of Ontario, Toronto, ON, Canada, 1 May 1917; pp. 73–87. [Google Scholar]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Beshai, R.A.; Barnes, E.E.; Murphy, S.M. The Role of Enemy-mediated Competition in Determining Fitness of a Generalist Herbivore. Southwest Entomol. 2019, 44, 69–77. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, P.; Yang, R.; Cao, R.; Hu, X.; Feng, J. Niche and Range Shifts of the Fall Webworm (Hyphantria cunea Dury) in Europe Imply Its Huge Invasion Potential in the Future. Insects 2023, 14, 316. https://doi.org/10.3390/insects14040316
Nie P, Yang R, Cao R, Hu X, Feng J. Niche and Range Shifts of the Fall Webworm (Hyphantria cunea Dury) in Europe Imply Its Huge Invasion Potential in the Future. Insects. 2023; 14(4):316. https://doi.org/10.3390/insects14040316
Chicago/Turabian StyleNie, Peixiao, Rujing Yang, Runyao Cao, Xiaokang Hu, and Jianmeng Feng. 2023. "Niche and Range Shifts of the Fall Webworm (Hyphantria cunea Dury) in Europe Imply Its Huge Invasion Potential in the Future" Insects 14, no. 4: 316. https://doi.org/10.3390/insects14040316
APA StyleNie, P., Yang, R., Cao, R., Hu, X., & Feng, J. (2023). Niche and Range Shifts of the Fall Webworm (Hyphantria cunea Dury) in Europe Imply Its Huge Invasion Potential in the Future. Insects, 14(4), 316. https://doi.org/10.3390/insects14040316



