The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants
Abstract
Simple Summary
Abstract
1. Introduction and Outlines
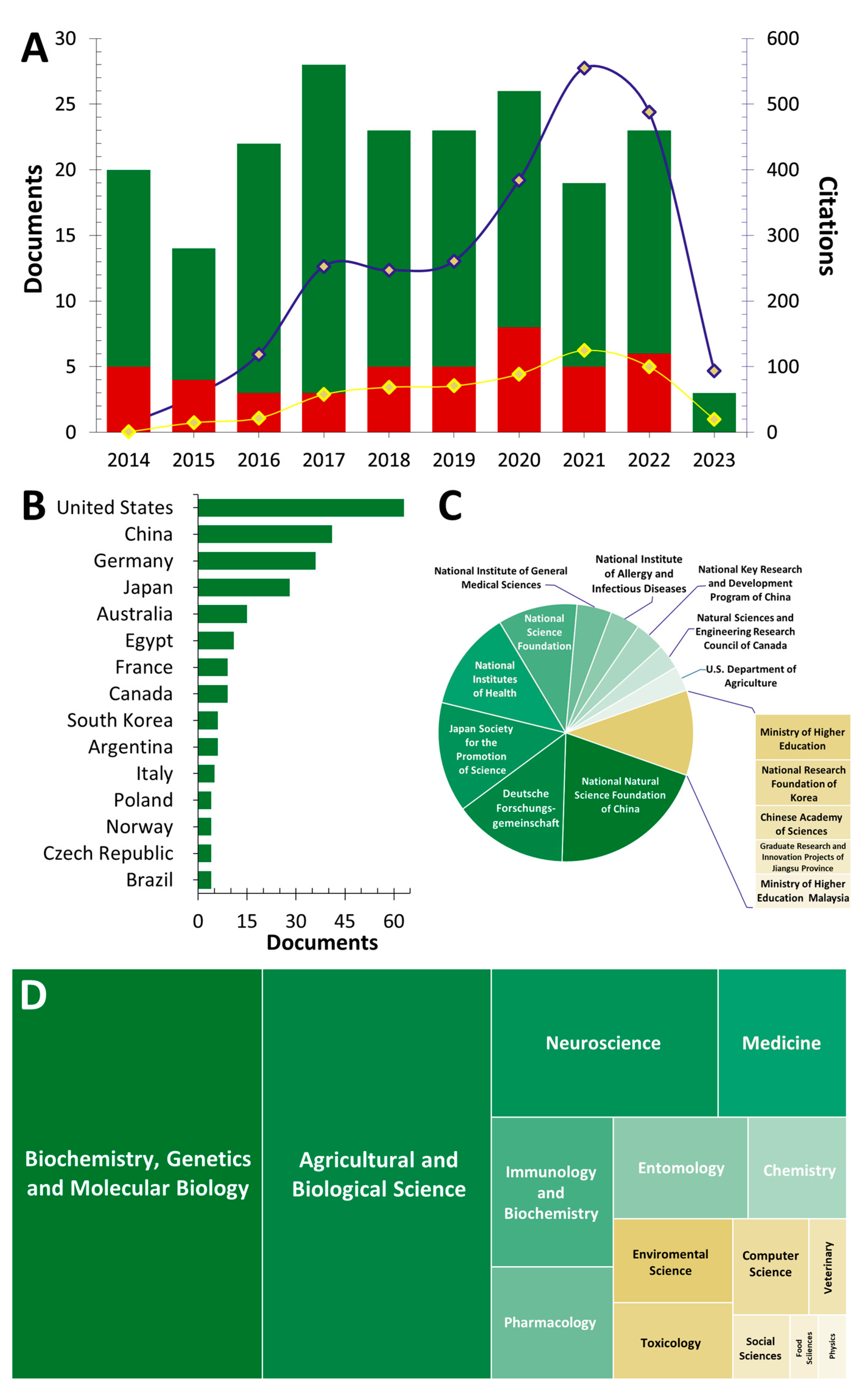
2. Bibliometric Analysis
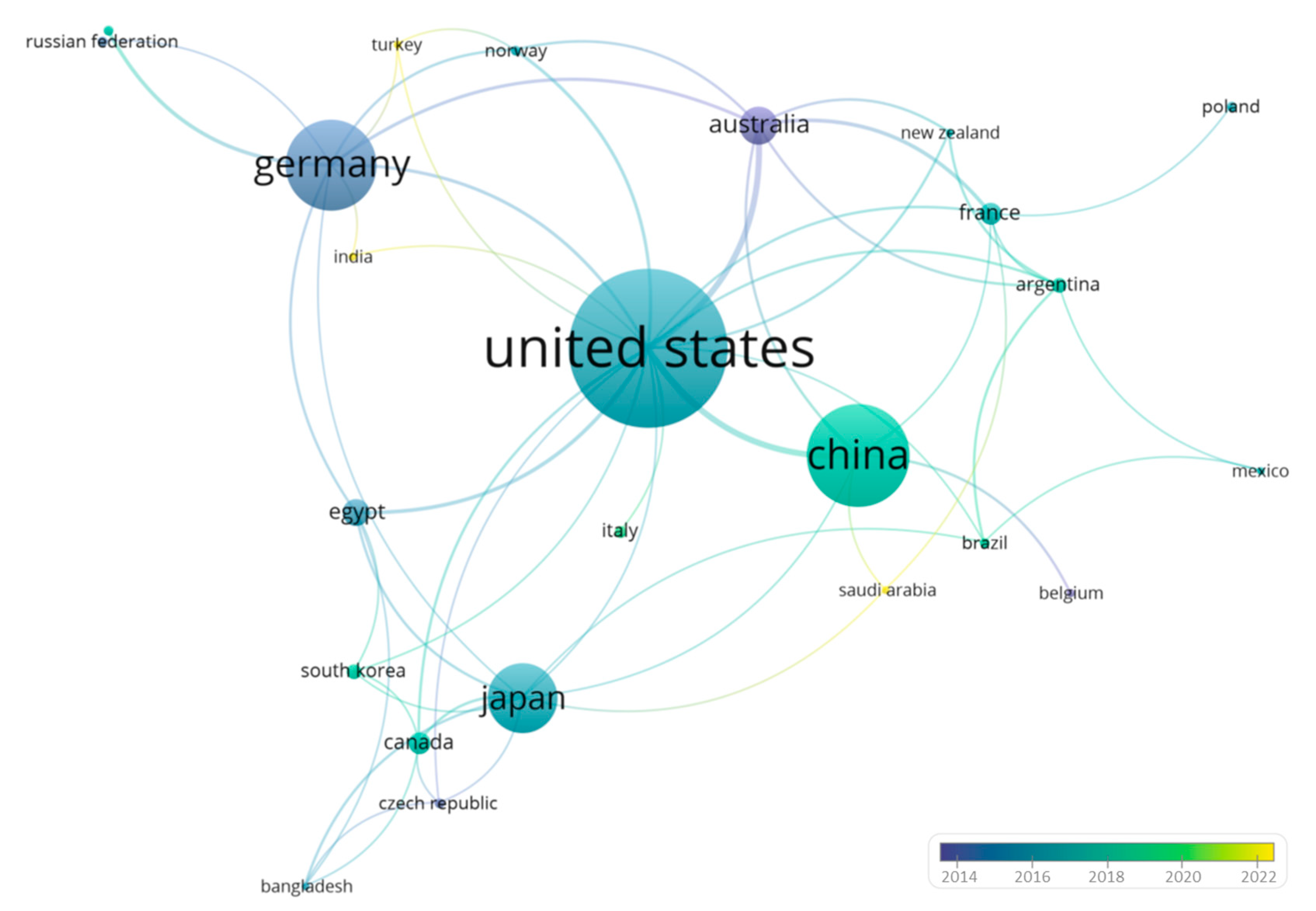
3. VosViewer Analysis
4. Functional Role of Biogenic Amines in Social Insects
4.1. Reproduction and Castes
4.2. Foraging, Aging, and Labor Division
4.3. Nestmate Recognition and Aggressive Behavior
4.4. Trophallaxis, Feeding, and Interspecific Interactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, E.O. The Insect Societies; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971; p. 548. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Richards, M.H. Social trait definitions influence evolutionary inferences: A phylogenetic approach to improving social terminology for bees. Curr. Opin. Insect Sci. 2019, 34, 97–104. [Google Scholar] [CrossRef]
- Knapp, R.A.; Norman, V.C.; Rouse, J.L.; Duncan, E.J. Environmentally responsive reproduction: Neuroendocrine signalling and the evolution of eusociality. Curr. Opin. Insect Sci. 2022, 53, 100951. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Hunt, J.H. Intracolony chemical communication in social insects. Insectes Sociaux 2013, 60, 275–291. [Google Scholar] [CrossRef]
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.H.; Richard, F.J. Intracolony vibroacoustic communication in social insects. Insectes Sociaux 2013, 60, 403–417. [Google Scholar] [CrossRef]
- Casacci, L.P.; Thomas, J.A.; Sala, M.; Treanor, D.; Bonelli, S.; Balletto, E.; Schönrogge, K. Ant pupae employ acoustics to communicate social status in their colony’s hierarchy. Curr. Biol. 2013, 23, 323–327. [Google Scholar] [CrossRef]
- Schönrogge, K.; Barbero, F.; Casacci, L.P.; Settele, J.; Thomas, J.A. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 2017, 134, 249–256. [Google Scholar]
- Cervo, R.; Cini, A.; Turillazzi, S. Visual recognition in social wasps. In Social Recognition in Invertebrates: The Knowns and the Unknowns; Aquiloni, L., Tricarico, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–145. [Google Scholar]
- Sheehan, M.J.; Tibbetts, E.A. Specialized face learning is associated with individual recognition in paper wasps. Science 2012, 334, 1272–1275. [Google Scholar]
- Leonhardt, S.D.; Menzel, F.; Nehring, V.; Schmitt, T. Ecology and evolution of communication in social insects. Cell 2016, 164, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454. [Google Scholar]
- Casacci, L.P.; Barbero, F.; Ślipiński, P.; Witek, M. The inquiline ant Myrmica karavajevi uses both chemical and vibroacoustic deception mechanisms to integrate into its host colonies. Biology 2021, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Okada, Y.; Shimoji, H.; Aonuma, H.; Miura, T.; Tsuji, K. Social evolution with decoupling of multiple roles of biogenic amines into different phenotypes in Hymenoptera. Front. Ecol. Evol. 2021, 9, 659160. [Google Scholar] [CrossRef]
- Anton, S.; Rössler, W. Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.R.; Shpigler, H.; Bloch, G.; Wheeler, D.E.; Robinson, G.E. Endocrine influences on insect societies. In Hormones, Brain and Behavior; Pfaff, D., Joels, M., Eds.; Academic Press: New York, NY, USA, 2017; pp. 421–451. [Google Scholar]
- Libersat, F.; Pflueger, H.J. Monoamines and the orchestration of behavior. BioScience 2004, 54, 17–25. [Google Scholar] [CrossRef]
- Roeder, T. Tyramine and octopamine: Ruling behavior and metabolism. Annu. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Traniello, J.F.A. Biogenic amines and collective organization in a superorganism: Neuromodulation of social behavior in ants. Brain. Behav. Evol. 2013, 82, 220–236. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Arganda, S.; Moreau, C.S.; Traniello, J.F.A. Origins of aminergic regulation of behavior in complex insect social systems. Front. Syst. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef]
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 2017, 62, 35–52. [Google Scholar] [CrossRef]
- Downer, R.G.H.; Hiripi, L. Biogenic amines in insects. In Insect Neurochemistry and Neurophysiology 1993; CRC Press: Boca Raton, FL, USA, 2019; pp. 23–38. ISBN 1351073591. [Google Scholar]
- Mannino, G.; Abdi, G.; Maffei, M.E.; Barbero, F. Origanum vulgare terpenoids modulate Myrmica scabrinodis brain biogenic amines and ant behaviour. PLoS ONE 2018, 13, e0209047. [Google Scholar] [CrossRef] [PubMed]
- Brandau, K.; Axelrod, J. The biosynthesis of octopamine. Naunyn-Schmiedeb. Arch. Pharmacol. 1972, 273, 123–133. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef]
- Welford, R.W.; Vercauteren, M.; Trébaul, A.; Cattaneo, C.; Eckert, D.; Garzotti, M.; Sieber, P.; Segrestaa, J.; Studer, R.; Groenen, P.M.; et al. Serotonin biosynthesis as a predictive marker of serotonin pharmacodynamics and disease-induced dysregulation. Sci. Rep. 2016, 6, 30059. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The Geomagnetic Field (GMF) is necessary for black garden ant (Lasius niger L.) foraging and modulates orientation potentially through aminergic regulation and MagR Expression. Int. J. Mol. Sci. 2023, 24, 4387. [Google Scholar] [CrossRef]
- Shin, M.; Friedman, D.A.; Gordon, D.M.; Venton, B.J. Measurement of natural variation of neurotransmitter tissue content in red harvester ant brains among different colonies. Anal. Bioanal. Chem. 2020, 412, 6167–6175. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, A.; Annangudi, S.P.; Richmond, T.A.; Ament, S.A.; Xie, F.; Southey, B.R.; Rodriguez-zas, S.R.; Robinson, G.E.; Sweedler, J.V. Quantitative peptidomics reveal brain peptide signatures of behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 2383–2388. [Google Scholar] [CrossRef]
- Pratavieira, M.; da Silva Menegasso, A.R.; Garcia, A.M.C.; dos Santos, D.S.; Gomes, P.C.; Malaspina, O.; Palma, M.S. MALDI imaging analysis of neuropeptides in the africanized honeybee (Apis mellifera) brain: Effect of ontogeny. J. Proteome Res. 2014, 13, 3054–3064. [Google Scholar] [CrossRef]
- Schmitt, F.; Vanselow, J.T.; Schlosser, A.; Kahnt, J.; Rössler, W.; Wegener, C. Neuropeptidomics of the carpenter ant Camponotus floridanus. J. Proteome Res. 2015, 14, 1504–1514. [Google Scholar] [CrossRef]
- Schmitt, F.; Vanselow, J.T.; Schlosser, A.; Wegener, C.; Rössler, W. Neuropeptides in the desert ant Cataglyphis fortis: Mass spectrometric analysis, localization, and age-related changes. J. Comp. Neurol. 2017, 525, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Vander Meer, R.K.; Chinta, S.P.; Jones, T.H.; O’Reilly, E.E.; Adams, R.M. Male fire ant neurotransmitter precursors trigger reproductive development in females after mating. Commun. Biol. 2021, 4, 1400. [Google Scholar] [CrossRef]
- Gospocic, J.; Shields, E.J.; Glastad, K.M.; Lin, Y.; Penick, C.A.; Yan, H. The neuropeptide corazonin controls social behavior and caste identity in ants. Cell 2017, 170, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Tlili, A.; Altinay, F.; Huang, R.; Altinay, Z.; Olivier, J.; Mishra, S.; Jemni, M.; Burgos, D. Are we there yet? A systematic literature review of Open Educational Resources in Africa: A combined content and bibliometric analysis. PLoS ONE 2022, 17, e0262615. [Google Scholar] [CrossRef] [PubMed]
- Bertocci, F.; Mannino, G. Pearls before swine: Plant-derived wastes to produce low-cholesterol meat from farmed pigs—A bibliometric analysis combined to meta-analytic studies. Foods 2023, 12, 571. [Google Scholar] [CrossRef]
- Rothballer, A.B. Aggression, defense and neurohumors. In Aggression and Defense; Clemente, C.D., Lindsley, D.B., Eds.; University of California: Los Angelos, CA, USA, 1967; pp. 135–171. [Google Scholar]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Bertocci, F.; Mannino, G. Can agri-food waste be a sustainable alternative in aquaculture? A bibliometric and meta-analytic study on growth performance, innate immune system, and antioxidant defenses. Foods 2022, 11, 1861. [Google Scholar] [CrossRef]
- Sasaki, K.; Watanabe, T. Sex-specific regulatory systems for dopamine production in the honey bee. Insects 2022, 13, 128. [Google Scholar] [CrossRef]
- Sasaki, K.; Matsuyama, H.; Morita, N.; Ono, M. Caste differences in the association between dopamine and reproduction in the bumble bee Bombus ignitus. J. Insect Physiol. 2017, 103, 107–116. [Google Scholar] [CrossRef]
- Sasaki, K.; Yamasaki, K.; Nagao, T. Neuro-endocrine correlates of ovarian development and egg-laying behaviors in the primitively eusocial wasp (Polistes chinensis). J. Insect Physiol. 2007, 53, 940–949. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yamada, Y.Y.; Sasaki, K. Identification of biogenic amines involved in photoperiod-dependent caste-fate determination during the adult stage in a temperate paper wasp. J. Insect Physiol. 2021, 131, 104223. [Google Scholar] [CrossRef] [PubMed]
- Farkhary, S.I.; Sasaki, K.; Hayashi, S.; Harano, K.; Koyama, S.; Satoh, T. Fighting and stinging responses are affected by a dopamine receptor blocker flupenthixol in honey bee virgin queens. J. Insect Behav. 2017, 30, 717–727. [Google Scholar] [CrossRef]
- Sasaki, K.; Harada, M. Dopamine production in the brain is associated with caste-specific morphology and behavior in an artificial intermediate honey bee caste. PLoS ONE 2020, 15, e0244140. [Google Scholar] [CrossRef]
- Harano, K.; Sasaki, M.; Nagao, T.; Sasaki, K. Dopamine influences locomotor activity in honeybee queens: Implications for a behavioural change after mating. Physiol. Entomol. 2008, 33, 395–399. [Google Scholar] [CrossRef]
- Farkhary, S.I.; Sasaki, K.; Hayashi, S.; Harano, K.; Koyama, S.; Satoh, T. Suppression of flight activity by a dopamine receptor antagonist in honey bee (Apis mellifera) virgin queens and workers. J. Insect Behav. 2019, 32, 218–224. [Google Scholar] [CrossRef]
- Cuvillier-Hot, V.; Lenoir, A. Biogenic amine levels, reproduction and social dominance in the queenless ant Streblognathus peetersi. Sci. Nat. 2006, 93, 149–153. [Google Scholar] [CrossRef]
- Penick, C.A.; Brent, C.S.; Dolezal, K.; Liebig, J. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 2014, 217, 1496–1503. [Google Scholar] [CrossRef]
- Okada, Y.; Sasaki, K.; Miyazaki, S.; Shimoji, H.; Tsuji, K.; Miura, T. Social dominance and reproductive differentiation mediated by dopaminergic signaling in a queenless ant. J. Exp. Biol. 2015, 218, 1091–1098. [Google Scholar] [CrossRef]
- Boulay, R.; Hooper-Bui, L.M.; Woodring, J. Oviposition and oogenesis in virgin fire ant females Solenopsis invicta are associated with a high level of dopamine in the brain. Physiol. Entomol. 2001, 26, 294–299. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Preston, C.A.; Hefetz, A. Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Sci. Nat. 2008, 95, 1155–1158. [Google Scholar] [CrossRef]
- Beggs, K.T.; Mercer, A.R. Dopamine receptor activation by honey bee queen pheromone. Curr. Biol. 2009, 19, 1206–1209. [Google Scholar] [CrossRef]
- Vergoz, V.; McQuillan, H.J.; Geddes, L.H.; Pullar, K.; Nicholson, B.J.; Paulin, M.G.; Mercer, A.R. Peripheral modulation of worker bee responses to queen mandibular pheromone. Proc. Natl. Acad. Sci. USA 2009, 106, 20930–20935. [Google Scholar] [CrossRef]
- Aonuma, H.; Watanabe, T. Octopaminergic system in the brain controls aggressive motivation in the ant, Formica japonica. Acta Biol. Hung. 2012, 63, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yokoi, K.; Toga, K. Bumble bee queens activate dopamine production and gene expression in nutritional signaling pathways in the brain. Sci. Rep. 2021, 11, 5526. [Google Scholar] [CrossRef]
- Sasaki, K.; Ugajin, A.; Harano, K. Caste-specific development of the dopaminergic system during metamorphosis in female honey bees. PLoS ONE 2018, 13, e0206624. [Google Scholar] [CrossRef]
- Hoyer, S.C.; Liebig, J.; Rössler, W. Biogenic amines in the ponerine ant Harpegnathos saltator: Serotonin and dopamine immunoreactivity in the brain. Arthropod Struct. Dev. 2005, 34, 429–440. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagao, T.; Sasaki, K. Consumption of tyrosine in royal jelly increases brain levels of dopamine and tyramine and promotes transition from normal to reproductive workers in queenless honey bee colonies. Gen. Comp. Endocrinol. 2015, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shimoji, H.; Aonuma, H.; Miura, T.; Tsuji, K.; Sasaki, K.; Okada, Y. Queen contact and among-worker interactions dually suppress worker brain dopamine as a potential regulator of reproduction in an ant. Behav. Ecol. Sociobiol. 2017, 71, 35. [Google Scholar] [CrossRef]
- Maák, I.; Camera, J.; Casacci, L.P.; Barbero, F.; Trigos-Peral, G.; Ślipiński, P.; Bonelli, S.; Zaccagno, M.; Witek, M. The influence of colony traits on the collective behaviour of Myrmica scabrinodis ants. Insect Conserv. Diver. 2019, 12, 481–491. [Google Scholar] [CrossRef]
- Gordon, D.M. The evolution of the algorithms for collective behavior. Cell Syst. 2016, 3, 514–520. [Google Scholar] [CrossRef]
- Schulz, D.J.; Robinson, G.E. Biogenic amines and division of labor in honey bee colonies: Behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 1999, 184, 481–488. [Google Scholar] [CrossRef]
- Seid, M.A.; Traniello, J.F.A. Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Sci. Nat. 2005, 92, 198–201. [Google Scholar] [CrossRef]
- Wnuk, A.; Wiater, M.; Godzińska, E.J. Effect of past and present behavioural specialization on brain levels of biogenic amines in workers of the red wood ant Formica polyctena. Physiol. Entomol. 2011, 36, 54–61. [Google Scholar] [CrossRef]
- Smith, A.R.; Muscedere, M.L.; Seid, M.A.; Traniello, J.F.A.; Hughes, W.O.H. BAs are associated with worker task but not patriline in the leaf-cutting ant Acromyrmex echinatior. J. Comp. Physiol. 2013, 199, 1117–1127. [Google Scholar] [CrossRef]
- Schulz, D.J.; Robinson, G.E. Octopamine influences division of labor in honey bee colonies. J. Comp. Physiol. A 2001, 87, 53–61. [Google Scholar] [CrossRef]
- Schulz, D.J.; Sullivan, J.P.; Robinson, G.E. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm. Behav. 2002, 42, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Søvik, E.; Perry, C.J.; Barron, A.B. Insect reward systems: Comparing flies and bees. In Advances in Insect Physiology: Genomics, Physiology and Behaviour of Social Insects; Zayed, A., Kent, C.F., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 189–226. [Google Scholar]
- Klappenbach, M.; Kaczer, L.; Locatelli, F. Dopamine interferes with appetitive long-term memory formation in honey bees. Neurobiol. Learn. Mem. 2013, 106, 230–237. [Google Scholar] [CrossRef]
- Lenschow, M.; Cordel, M.; Pokorny, T.; Mair, M.M.; Hofferberth, J.; Ruther, J. The post-mating switch in the pheromone response of Nasonia females is mediated by dopamine and can be reversed by appetitive learning. Front. Behav. Neurosci. 2018, 12, 14. [Google Scholar] [CrossRef]
- Baracchi, D.; Cabirol, A.; Devaud, J.M.; Haase, A.; d’Ettorre, P.; Giurfa, M. Pheromone components affect motivation and induce persistent modulation of associative learning and memory in honey bees. Commun. Biol. 2020, 3, 447. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, M.L.; Johnson, N.; Gillis, B.C.; Kamhi, J.F.; Traniello, J.F.A. Serotonin modulates worker responsiveness to trail pheromone in the ant Pheidole dentata. J. Comp. Physiol. A 2012, 198, 219–227. [Google Scholar] [CrossRef]
- Crozier, R.H.; Dix, M.W. Analysis of two genetic models for the innate components of colony odour in social Hymenoptera. Behav. Ecol. Sociobiol. 1979, 4, 217–224. [Google Scholar] [CrossRef]
- Obin, M.S.; Vander Meer, R.K. Mechanism of template-label matching in fire ant, Solenopsis invicta Buren, nestmate recognition. Anim. Behav. 1989, 38, 430–435. [Google Scholar] [CrossRef]
- Brandstaetter, A.S.; Kleineidam, C.J. Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. J. Neurophysiol. 2011, 106, 2437–2449. [Google Scholar] [CrossRef]
- Menzel, F.; Schmitt, T.; Bluthgen, N. Intraspecific nestmate recognition in two parabiotic ant species: Acquired recognition cues and low inter-colony discrimination. Insectes Sociaux 2009, 56, 251–260. [Google Scholar] [CrossRef]
- Robinson, G.E.; Heuser, L.M.; LeConte, Y.; Lenquette, F.; Hollingworth, R.M. Neurochemicals aid bee nestmate recognition. Nature 1999, 399, 534–535. [Google Scholar] [CrossRef]
- Nouvian, M.; Mandal, S.; Jamme, C.; Claudianos, C.; D’Ettorre, P.; Reinhard, J.; Barron, A.B.; Giurfa, M. Cooperative defence operates by social modulation of biogenic amine levels in the honey bee brain. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172653. [Google Scholar] [CrossRef] [PubMed]
- Bubak, A.N.; Yaeger, J.D.; Renner, K.J.; Swallow, J.G.; Greene, M.J. Neuromodulation of nestmate recognition decisions by pavement ants. PLoS ONE 2016, 11, e0166417. [Google Scholar] [CrossRef]
- Yakovlev, I.K. Effects of octopamine on aggressive behavior in red wood ants. Neurosci. Behav. Physiol. 2018, 48, 279–288. [Google Scholar] [CrossRef]
- Szczuka, A.; Korczyńska, J.; Wnuk, A.; Symonowicz, B.; Szwacka, A.G.; Mazurkiewicz, P.; Kostowski, W.; Godzińska, E.J. The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta Neurobiol. Exp. 2013, 73, 495–520. [Google Scholar]
- Aonuma, H. Serotonergic control in initiating defensive responses to unexpected tactile stimuli in the trap-jaw ant Odontomachus kuroiwae. J. Exp. Biol. 2020, 223, jeb228874. [Google Scholar] [CrossRef] [PubMed]
- Kamhi, J.F.; Nunn, K.; Robson, S.K.; Traniello, J.F. Polymorphism and division of labour in a socially complex ant: Neuromodulation of aggression in the Australian weaver ant, Oecophylla smaragdina. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150704. [Google Scholar] [CrossRef] [PubMed]
- Boulay, R.; Soroker, V.; Godziñska, E.J.; Hefetz, A.; Lenoir, A. Octopamine reverses the isolation-induced increase in trophallaxis in the carpenter ant Camponotus fellah. J. Exp. Biol. 2000, 203, 513–520. [Google Scholar] [CrossRef]
- Boulay, R.; Lenoir, A. Social isolation of mature workers affects nestmate recognition in the ant Camponotus fellah. Behav. Process. 2001, 15, 67–73. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Yamaoka, R.; Aonuma, H. Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J. Exp. Biol. 2011, 214, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Falibene, A.; Rössler, W.; Josens, R. Serotonin depresses feeding behaviour in ants. J. Insect Physiol. 2012, 58, 7–17. [Google Scholar] [CrossRef]
- Hojo, M.K.; Pierce, N.E.; Tsuji, K. Lycaenid caterpillar secretions manipulate attendant ant behavior. Curr. Biol. 2015, 25, 2260–2264. [Google Scholar] [CrossRef]
- Kudo, T.; Aonuma, H.; Hasegawa, E. A symbiotic aphid selfishly manipulates attending ants via dopamine in honeydew. Sci. Rep. 2021, 11, 18569. [Google Scholar] [CrossRef]
- Patricelli, D.; Barbero, F.; Occhipinti, A.; Bertea, C.M.; Bonelli, S.; Casacci, L.P.; Zebelo, S.A.; Crocoll, C.; Gershenzon, J.; Maffei, M.E.; et al. Plant defences against ants provide a pathway to social parasitism in butterflies. Proc. R. Soc. 2015, 282, 1811. [Google Scholar] [CrossRef]
- Heil, M.; McKey, D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 425–453. [Google Scholar] [CrossRef]
- LeBoeuf, A.C.; Benton, R.; Keller, L. The molecular basis of social behavior: Models, methods and advances. Curr. Opin. Neurobiol. 2013, 23, 3–10. [Google Scholar] [CrossRef]
- Hoyer, S.C.; Eckart, A.; Herrel, A.; Zars, T.; Fischer, S.A.; Hardie, S.L.; Heisenberg, M. Octopamine in male aggression of Drosophila. Curr. Biol. 2008, 18, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, A.; Suetsugu, E.; Hirokado, S.; Kuwano, E.; Taniguchi, E.; Eto, M. Effect of octopamine on the activity of juvenile-hormone esterase in the silkworm Bombyx mori and the red flour beetle Tribolium freemani. Gen. Comp. Endocrinol. 1999, 116, 373–381. [Google Scholar] [CrossRef]
- Rogers, S.M.; Matheson, T.; Despland, E.; Dodgson, T.; Burrows, M.; Simpson, S.J. Mechanosensory-induced behavioural gregarization in the desert locust Schistocerca gregaria. J. Exp. Biol. 2003, 206, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, X.; Lei, H.; Li, T.; Hao, S.; Kang, L. Octopamine and Tyramine Respectively Regulate Attractive and Repulsive Behavior in Locust Phase Changes. Sci. Rep. 2015, 5, 8036. [Google Scholar] [CrossRef]
- Alessi, A.M.; O’Connor, V.; Aonuma, H.; Newland, P.L. Dopaminergic modulation of phase reversal in desert locusts. Front. Behav. Neurosci. 2014, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, Z.; Kang, L. Two dopamine receptors play different roles in phase change of the migratory locust. Front. Behav. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Hewlett, S.E.; Delahunt Smoleniec, J.D.; Wareham, D.M.; Pyne, T.M.; Barron, A.B. Biogenic amine modulation of honey bee sociability and nestmate affiliation. PLoS ONE 2018, 13, e0205686. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.B.; Maleszka, J.; Vander Meer, R.K.; Robinson, G.E.; Maleszka, R. Comparing injection, feeding and topical application methods for treatment of honeybees with octopamine. J. Insect Physiol. 2007, 53, 187–194. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, F.; Mannino, G.; Casacci, L.P. The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants. Insects 2023, 14, 386. https://doi.org/10.3390/insects14040386
Barbero F, Mannino G, Casacci LP. The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants. Insects. 2023; 14(4):386. https://doi.org/10.3390/insects14040386
Chicago/Turabian StyleBarbero, Francesca, Giuseppe Mannino, and Luca Pietro Casacci. 2023. "The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants" Insects 14, no. 4: 386. https://doi.org/10.3390/insects14040386
APA StyleBarbero, F., Mannino, G., & Casacci, L. P. (2023). The Role of Biogenic Amines in Social Insects: With a Special Focus on Ants. Insects, 14(4), 386. https://doi.org/10.3390/insects14040386









