Quantitative Effects of Temperature and Exposure Duration on the Occurrence and Repair of Indirect Chilling Injury in the Fall Armyworm Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Used
2.2. Comparison of Cold Tolerance among Stages and Temperatures
2.3. Effects of Temperature on Repair of Chilling Injury in Adults
2.4. Fitting Survival Rates to a Time–Temperature Model
2.5. Statistical Analyses
3. Results
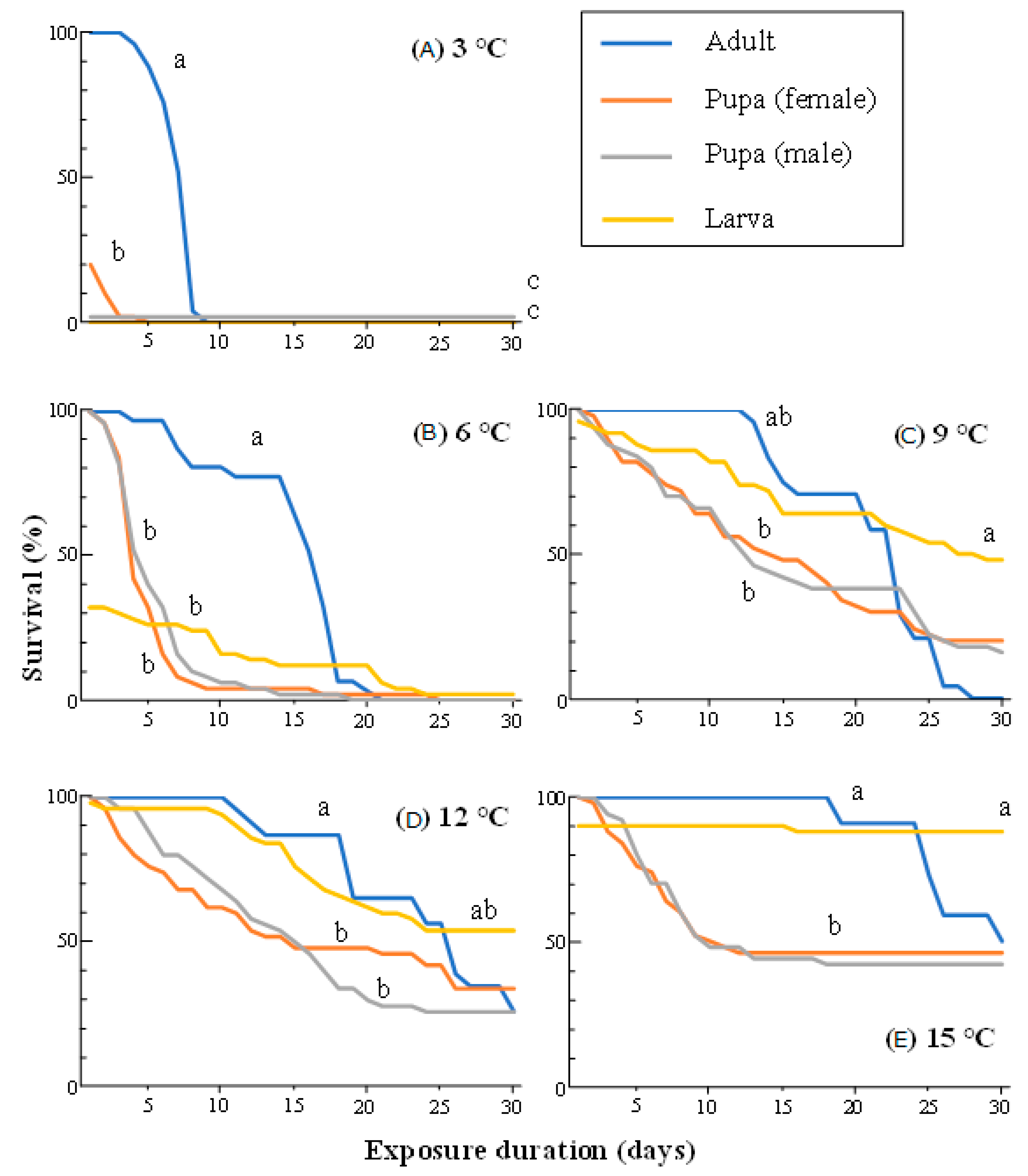
3.1. Survival of Adults, Pupae, and Larvae at Constant Low Temperatures
3.2. Survival of Adults at Different Constant Low Temperatures
3.3. Repair of Chilling Injury in Adults at Different Thermal Regimes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrews, K.L. The whorlworm, Spodoptera frugiperda, in central America and neighboring areas. Fla. Entomol. 1980, 63, 456–467. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennila, S.; Wang, Z.; Young, K.; Khurana, J.; Cruz, I.; Chen, J.; Reynaud, B.; Delatte, H.; Baufeld, P.; Rajan, R.P.; et al. G20 Discussion group on Fall Armyworm Spodoptera frugiperda (J.E.Smith) [Lepidoptera: Noctuidae]. In Proceedings of the International Workshop on Facilitatng International Research Collaboration on Transboundary Plant Pests, Tsukuba, Japan, 27–29 November 2019; pp. 27–29. [Google Scholar]
- Zhou, Y.; Wu, Q.; Zhang, H.; Wu, K. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Wu, M.F.; Qi, G.J.; Chen, H.; Ma, J.; Liu, J.; Jiang, Y.Y.; Lee, G.S.; Otuka, A.; Hu, G. Overseas immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), invading Korea and Japan in 2019. Insect Sci. 2022, 29, 505–520. [Google Scholar] [CrossRef]
- Hokkaido Plant Protection Office. Confirmation of injury on maize by larvae of the fall armyworm Spodoptera frugiperda. Spec. Not. Pest Forecast. Inf. 2020, 2, 1–2. [Google Scholar]
- Johnson, S.J. Migration and the life history of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Incest Sci. Appl. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Future climate scenarios project a decrease in risk of fall armyworm outbreaks. J. Agric. Sci. 2017, 155, 1219–1238. [Google Scholar] [CrossRef]
- Baloch, M.N.; Fan, J.; Muhammad, H.; Runzhi, Z. Mapping potential distribution of Spodoptera frugiperda (Lepidoptera; Noctidiae) in Central Asia. Insects 2020, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Jiang, C.; Guo, X.; Chen, D.; You, C.; Zhang, Y.; Wang, M.; Li, Q. Potential distribution of Spodoptera frugiperda (J.E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Conserv. 2020, 21, e00865. [Google Scholar] [CrossRef]
- Gilioli, G.; Sperandio, G.; Simonetto, A.; Ciampitti, M.; Gervasio, P. Assessing the risk of establishment and transient populations of Spodoptera frugiperda in Europe. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Lee, R.E., Jr. Principles of insect low temperature tolerance. In Insects at Low Temperature; Lee, R.E., Jr., Denlinger, D.L., Eds.; Chapman & Hall: New York, NY, USA, 1991; pp. 17–46. [Google Scholar]
- Bayley, J.S.; Winter, C.B.; Andersen, M.K.; Overgaard, J. Cold exposure causes cell death by depolarization-mediated Ca2+ overload in a chill-susceptible insect. Proc. Natl. Acad. Sci. USA 2018, 115, E9737–E9744. [Google Scholar] [CrossRef] [Green Version]
- Koštál, V.; Yanagimoto, M.; Bastl, J. Chilling-injury and disturbance of ion homeostasis in the coxal muscle of the tropical cockroach (Nauphoeta cinerea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 143, 171–179. [Google Scholar] [CrossRef]
- Wood, J.R.; Poe, S.L.; Leppla, N.C. Winter Survival of Fall Armyworm Pupae in Florida. Environ. Entomol. 1979, 8, 249–252. [Google Scholar] [CrossRef]
- Malekera, M.J.; Acharya, R.; Hwang, H.S.; Lee, K.Y. Effect of cold acclimation and rapid cold-hardening on the survival of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) under cold stress. J. Asia-Pac. Entomol. 2022, 25, 101862. [Google Scholar] [CrossRef]
- Keosentse, O.; Mutamiswa, R.; Plessis, H.D.; Nyamukondiwa, C. Developmental stage variation in Spodoptera frugiperda (Lepidoptera:Noctuidae) low temperature tolerance: Implications for overwintering. Austral. Entomol. 2021, 60, 400–410. [Google Scholar] [CrossRef]
- Perkins, W.D. Laboratory rearing of the fall armyworm. Fla. Entomol. 1979, 62, 87–91. [Google Scholar] [CrossRef]
- Vatanparast, M.; Ahmed, S.; Lee, D.H.; Hwang, S.H.; Hammock, B.; Kim, Y. EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua. Sci. Rep. 2022, 10, 20183. [Google Scholar] [CrossRef]
- Matsukura, K.; Tsumuki, H.; Izumi, Y.; Wada, T. Physiological response to low temperature in freshwater apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae). J. Exp. Biol. 2009, 212, 2558–2563. [Google Scholar] [CrossRef] [Green Version]
- Matsukura, K.; Izumi, Y.; Kumashiro, S.; Matsumura, M. Cold tolerance of the maize orange leafhopper, Cicadulina bipunctata. J. Insect. Physiol. 2014, 67, 114–119. [Google Scholar] [CrossRef]
- Yoshida, K.; Wada, T.; Matsukura, K.; Shiba, T. Potential overwintering areas of the alien apple snail, Pomacea canaliculata, in Japan at its northern distribution limit. Aquat. Invasions 2022, 17, 402–414. [Google Scholar] [CrossRef]
- Nedvěd, O.; Lavy, D.; Verhoef, H.A. Modelling the time–temperature relationship in cold injury and effect of high-temperature interruptions on survival in a chill-sensitive collembolan. Func. Ecol. 1998, 12, 816–824. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Zhang, Z.; Huang, J.; Zhang, J.; Hafeez, M.; Wang, L.; Guo, W.; Lu, Y. Supercooling capacity and cold tolerance of the South American tomato pinworm, Tuta absoluta, a newly invaded pest in China. J. Pest Sci. 2021, 94, 845–858. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, S.; Wu, Q.; Li, Y.; Wu, K. Cold hardiness of the invasive fall armyworm, Spodoptera frugiperda in China. J. Integr. Agric. 2021, 20, 764–771. [Google Scholar] [CrossRef]
- Grumiaux, C.; Andersen, M.K.; Colinet, H.; Overgaard, J. Fluctuating thermal regime preserves physiological homeostasis and reproductive capacity in Drosophila suzukii. J. Insect Physiol. 2019, 113, 33–41. [Google Scholar] [CrossRef]
- Colinet, H.; Renault, D.; Javal, M.; Berková, P.; Šimek, P.; Koštál, V. Uncovering the benefits of fluctuating thermal regimes on cold tolerance of Drosophila flies by combined metabolomic and lipidomic approach. Biochim. Biophys. Acta 2016, 1861, 1736–1745. [Google Scholar] [CrossRef]
- Rogers, R.; Leopold, R. Chilling Injury in the Housefly: Evidence for the Role of Oxidative Stress between Pupariation and Emergence. Cryobiol. 1996, 33, 447–458. [Google Scholar]
- Macmillan, H.; Sinclair, B. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011, 57, 12–20. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Matsukura, K. Quantitative Effects of Temperature and Exposure Duration on the Occurrence and Repair of Indirect Chilling Injury in the Fall Armyworm Spodoptera frugiperda. Insects 2023, 14, 356. https://doi.org/10.3390/insects14040356
Tanaka Y, Matsukura K. Quantitative Effects of Temperature and Exposure Duration on the Occurrence and Repair of Indirect Chilling Injury in the Fall Armyworm Spodoptera frugiperda. Insects. 2023; 14(4):356. https://doi.org/10.3390/insects14040356
Chicago/Turabian StyleTanaka, Yoshiaki, and Keiichiro Matsukura. 2023. "Quantitative Effects of Temperature and Exposure Duration on the Occurrence and Repair of Indirect Chilling Injury in the Fall Armyworm Spodoptera frugiperda" Insects 14, no. 4: 356. https://doi.org/10.3390/insects14040356
APA StyleTanaka, Y., & Matsukura, K. (2023). Quantitative Effects of Temperature and Exposure Duration on the Occurrence and Repair of Indirect Chilling Injury in the Fall Armyworm Spodoptera frugiperda. Insects, 14(4), 356. https://doi.org/10.3390/insects14040356





