Evaluation of Transgenerational Effects of Sublethal Imidacloprid and Diversity of Symbiotic Bacteria on Acyrthosiphon gossypii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Insecticide
2.2. Bioassay of Imidacloprid Toxicity to A. gossypii
2.3. Sublethal Effects of Imidacloprid on A. gossypii
2.4. Effect of Imidacloprid on the Symbiotic Bacteria of A. gossypii
3. Results
3.1. Toxicity of Imidacloprid to A. gossypii Adults
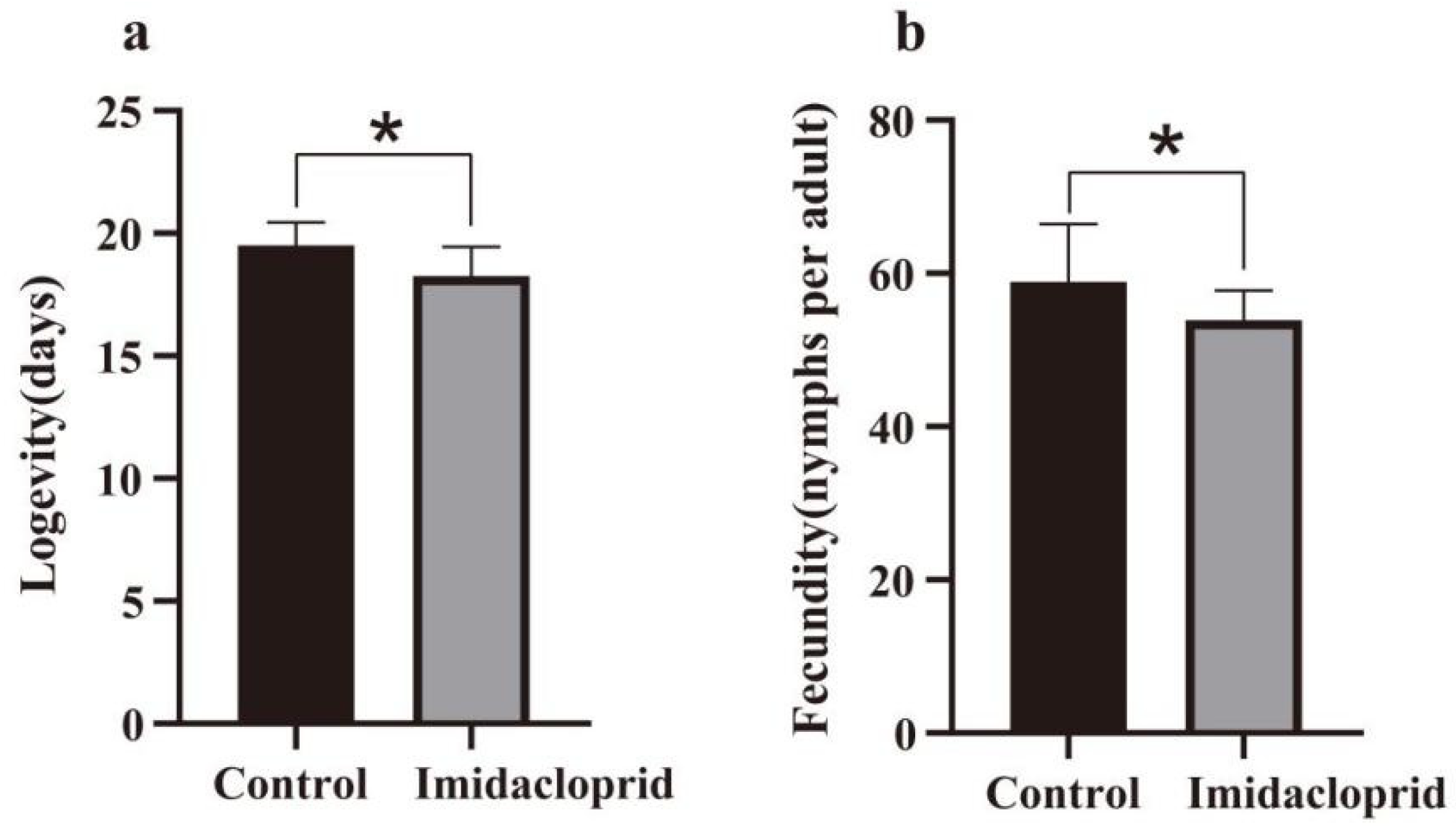
3.2. Sublethal Effects of Imidacloprid on the G0 Generation of A. gossypii
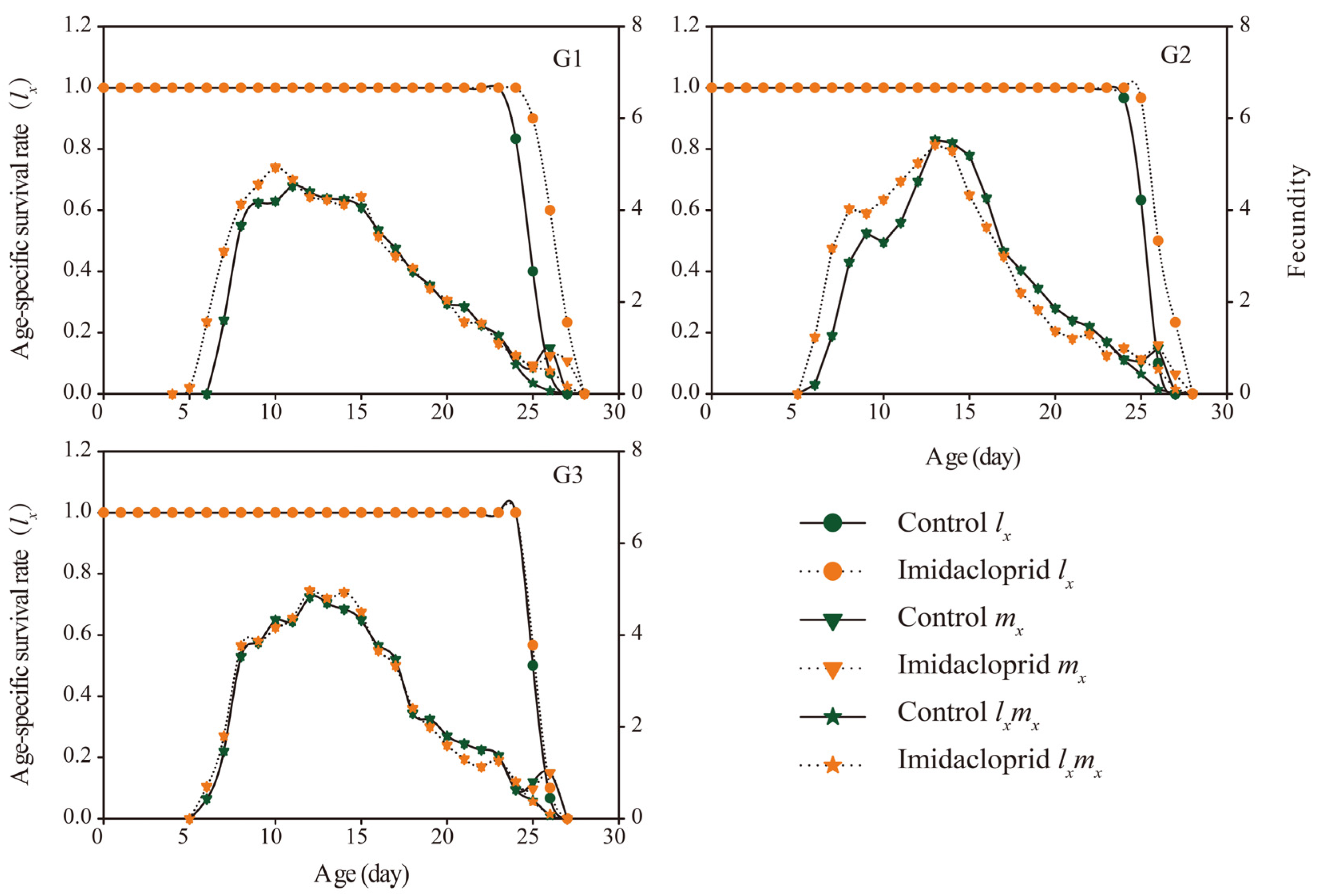
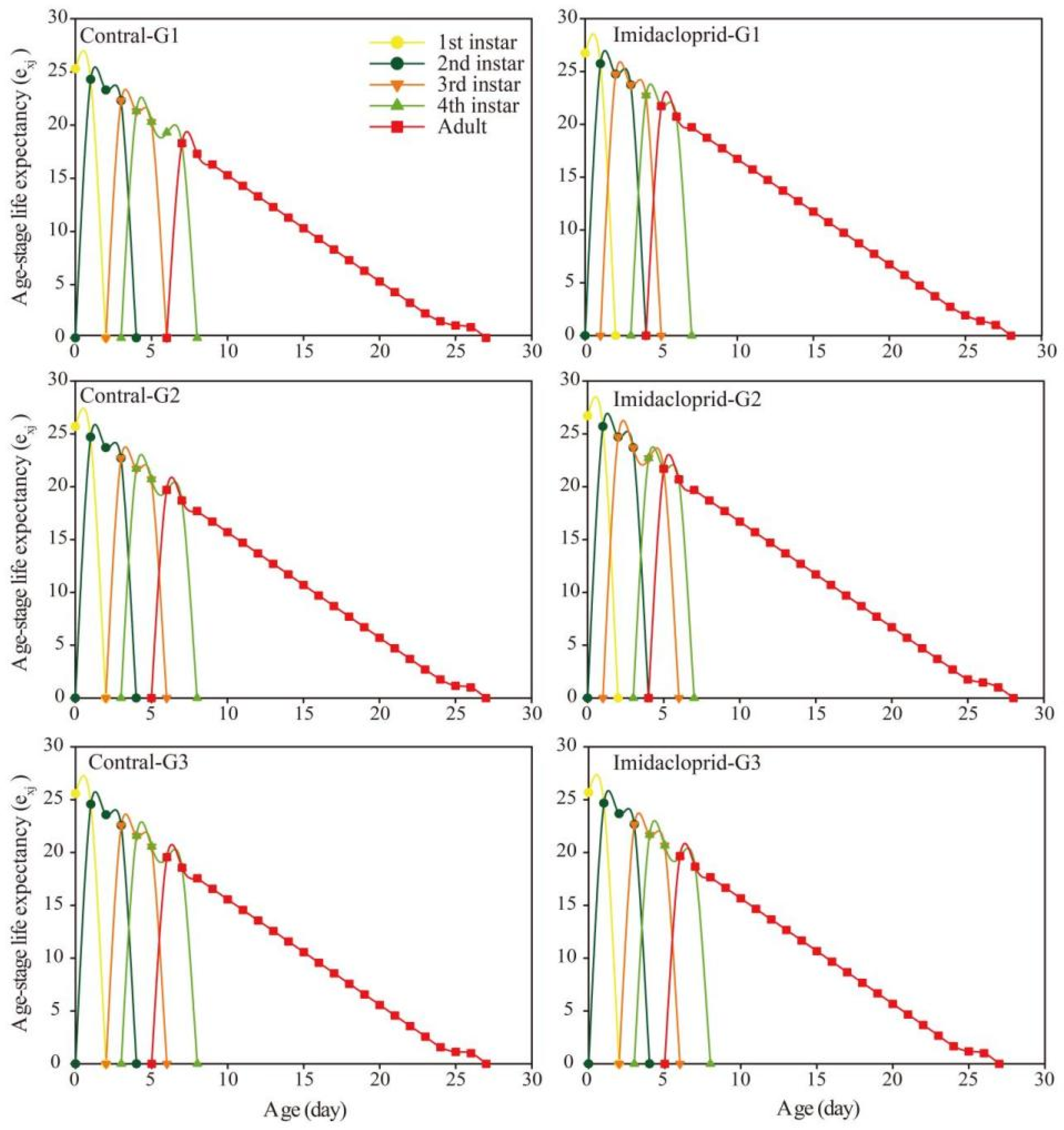
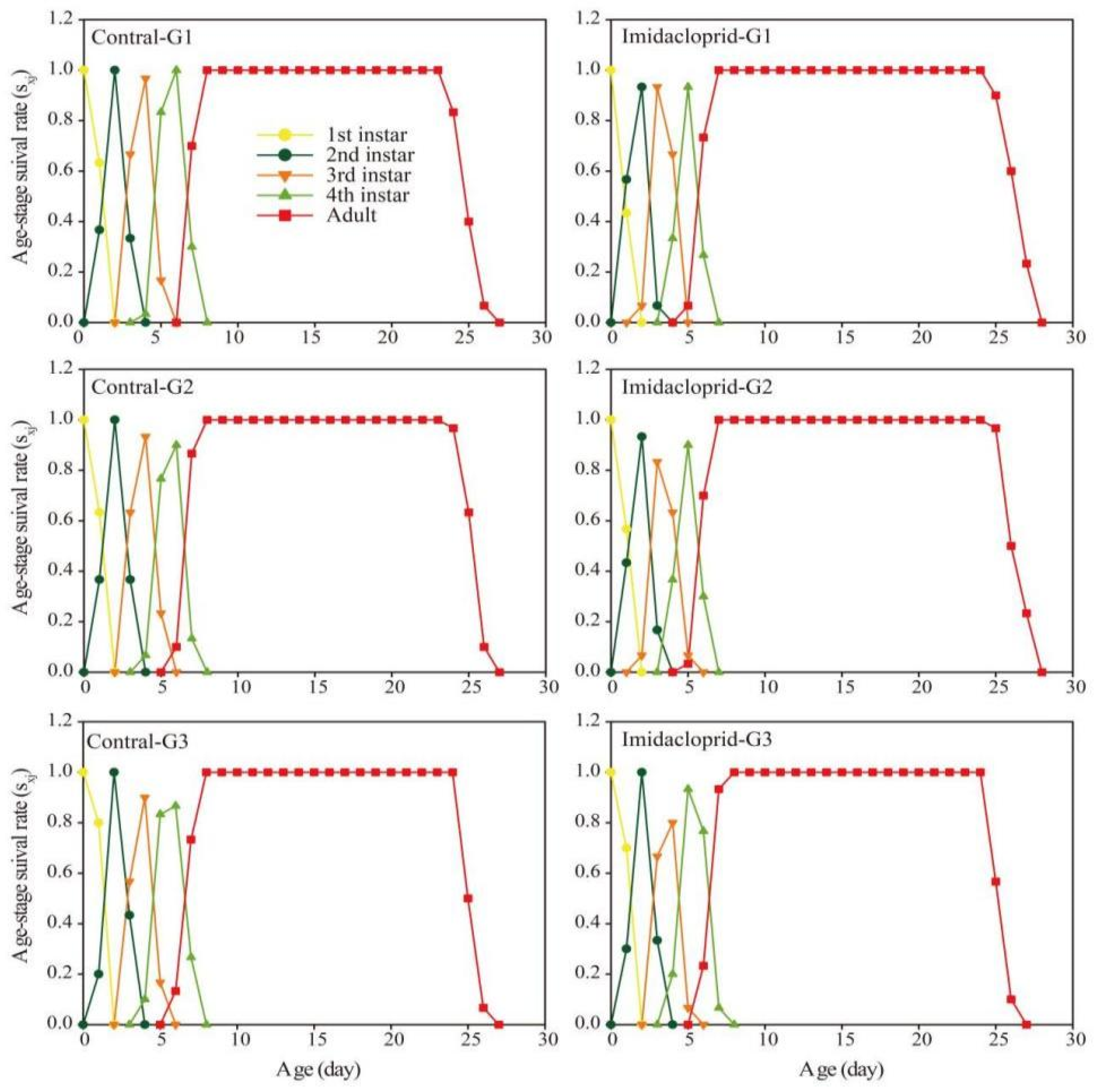
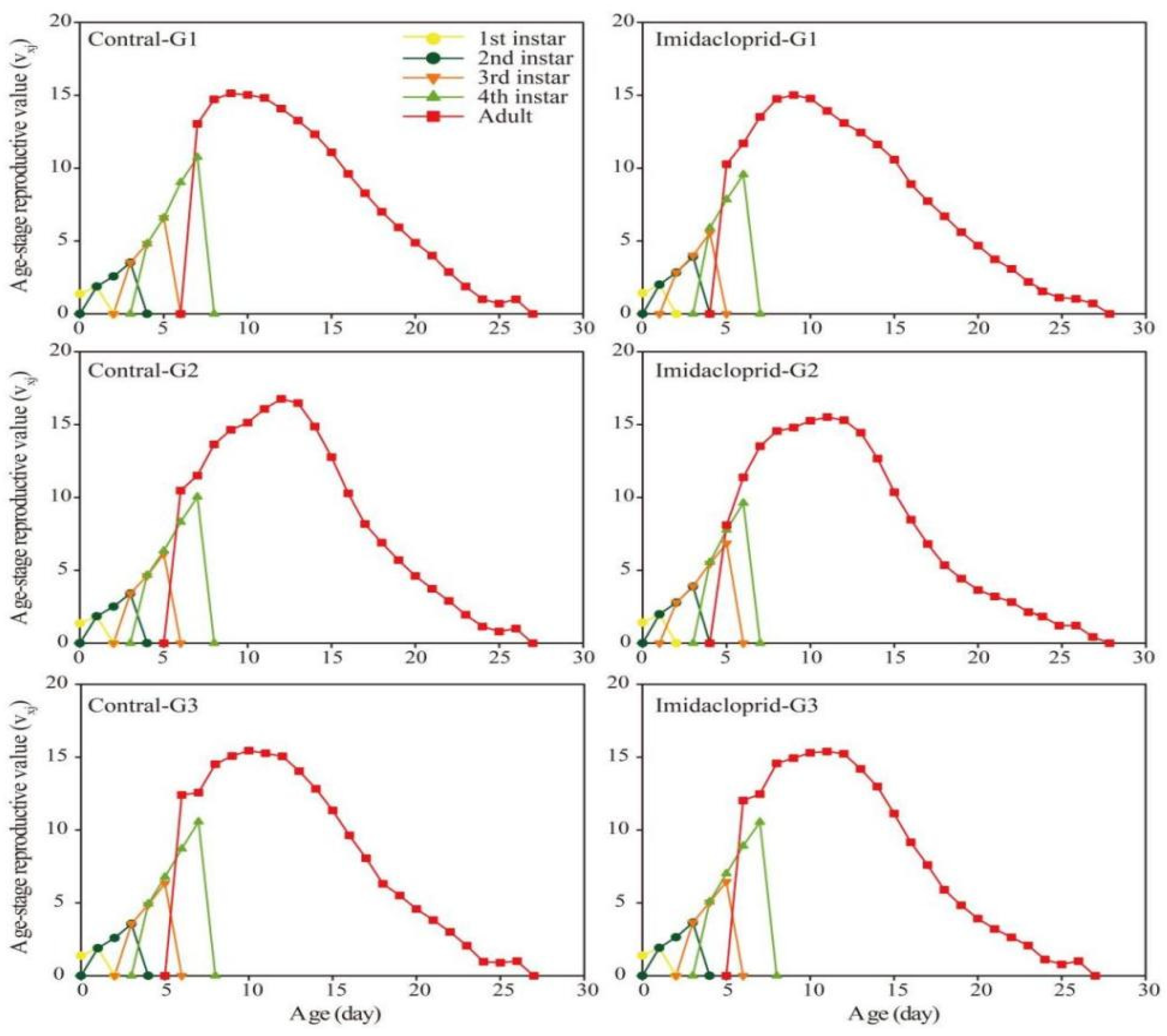
3.3. Transgenerational Effects of Imidacloprid on the G1–G3 Generation of A. gossypii
3.4. Sequencing Data
3.5. Diversity Analysis of Bacterial Populations
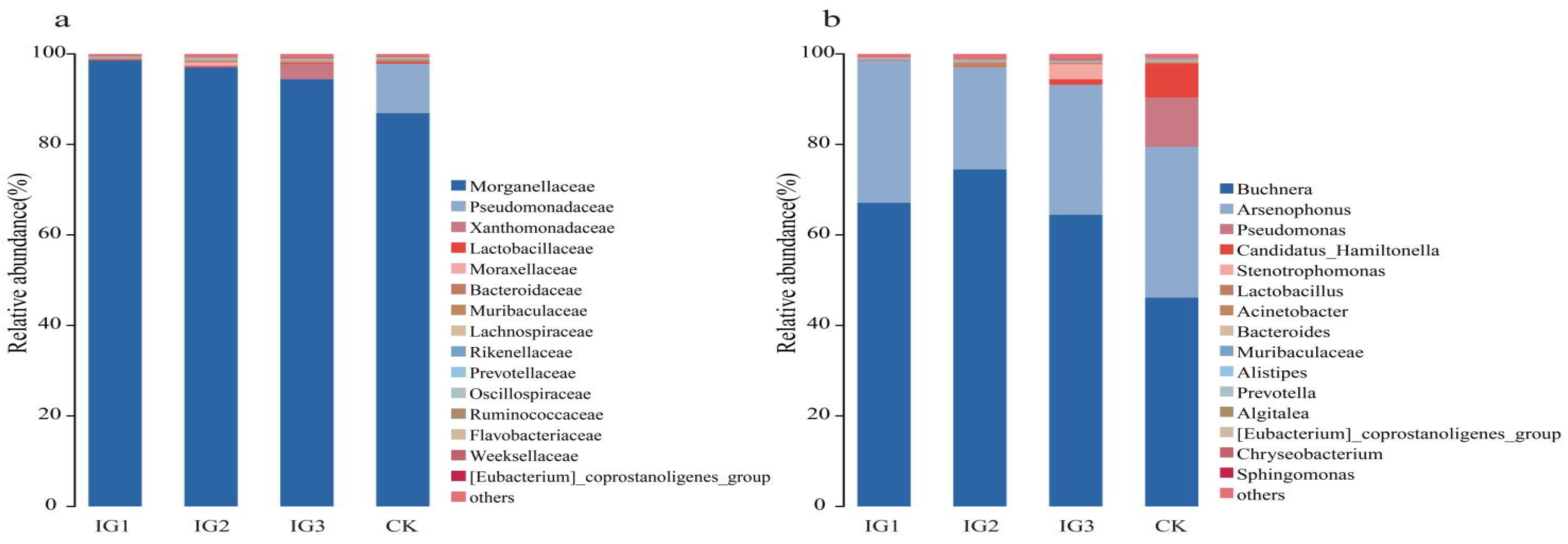
3.6. Taxonomic Composition of Bacteria in A. gossypii
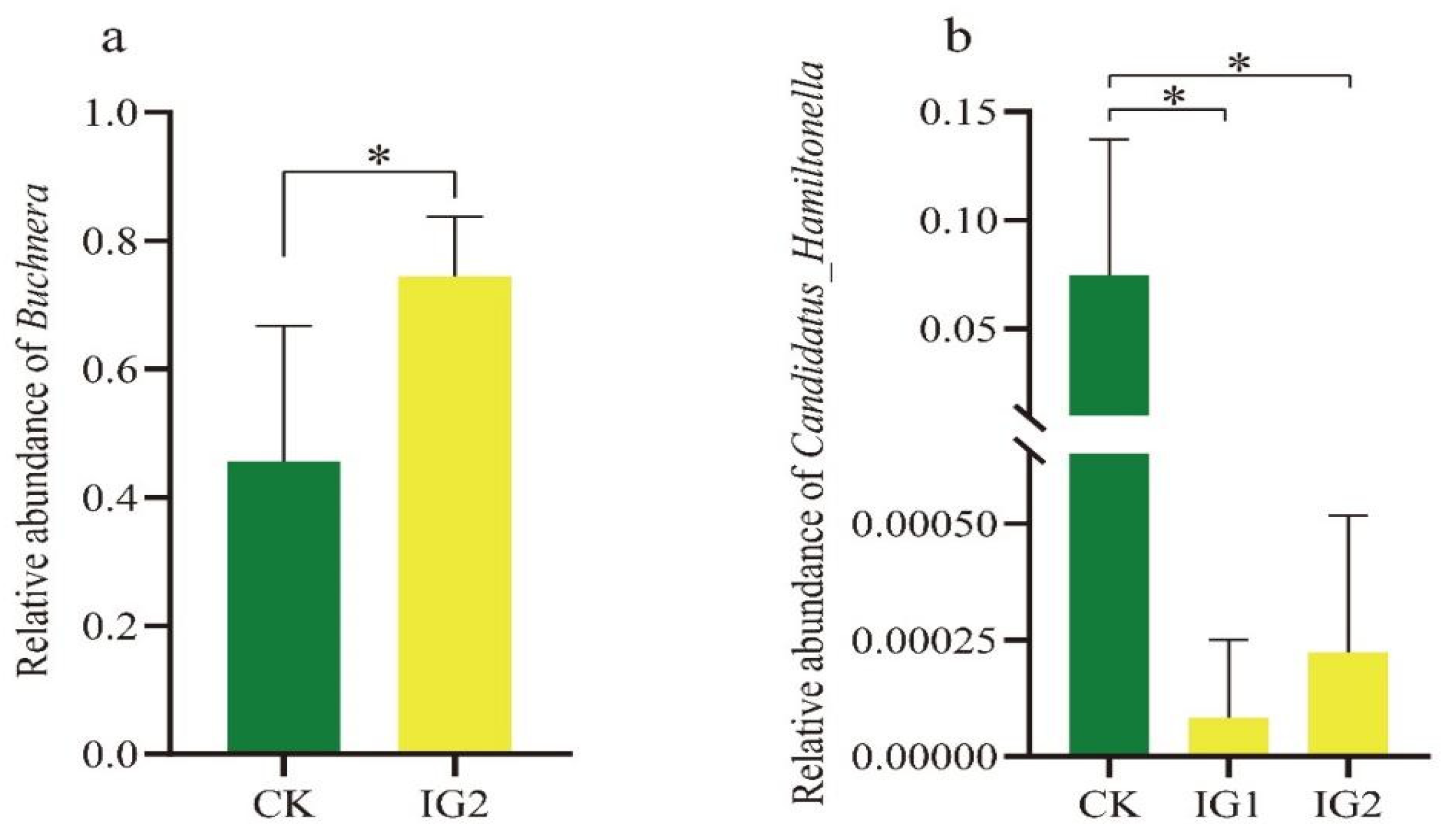
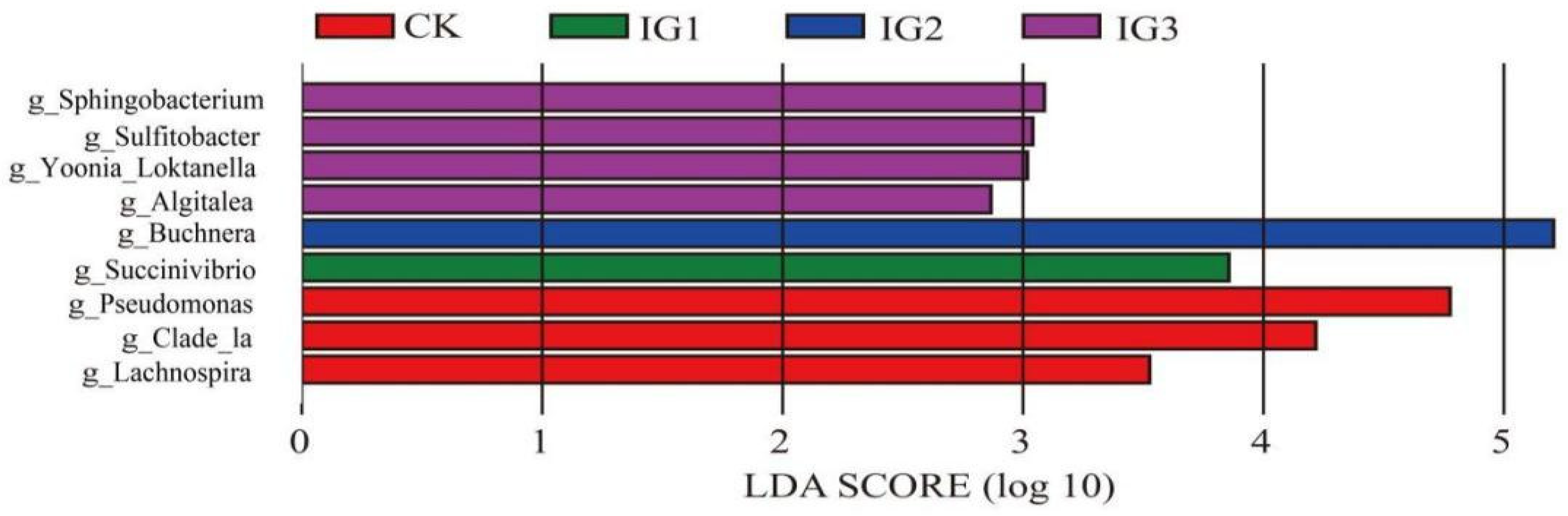
3.7. Effects of Imidacloprid Treatment on Three Successive Generations of the Bacterial Community in A. gossypii
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Taxonomic Categories | Taxa | CK | IG1 | CK | IG2 | CK | IG3 |
|---|---|---|---|---|---|---|---|
| Phylum | Proteobacteria | 98.10 | 98.68 | 98.10 | 98.15 | 98.10 | 98.19 |
| Class | Gammaproteobacteria | 97.96 | 98.63 | 97.96 | 98.06 | 97.96 | 98.07 |
| Order | Enterobacterales | 86.94 | 98.54 | 86.94 | 97.10 | 86.94 | 94.44 |
| Family | Morganellaceae | 86.91 | 98.53 | 86.91 | 97.00 | 86.91 | 94.40 |
| Genus | Buchnera | 46.14 | 67.09 | 46.14 | 74.47 | 46.14 | 64.45 |
| Arsenophonus | 33.33 | 31.42 | 33.33 | 22.49 | 33.33 | 28.74 |
| Taxon | CK | IG1 | CK | IG2 | CK | IG3 |
|---|---|---|---|---|---|---|
| Morganellaceae | 0.87 | 0.99 | 0.87 | 0.97 | 0.87 | 0.94 |
| Pseudomonadaceae | 0.11 | 0.00 | 0.11 | 4.38 × 10−4 | 0.11 | 3.30 × 10−5 |
| Xanthomonadaceae | 1.40 × 10−4 | 2.14 × 10−5 | 1.40 × 10−4 | 2.79 × 10−4 | 1.40 × 10−4 | 0.03 |
| Lactobacillaceae | 3.56 × 10−3 | 2.06 × 10−3 | 3.56 × 10−3 | 2.58 × 10−3 | 3.56 × 10−3 | 2.49 × 10−3 |
| Moraxellaceae | 2.79 × 10−4 | 6.76 × 10−5 | 2.79 × 10−4 | 8.22 × 10−3 | 2.79 × 10−4 | 5.06 × 10−4 |
| Bacteroidaceae | 3.05 × 10−3 | 1.47 × 10−3 | 3.05 × 10−3 | 2.23 × 10−3 | 3.05 × 10−3 | 1.90 × 10−3 |
| Muribaculaceae | 2.52 × 10−3 | 9.15 × 10−4 | 2.52 × 10−3 | 1.72 × 10−3 | 2.52 × 10−3 | 1.51 × 10−3 |
| Lachnospiraceae | 1.85 × 10−3 | 1.36 × 10−3 | 1.85 × 10−3 | 1.90 × 10−3 | 1.85 × 10−3 | 1.28 × 10−3 |
| Rikenellaceae | 1.12 × 10−3 | 1.38 × 10−3 | 1.12 × 10−3 | 1.07 × 10−3 | 1.12 × 10−3 | 7.66 × 10−4 |
| Prevotellaceae | 8.19 × 10−4 | 9.61 × 10−4 | 8.19 × 10−4 | 1.04 × 10−3 | 8.19 × 10−4 | 7.88 × 10−4 |
| Oscillospiraceae | 8.34 × 10−4 | 5.87 × 10−4 | 8.34 × 10−4 | 1.21 × 10−3 | 8.34 × 10−4 | 8.14 × 10−4 |
| Ruminococcaceae | 7.66 × 10−4 | 9.22 × 10−4 | 7.66 × 10−4 | 8.97 × 10−4 | 7.66 × 10−4 | 7.44 × 10−4 |
| Flavobacteriaceae | 2.30 × 10−4 | 7.48 × 10−5 | 2.30 × 10−4 | 8.51 × 10−4 | 2.30 × 10−4 | 1.43 × 10−3 |
| Weeksellaceae | 1.21 × 10−4 | 4.27 × 10−5 | 1.21 × 10−4 | 2.12 × 10−5 | 1.21 × 10−4 | 1.88 × 10−3 |
| [Eubacterium]_coprostanoligenes_group | 6.07 × 10−4 | 5.77 × 10−4 | 6.07 × 10−4 | 5.19 × 10−4 | 6.07 × 10−4 | 3.08 × 10−4 |
| others | 6.03 × 10−3 | 4.29 × 10−3 | 6.03 × 10−3 | 7.02 × 10−3 | 6.03 × 10−3 | 8.13 × 10−3 |
| Taxon | CK | IG1 | CK | IG2 | CK | IG3 |
|---|---|---|---|---|---|---|
| Buchnera | 0.46 | 0.67 | 0.46 | 0.74 | 0.46 | 0.64 |
| Arsenophonus | 0.33 | 0.31 | 0.33 | 0.22 | 0.33 | 0.29 |
| Pseudomonas | 0.11 | 0.00 | 0.11 | 4.38 × 10−4 | 0.11 | 3.30 × 10−5 |
| Candidatus_Hamiltonella | 0.07 | 8.54 × 10−5 | 0.07 | 2.22 × 10−4 | 0.07 | 0.01 |
| Stenotrophomonas | 8.30 × 10−5 | 2.14 × 10−5 | 8.30 × 10−5 | 2.68 × 10−4 | 8.30 × 10−5 | 0.03 |
| Lactobacillus | 3.56 × 10−3 | 2.06 × 10−3 | 3.56 × 10−3 | 2.58 × 10−3 | 3.56 × 10−3 | 2.49 × 10−3 |
| Acinetobacter | 2.79 × 10−4 | 1.07 × 10−5 | 2.79 × 10−4 | 8.22 × 10−3 | 2.79 × 10−4 | 5.06 × 10−4 |
| Bacteroides | 3.05 × 10−3 | 1.47 × 10−3 | 3.05 × 10−3 | 2.23 × 10−3 | 3.05 × 10−3 | 1.90 × 10−3 |
| Muribaculaceae | 2.52 × 10−3 | 9.15 × 10−4 | 2.52 × 10−3 | 1.70 × 10−3 | 2.52 × 10−3 | 1.51 × 10−3 |
| Alistipes | 7.73 × 10−4 | 1.03 × 10−3 | 7.73 × 10−4 | 8.33 × 10−4 | 7.73 × 10−4 | 5.28 × 10−4 |
| Prevotella | 5.92 × 10−4 | 6.91 × 10−4 | 5.92 × 10−4 | 8.62 × 10−4 | 5.92 × 10−4 | 5.57 × 10−4 |
| Algitalea | 1.81 × 10−4 | 0.00 | 1.81 × 10−4 | 7.91 × 10−4 | 1.81 × 10−4 | 1.34 × 10−3 |
| [Eubacterium]_coprostanoligenes_group | 6.07 × 10−4 | 5.77 × 10−4 | 6.07 × 10−4 | 5.19 × 10−4 | 6.07 × 10−4 | 3.08 × 10−4 |
| Chryseobacterium | 8.68 × 10−5 | 0.00 | 8.68 × 10−5 | 2.12 × 10−5 | 8.68 × 10−5 | 1.83 × 10−3 |
| Sphingomonas | 2.75 × 10−4 | 4.56 × 10−4 | 2.75 × 10−4 | 4.98 × 10−4 | 2.75 × 10−4 | 3.85 × 10−4 |
| others | 9.97 × 10−3 | 7.55 × 10−3 | 9.97 × 10−3 | 0.01 | 9.97 × 10−3 | 0.01 |
References
- Barros, M.A.L.; Silva, C.R.C.D.; Lima, L.M.D.; Farias, F.J.C.; Ramos, G.A.; Santos, R.C.D. A review on evolution of cotton in Brazil: GM, white, and colored cultivars. J. Nat. Fibers 2022, 19, 209–221. [Google Scholar] [CrossRef]
- Gao, G.Z.; Feng, L.K.; Perkins, L.E.; Sharma, S.S.; Lu, Z.Z. Effect of the frequency and magnitude of extreme temperature on the life history traits of the large cotton aphid, Acyrthosiphon gossypii (Hemiptera: Aphididae): Implications for their population dynamics under global warming. Entomol. Gen. 2018, 37, 103–113. [Google Scholar] [CrossRef]
- Müller, F.P. Incidence of the aphid Acyrthosiphon gossypii Mordvilko on legumes and on cotton (Homoptera: Aphididae). Beitr. Zur Entomol. Contrib. Entomol. 1975, 25, 257–260. [Google Scholar]
- Gao, G.Z.; Perkins, L.E.; Zalucki, M.P.; Lu, Z.Z.; Ma, J.H. Effect of temperature on the biology of Acyrthosiphon gossypii Mordvilko (Homoptera: Aphididae) on cotton. J. Pest Sci. 2013, 86, 167–172. [Google Scholar] [CrossRef]
- Liu, J.P.; Wang, C.; Desneux, N.; Lu, Y.H. Impact of temperature on survival rate, fecundity, and feeding behavior of two aphids, Aphis gossypii and Acyrthosiphon gossypii, when reared on cotton. Insects 2021, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.Y.; Zhang, S.; Liu, Y.; Ma, L.; Li, X.Q.; Zhang, Y.X.; Fan, Y.J.; Song, D.L.; Gao, X.W. Slow resistance evolution to neonicotinoids in field populations of wheat aphids revealed by insecticide resistance monitoring in China. Pest Manag. Sci. 2022, 78, 1428–1437. [Google Scholar] [CrossRef]
- Zhang, A.N.; Xu, L.; Liu, Z.Q.; Zhang, J.B.; Han, L.L.; Zhao, K.J. The effects of acetamiprid multigeneration stress on metabolism and physiology of Aphis glycines Matsumura (Hemiptera: Aphididae). Arch. Insect Biochem. Physiol. 2022, 110, e21903. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, M.; Huang, Y.N.; Yang, Z.L.; Su, S.; Chen, M.H. Characterisation of imidacloprid resistance in the bird cherry-oat aphid, Rhopalosiphum padi, a serious pest on wheat crops. Pest Manag. Sci. 2018, 74, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.A.; Christofoletti, C.A.; Pedro, J.; Bueno, O.C.; Malaspina, O.; Ferreira, R.A.; Fontanetti, C.S. Allium cepa and Tradescantia pallida bioassays to evaluate effects of the insecticide imidacloprid. Chemosphere 2015, 120, 438–442. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Nishimura, K.; Kanda, Y.; Okazawa, A.; Ueno, T. Relationship between insecticidal and neurophysiological activities of imidacloprid and related compounds. Pestic. Biochem. Physiol. 1994, 50, 51–59. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Skouras, P.J.; Darras, A.I.; Mprokaki, M.; Demopoulos, V.; Margaritopoulos, J.T.; Delis, C.; Stathas, G.J. Toxicity, sublethal and low dose effects of imidacloprid and deltamethrin on the aphidophagous predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae). Insects 2021, 12, 696. [Google Scholar] [CrossRef]
- Wumuerhan, P.; Yuntao, J.; Deying, M. Effects of exposure to imidacloprid direct and poisoned cotton aphids Aphis gossypii on ladybird Hippodamia variegata feeding behavior. J. Pestic. Sci. 2020, 45, 24–28. [Google Scholar] [CrossRef]
- Saber, M. Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology 2011, 20, 1476–1484. [Google Scholar] [CrossRef]
- Gao, J.; Jin, S.S.; He, Y.; Luo, J.H.; Xu, C.Q.; Wu, Y.Y.; Hou, C.S.; Wang, Q.; Diao, Q.Y. Physiological analysis and transcriptome analysis of Asian honey bee (Apis cerana cerana) in response to sublethal neonicotinoid imidacloprid. Insects 2020, 11, 753. [Google Scholar] [CrossRef]
- Delkash-Roudsari, S.; Goldansaz, S.H.; Talebi Jahromi, K.; Ashouri, A.; Abramson, C.I. Side effects of imidacloprid, ethion, and hexaflumuron on adult and larvae of honey bee Apis mellifera (Hymenoptera, Apidae). Apidologie 2022, 53, 17. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zheng, X.S.; Gao, X.W. Sublethal effects of imidacloprid on the fecundity, longevity, and enzyme activity of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus). Bull. Entomol. Res. 2016, 106, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.J.; Cheng, Y.B.; Li, Y.M.; Li, W.R.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic hormone regulates cytochrome P450-mediated imidacloprid resistance in the brown planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef]
- Jie, M.L.; Gao, Y.H.; Kuang, D.H.; Shi, Y.S.; Wang, H.H.; Jing, W.W. Relationship between imidacloprid residues and control effect on cotton aphids in arid region. Environ. Geochem. Health 2021, 43, 1941–1952. [Google Scholar] [CrossRef]
- Liu, X.L.; Fu, Z.X.; Zhu, Y.F.; Gao, X.W.; Liu, T.X.; Liang, P. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Physiol. 2022, 180, 104981. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.B.; Liu, S.H.; Gu, J.H.; Wang, X.Z.; Liang, X.L.; Liu, Z.W. Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2009, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.B.; Dou, T.; Gao, F.T.; Wang, G.H.; Dong, Y.C.; Song, N.; An, S.H.; Yin, X.M.; Liu, X.Y.; Ren, Y.D. Sublethal effects of thiamethoxam on biological traits and detoxification enzyme activities in the small brown planthopper, Laodelphax striatellus (Fallén). J. Econ. Entomol. 2022, 115, 2051–2060. [Google Scholar] [CrossRef]
- Ma, K.S.; Tang, Q.L.; Liang, P.Z.; Li, J.H.; Gao, X.W. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Ayyanath, M.M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Rix, R.R.; Cutler, G.C. Pesticide-induced hormesis in arthropods: Towards biological systems. Curr. Opin. Toxicol. 2022, 29, 43–50. [Google Scholar] [CrossRef]
- Wu, J.C.; Ge, L.Q.; Liu, F.; Song, Q.S.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Cheng, S.Y.; Desneux, N.; Gao, X.W.; Xiu, X.J.; Wang, F.L.; Hou, M.L. Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest Sci. 2022, 96, 161–180. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Does multigenerational exposure to hormetic concentrations of imidacloprid precondition aphids for increased insecticide tolerance? Pest Manag. Sci. 2018, 74, 314–322. [Google Scholar] [CrossRef]
- Ding, Z.P.; Wen, Y.C.; Yang, B.J.; Zhang, Y.X.; Liu, S.H.; Liu, Z.W.; Han, Z.J. Biochemical mechanisms of imidacloprid resistance in Nilaparvata lugens: Over-expression of cytochrome P450 CYP6AY1. Insect Biochem. Mol. Biol. 2013, 43, 1021–1027. [Google Scholar] [CrossRef]
- Shang, J.; Yao, Y.S.; Chen, L.L.; Zhu, X.Z.; Niu, L.; Gao, X.K.; Luo, J.Y.; Ji, J.C.; Cui, J.J. Sublethal exposure to deltamethrin stimulates reproduction and alters symbiotic bacteria in Aphis gossypii. J. Agric. Food Chem. 2021, 69, 15097–15107. [Google Scholar] [CrossRef]
- Ramya, S.L.; Venkatesan, T.; Srinivasa Murthy, K.; Jalali, S.K.; Verghese, A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016, 47, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, J.X.; Qin, Y.G.; Fan, J.; Zhang, Y.; Tan, X.L.; Hou, M.L.; Chen, J.L. Reduced insecticide susceptibility of the wheat aphid Sitobion miscanthi after infection by the secondary bacterial symbiont Hamiltonella defensa. Pest Manag. Sci. 2021, 77, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qi, H.L.; Yang, D.B.; Yuan, H.Z.; Rui, C.H. Cycloxaprid: A novel cis-nitromethylene neonicotinoid insecticide to control imidacloprid-resistant cotton aphid (Aphis gossypii). Pestic. Biochem. Physiol. 2016, 132, 96–101. [Google Scholar] [CrossRef]
- Harcourt, D.G. The development and use of life tables in the study of natural insect populations. Annu. Rev. Entomol. 1969, 14, 175–196. [Google Scholar] [CrossRef]
- Brownstone, D.; Valletta, R. The bootstrap and multiple imputations: Harnessing increased computing power for improved statistical tests. J. Econ. Perspect. 2001, 15, 129–141. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Xiao, D.; Li, J.Y.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Guo, Y.Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Shi, X.B.; Jiang, L.L.; Wang, H.Y.; Qiao, K.; Wang, D.; Wang, K.Y. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Wu, J.C. Mechanisms on pesticide-induced resurgence of pests. Chin. J. Appl. Entomol. 2011, 48, 799–803. [Google Scholar]
- Ullah, F.; Xu, X.; Gul, H.; Güncan, A.; Hafeez, M.; Gao, X.W.; Song, D.L. Impact of imidacloprid resistance on the demographic traits and expressions of associated genes in Aphis gossypii Glover. Toxics 2022, 10, 658. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.S.; Liu, X.X.; Liang, G.M. Differences in the sublethal effects of sulfoxaflor and acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) are related to its basic sensitivity level. Insects 2022, 13, 498. [Google Scholar] [CrossRef]
- Zhang, A.N.; Xu, L.; Liu, Z.Q.; Zhang, J.B.; Zhao, K.J.; Han, L.L. Effects of acetamiprid at low and median lethal concentrations on the development and reproduction of the soybean aphid Aphis glycines. Insects 2022, 13, 87. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Guedes, R.N.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.W.; Song, D.L. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Xiang, M.; Hu, H.M.; An, C.J.; Gao, X.W. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J. Econ. Entomol. 2015, 108, 2720–2728. [Google Scholar] [CrossRef]
- Tang, Q.L.; Ma, K.S.; Chi, H.; Hou, Y.M.; Gao, X.W. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef]
- Fouad, E.A.; El-Sherif, S.A.N.; Mokbel, E.M.S. Flupyradifurone induces transgenerational hormesis effects in the cowpea aphid, Aphis craccivora. Ecotoxicology 2022, 31, 909–918. [Google Scholar] [CrossRef]
- Forbes, V.E. Is hormesis an evolutionary expectation? Funct. Ecol. 2000, 14, 12–24. [Google Scholar] [CrossRef]
- Aksoy, S. Tsetse-haven for microorganisms. Parasitol. Today 2000, 16, 114–118. [Google Scholar] [CrossRef]
- Fouda, M.A.; Hassan, M.I.; Al-Daly, A.G.; Hammad, K.M. Effect of midgut bacteria of Culex pipiens L. on digestion and reproduction. J. Egypt. Soc. Parasitol. 2001, 31, 767–780. [Google Scholar]
- Renoz, F.; Pons, I.; Hance, T. Evolutionary responses of mutualistic insect-bacterial symbioses in a world of fluctuating temperatures. Curr. Opin. Insect Sci. 2019, 35, 20–26. [Google Scholar] [CrossRef]
- Vorburger, C. The evolutionary ecology of symbiont-conferred resistance to parasitoids in aphids. Insect Sci. 2014, 21, 251–264. [Google Scholar] [CrossRef]
- Aswathi, A.; Pandey, A.; Sukumaran, R.K. Rapid degradation of the organophosphate pesticide-Chlorpyrifos by a novel strain of Pseudomonas nitroreducens AR-3. Bioresour. Technol. 2019, 292, 122025. [Google Scholar] [CrossRef]
- van den Bosch, T.J.M.; Welte, C.U. Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 2017, 10, 531–540. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, L.; Wang, S.H.; Qu, J.; Li, K.; Xu, L.L.; Shi, Y.H.; Yan, Y.C. Biodegradation of beta-cypermethrin by two Serratia spp. with different cell surface hydrophobicity. Bioresour. Technol. 2010, 101, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luo, J.Y.; Wang, L.; Zhang, L.J.; Zhu, X.Z.; Jiang, W.L.; Cui, J.J. Bacterial communities in natural versus pesticide-treated Aphis gossypii populations in North China. Microbiologyopen 2019, 8, e00652. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.N.; Jin, D.C.; Gong, P.P.; Li, Q.C.; Zhang, Y.H.; Li, X.R.; Deng, Y.; Cernava, T.; Zhu, X. Implications of environmentally shaped microbial communities for insecticide resistance in Sitobion miscanthi. Environ. Res. 2022, 215, 114409. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.K.; Gong, Y.J.; Chen, J.C.; Shi, P.; Cao, L.J.; Yang, Q.; Hoffmann, A.A.; Wei, S.J. Increased density of endosymbiotic Buchnera related to pesticide resistance in yellow morph of melon aphid. J. Pest Sci. 2020, 93, 1281–1294. [Google Scholar] [CrossRef]
- Huang, Y.N. Insecticide Resistance Monitoring and Symbionts Detection in Rhopalosiphum padi Field Populations from Guan Zhong Area; Shaanxi Province Northwest A&F University: Xianyang, China, 2018. [Google Scholar]

| Treatment | Slope ± SE | LC15 (mg·L−1) (95% CL) | LC50 (mg·L−1) (95% CL) | LC90 (mg·L−1) (95% CL) | R2 | χ2 (df) |
|---|---|---|---|---|---|---|
| Imidacloprid | 2.53 ± 0.21 | 0.54 (0.43–0.65) | 1.46 (1.27–1.68) | 4.97 (3.94–6.79) | 0.97 | 4.08 (12) |
| Treatments | First Instar (d) | Second Instar (d) | Third Instar (d) | Fourth Instar (d) | Pre-Adult (d) | Adult (d) | APOP (d) | TPOP (d) | Longevity (d) | Fecundity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | Control | 1.63 ± 0.09 | N | 1.70 ± 0.08 | N | 1.80 ± 0.09 | N | 2.17 ± 0.08 | * | 7.30 ± 0.08 | * | 18.00 ± 0.12 | * | 0.07 ± 0.05 | * | 7.37 ± 0.09 | * | 25.30 ± 0.15 | * | 54.47 ± 0.89 | * |
| Imidacloprid | 1.43 ± 0.09 | 1.57 ± 0.09 | 1.67 ± 0.09 | 1.53 ± 0.09 | 6.20 ± 0.10 | 20.53 ± 0.11 | 0.47 ± 0.12 | 6.67 ± 0.16 | 26.73 ± 0.17 | 59.83 ± 1.05 | |||||||||||
| G2 | Control | 1.63 ± 0.08 | N | 1.73 ± 0.08 | N | 1.80 ± 0.10 | N | 1.87 ± 0.10 | N | 7.03 ± 0.09 | * | 18.67 ± 0.13 | * | 0.43 ± 0.11 | N | 7.47 ± 0.13 | * | 25.70 ± 0.13 | * | 55.40 ± 0.91 | * |
| Imidacloprid | 1.57 ± 0.09 | 1.53 ± 0.09 | 1.60 ± 0.09 | 1.57 ± 0.09 | 6.27 ± 0.09 | 20.43 ± 0.10 | 0.60 ± 0.11 | 6.87 ± 0.13 | 26.70 ± 0.16 | 59.07 ± 0.64 | |||||||||||
| G3 | Control | 1.80 ± 0.07 | N | 1.63 ± 0.09 | N | 1.63 ± 0.10 | N | 2.07 ± 0.08 | N | 7.13 ± 0.11 | * | 18.83 ± 0.14 | * | 0.17 ± 0.07 | N | 7.30 ± 0.13 | N | 25.57 ± 0.11 | N | 55.43 ± 0.89 | N |
| Imidacloprid | 1.70 ± 0.08 | 1.63 ± 0.09 | 1.53 ± 0.09 | 1.97 ± 0.09 | 6.83 ± 0.10 | 18.43 ± 0.13 | 0.40 ± 0.10 | 7.23 ± 0.15 | 25.67 ± 0.12 | 55.83 ± 0.87 | |||||||||||
| Treatments | R0 | rm | λ | T | GRR | DT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | Control | 54.47 ± 0.89 | * | 0.31 ± 0.003 | * | 1.37 ± 0.004 | * | 12.72 ± 0.13 | * | 55.88 ± 0.96 | * | 2.21 ± 0.02 | * |
| Imidacloprid | 59.83 ± 1.05 | 0.35 ± 0.006 | 1.41 ± 0.008 | 11.80 ± 0.18 | 60.77 ± 1.03 | 2.00 ± 0.03 | |||||||
| G2 | Control | 55.40 ± 0.91 | * | 0.31 ± 0.004 | * | 1.36 ± 0.005 | * | 13.11 ± 0.16 | * | 56.58 ± 0.91 | * | 2.26 ± 0.03 | * |
| Imidacloprid | 59.07 ± 0.64 | 0.34 ± 0.005 | 1.41 ± 0.006 | 11.97 ± 0.15 | 59.95 ± 0.65 | 2.03 ± 0.03 | |||||||
| G3 | Control | 55.43 ± 0.89 | N | 0.32 ± 0.004 | N | 1.37 ± 0.006 | N | 12.66 ± 0.17 | N | 56.77 ± 0.91 | N | 2.19 ± 0.03 | N |
| Imidacloprid | 55.83 ± 0.87 | 0.32 ± 0.005 | 1.38 ± 0.007 | 12.43 ± 0.19 | 57.01 ± 0.89 | 2.14 ± 0.03 | |||||||
| Samples | Raw Reads | Fitted | ASV_Counts | Observed Species | Chao1 | Shannon | Simpson | ACE | Goods Coverage |
|---|---|---|---|---|---|---|---|---|---|
| IG1 | 79,877 | 73,689 | 109 | 108.28 | 108.57 | 1.29 | 0.50 | 108.81 | 1.00 |
| IG2 | 80,222 | 74,207 | 134 | 133.50 | 133.95 | 1.19 | 0.41 | 134.21 | 1.00 |
| IG3 | 79,337 | 72,337 | 131 | 130.30 | 130.65 | 1.41 | 0.50 | 131.38 | 1.00 |
| CK | 79,825 | 73,570 | 143 | 142.88 | 143.08 | 1.74 | 0.60 | 143.35 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Su, Y.; Han, X.; Guo, W.; Zhu, Y.; Yao, Y. Evaluation of Transgenerational Effects of Sublethal Imidacloprid and Diversity of Symbiotic Bacteria on Acyrthosiphon gossypii. Insects 2023, 14, 427. https://doi.org/10.3390/insects14050427
Wei Y, Su Y, Han X, Guo W, Zhu Y, Yao Y. Evaluation of Transgenerational Effects of Sublethal Imidacloprid and Diversity of Symbiotic Bacteria on Acyrthosiphon gossypii. Insects. 2023; 14(5):427. https://doi.org/10.3390/insects14050427
Chicago/Turabian StyleWei, Yindi, Yue Su, Xu Han, Weifeng Guo, Yue Zhu, and Yongsheng Yao. 2023. "Evaluation of Transgenerational Effects of Sublethal Imidacloprid and Diversity of Symbiotic Bacteria on Acyrthosiphon gossypii" Insects 14, no. 5: 427. https://doi.org/10.3390/insects14050427





