Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Dissections and Measurements
2.3. Images and Image Processing
2.4. Terminology
2.5. DNA Barcoding
3. Results
3.1. Triatoma picta Zhao & Cai sp. nov. (Figure 1, Figure 2 and Figure 3)
3.1.1. Diagnosis
3.1.2. Description



3.1.3. Etymology
3.1.4. Distribution
3.1.5. Measurements
3.1.6. Materials Examined
3.2. Triatoma atrata Zhao & Cai sp. nov. (Figure 5, Figure 6 and Figure 7)
3.2.1. Diagnosis
3.2.2. Description



3.2.3. Etymology
3.2.4. Distribution
3.2.5. Materials Examined
3.3. Triatoma sinica Hsiao, 1965 (Figure 8, Figure 9 and Figure 10)
3.3.1. Diagnosis
3.3.2. Redescripition
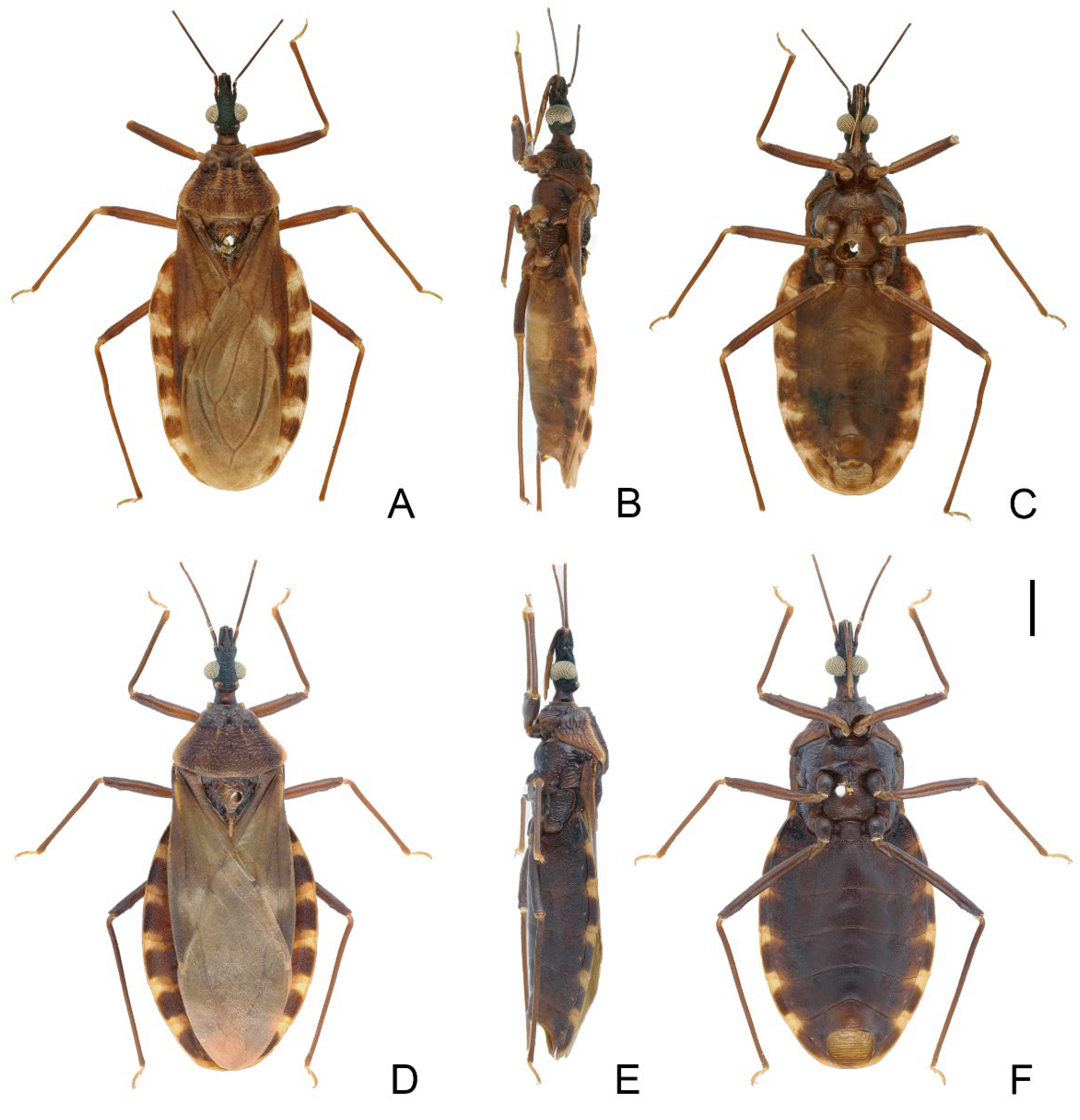


3.3.3. Distribution
3.3.4. Measurements
3.3.5. Materials Examined
3.4. Triatoma rubrofasciata (De Geer, 1773) (Figure 11)
3.4.1. Remarks

3.4.2. Materials Examined
3.5. DNA Barcoding Analysis
3.6. Key to the Species of Triatominae from China
- 1.
- First antennal segment longer than maxillary plate. Pronotum with reddish lateral margins. Medial basal sclerite of phallosoma eagle’s beak-like shape (Figure 11H–J)......................................................................... T. rubrofasciata (De Geer, 1773)
- −
- First antennal segment not longer than maxillary plate. Pronotum with yellowish strips or markings. Medial basal sclerite of phallosoma not eagle’s beak-like shape....................................................................................................... 2
- 2.
- Anterolateral angles of pronotum prominent and tips sharp (Figure 2D,E). Bottom third of corium entirely yellowish (Figure 2F,G). Medial basal sclerite of phallosoma subtriangular in the lateral view (Figure 3I). Basal lateral sclerites of endosoma without denticles (Figure 3G)......................................................................... Triatoma picta Zhao & Cai sp. nov.
- −
- Anterolateral angles of pronotum slightly prominent and tips round. Not all of bottom third of corium entirely yellowish. Medial basal sclerite of phallosoma oblong in lateral view. Basal lateral sclerites of endosoma with denticles....................................................................................................... 3
- 3.
- Width of eye equal to synthlipsis in dorsal view (Figure 9A,E). Body generally brown; rectangular area in middle of each connexival segment black (Figure 8D). Basal lateral sclerites of endosoma sub-semicircular (Figure 10I–J)......................................................................... Triatoma sinica Hsiao, 1965
- −
- Width of eye shorter than synthlipsis in dorsal view (Figure 6A). Body generally black; connexivum generally black with joint area of adjacent segments light yellow (Figure 5D). Basal lateral sclerites of endosoma rounded rectangular (Figure 7G) ......................................................................... Triatoma atrata Zhao & Cai sp. nov.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, A.M.; Roque, A.L.R. Domestic and wild mammalian reservoirs. In American Trypanosomiasis; Telleria, J., Tibyarenc, M., Eds.; Elsevier: London, UK, 2010; pp. 249–276. [Google Scholar] [CrossRef]
- WHO. Chagas Disease (Also Known as American Trypanosomiasis). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 27 March 2023).
- PAHO. Chagas Disease. 2023. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 27 March 2023).
- Galvão, C. Taxonomy. In Triatominae—The Biology of Chagas Disease Vectores; Guarneri, A., Lorenzo, M., Eds.; Entomology in Focus 5; Springer International Publishing: Cham, Switzerland, 2021; pp. 15–38. [Google Scholar] [CrossRef]
- Gil-Santana, H.R.; Chavez, T.; Pita, S.; Panzera, F.; Galvão, C. Panstrongylus noireaui, a remarkable new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 2022, 1104, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Correia, J.P.S.; Gil-Santana, H.R.; Dale, C.; Galvão, C. Triatoma guazu Lent and Wygodzinsky is a junior synonym of Triatoma williami Galvão, Souza and Lima. Insects 2022, 13, 591. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Rendón, J.; Esteban, L.; Rengifo-Correa, L.; Díaz-Albiter, H.; Huerta, H.; Dale, C. Triatoma yelapensis sp. nov. (Hemiptera: Reduviidae) from Mexico, with a Key of Triatoma Species Recorded in Mexico. Insects 2023, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Lent, H.; Wygodzinsky, P. Revision of the Triatominae (Hemiptera, Reduvidae) and Their Significance as Vectors of Chagas’ Disease; Bulletin of the American Museum of Natural History: New York, NY, USA, 1979; Volume 163, pp. 127–520. Available online: http://digitallibrary.amnh.org/handle/2246/1282 (accessed on 10 April 2023).
- Hsiao, T.; Ren, S.; Zheng, L.; Jing, X.; Zou, H.; Liu, S. A Handbook for the Determination of the Chinese Hemiptera—Heteroptera (I); Science Press: Beijing, China, 1981; pp. 1–639. [Google Scholar]
- Hsiao, T. A new species of Triatoma Laporte (Heteroptera: Reduviidae). Acta Polym. Sin. 1965, 2, 197–200. [Google Scholar]
- Qian, Y.; Li, S.; Wang, Q.; Zhang, L.; Liu, W.; Chen, J.; Wang, J.; Xiao; Zhou, X. Rapid risk assessment on the import of American Trypanosomiasis to China. Chin. J. Parasitol. Parasit. Dis. 2013, 31, 57–59. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Yoo, W.G.; Park, H.C.; Yoo, H.S.; Kang, D.W.; Jin, S.D.; Min, H.K.; Paek, W.K.; Lim, J. DNA barcoding of fish, insects, and shellfish in Korea. Genom. Inform. 2012, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Deng, J.; Chen, C.; Zeng, L.; Lin, X.; Cheng, Z.; Qiao, G.; Huang, X. DNA barcoding subtropical aphids and implications for population differentiation. Insects 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Vendrami, D.P.; Ceretti-Junior, W.; Obara, M.T.; Marrelli, M.T. Mitochondrial PCR-RFLP Assay to Distinguish Triatoma brasiliensis macromelasoma from Triatoma brasiliensis brasiliensis Subspecies (Hemiptera: Reduviidae). J. Trop. Med. 2013, 2013, 305198. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Moreira de Souza, R.; Campolina-Silva, G.H.; Bezerra, C.M.; Diotaiuti, L.; Gorla, D.E. Does Triatoma brasiliensis occupy the same environmental niche space as Triatoma melanica? Parasit. Vectors 2015, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Galvão, C.; Cai, W. Rhodnius micki, a new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys 2021, 1012, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.; Weirauch, C. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History, 2nd ed.; Siri Scientific Press: Manchester, UK, 2020; pp. 97–127. [Google Scholar]
- Rosa, J.A.; Mendonça, V.J.; Rocha, C.S.; Gardim, S.; Cilense, M. Characterization of the external female genitalia of six species of Triatominae (Hemiptera: Reduviidade) by scanning electron microscopy. Mem. Inst. Oswaldo Cruz 2010, 105, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Fan, M.; Li, H.; Cai, W. Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species. Insects 2023, 14, 450. https://doi.org/10.3390/insects14050450
Zhao Y, Fan M, Li H, Cai W. Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species. Insects. 2023; 14(5):450. https://doi.org/10.3390/insects14050450
Chicago/Turabian StyleZhao, Yisheng, Mingyuan Fan, Hu Li, and Wanzhi Cai. 2023. "Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species" Insects 14, no. 5: 450. https://doi.org/10.3390/insects14050450
APA StyleZhao, Y., Fan, M., Li, H., & Cai, W. (2023). Review of Kissing Bugs (Hemiptera: Reduviidae: Triatominae) from China with Descriptions of Two New Species. Insects, 14(5), 450. https://doi.org/10.3390/insects14050450






